ABSTRACT
Raspberry (Rubus idaeus L.) explants were cultured in vitro under two boron concentrations (0.1 and 0.5 mM) and three levels of NaCl salinity (0, 5, and 10 mM) on an MS medium. The high boron and salt treatment (0.5 mM B and 10 mM NaCl) diminished mean shoot length and fresh and dry weights of the explants; however, fresh-to-dry weight ratio was positively affected. When B increased in the medium, leaf chlorophyll content and fluorescence were reduced under saline conditions. The absorption of boron, chloride, and sodium was enhanced by increased concentrations of boron and NaCl in the medium. Moreover, B enhanced the uptake and accumulation of chloride and sodium in the explants, but the reverse was not true. Finally, the relative growth rate and relative performance of the explants, under the combined effect of B and NaCl, showed a rapid decline at high boron and salt treatment.
INTRODUCTION
Boron is an essential element implicated in cell wall synthesis and structure, membrane stucture and function, metabolism of phenolic acids and auxins, and even in nitrogen metabolism (CitationRuiz et al., 1998). Plant requirements for boron differ widely among species, and the concentration range between deficient and toxic levels is narrower for this micronutrient than for any other (CitationBastías et al., 2004). High concentration of boron (B) may occur naturally in the soil or in the ground water or be added to the soil from fertilizers or irrigation water (CitationNable et al., 1997). Also, boron is often found in high concentrations in association with saline soils and saline water, and this kind of stress is frequently associated with arid and semiarid environments (CitationLäuchli, 2002; CitationStemberg et al., 2001). Moreover, because boron is removed more slowly than salinity, it may be still present at excessive levels even after salt leaching (CitationAlpaslam and Gunes, 2001). There is currently limited information on the response of plants to the combination of B toxicity and salinity, and there is no consensus about the mutual relations between salinity stress and B toxicity (Yerminahu et al., 2003). CitationBingham et al. (1987) reported that wheat plants respond independently of soil salinity levels and, according to CitationHolloway and Alston (1992), excess external B and salinity interact to limit growth, yield, and yield components of wheat. A similar tendency was found by CitationAlpaslam and Gunes (2001) in tomato. In contrast to this, an antagonistic effect between salinity and B toxicity was observed in 12 different kinds of plants (CitationFerreyra et al., 1997).
The increasing interest in the cultivation of red raspberry in warmer climates and in the mild southern parts of Europe (CitationNeocleous et al., 2005a, Citation2005b; CitationOliveira et al., 1996, Citation1999), where the water salinity is one of the major limiting factors in agricultural production, requires a better understanding about the combined effect of salt with nutrients that have key role in plant development, such as boron. There are only a few studies carried out in comprehensive understanding of the principal physiological mechanisms of salt damage in red raspberries in vivo (CitationNeocleous and Vasilakakis, 2007) and in vitro (CitationNeocleous and Minas, 2007). Since no information was found on the the combined effect of salt and boron concentrations on red raspberry, the aim of this work was an effort to investigate the plant response and adaptation mechanisms in vitro and gather useful information to be used in open field cultivation.
MATERIALS AND METHODS
Apical shoots of red raspberry (Rubus idaeus cv. Autumn Bliss) 20 mm long were collected from actively growing plants in a greenhouse and they were used as explants. Explants were rinsed under running tap water followed by continuous agitation for 15 min in 15% commercial bleach (0.78% sodium hypochlorite) plus 0.1% (v/v) Tween 20 and rinsed in sterile distilled water. Each explant was transferred into a 330-mL fully transparent polycarbonate jar containing 60 mL of culture medium and placed on the upper surface of a solid MS (CitationMurashige and Skoog, 1962) modified medium. The medium contained MS basic salts and vitamins, supplemented with 3% sucrose, 4.5 mg L−1 BAP, 0.009 mg L−1 IBA, and 55.7 mg L−1 ascorbic acid and was solidified with 2.5 g L−1 phytagel. Two B concentrations were used, the original concentration in MS (0.1 mM) and a 5 × concentration (0.5 mM) combined with three NaCl concentrations (0, 5, and 10 mM). The pH of the medium was adjusted at 5.6 before autoclaving at 1.5 atm and 120°C for 20 min. Cultures were incubated in a growth room at 23°C and 16 h photoperiod (2000 lux) for 20 days before evaluation for boron and salt concentrations effects.
The length, fresh, and dry weights (after drying at 68°C) of the micro shoots were measured and the ratio fresh:dry weight was calculated. Leaf chlorophyll fluorescence was estimated using chlorophyll fluorometer OS-30p (Opti-Sciences, Hudson). The OS-30p (Opti-Science, Hudson, NH, USA) measures chlorophyll fluorescence parameters F 0 and Fm where F 0 is the initial fluorescence, Fm is the maximum fluorescence, and the variable fluorescence Fv is calculated as: Fv = Fm − F 0. To estimate the leaf chlorophyll content, three leaf disks of 2.5 cm2 area were weighed and ground in 10 cm3 of N,N dimethylformamide. After 48 h storage in the dark at 5°C, the absorbance of the supernatant was measured at 647 and 664 nm with a Campesc M350 UV-Vis spectrophotometer (Campesc Ltd, Cambridge, U.K.) and chlorophyll content—a, b, and a + b—was computed according to CitationMoran (1982).
To determine the mineral composition, plantlets were harvested and rinsed with distilled water. After drying, chloride concentration in the micro shoots was determined by titration against silver nitrate (CitationRichards, 1954) and sodium by flame photometer Sherwood model 420, (Sherwood Scientific Ltd, Cambridge, U.K.). Boron was determined by the carmine method according to CitationHatcher and Wilcox (1950). The relative growth rate (RGR) was calculated as: RGR = (lnW 2 − lnW 1)/(t 2 − t 1) (CitationBlackman, 1919), where W 1 and W 2 are plant dry weights at times t 1 and t 2. Finally, to compare the relative performance (RP) between treatments, the following equation was used to estimate the comparative changes dry weight: RP = [(Performance under stress)/(Performance under control conditions)] × 100.
Each treatment included 25 replicates (jars) randomly assigned. Differences between means were evaluated using Duncan's multiple range test at P ≤ 0.05.
RESULTS
B and NaCl Effect on Visual Symptoms
No visual symptoms were observed on micro shoots grown in culture media supplemented with various concentrations of B and NaCl in the present study.
B and NaCl Effect on Growth Parameters
As shown in , the presence of B and NaCl in the growing medium considerably affected the length and fresh and dry weights of the micro shoots. Particularly, the reduced growth occurred when explants were grown on culture medium containing 0.5 mM B and 10 mM NaCl. In contrast, the same concentrations increased the fresh-to–dry weight ratio ().
TABLE 1. Effect of B and NaCl concentration of the culture medium on the length, fresh and dry weights, and fresh-to–dry weight ratio per explant (Rubus idaeus L. ‘Autumn Bliss’)
B and NaCl Effect on Chlorophyll Content and Chlorophyll Fluorescence
In comparison with the medium containing 0.1 mM B and 0 mM NaCl, the reductions in chlorophyll content and fluorescence were statistically significant when B concentration in the medium was 0.5 mM and NaCl 5 and 10 mM (). The maximum reduction was observed at 0.5 mM B and 10 mM NaCl and amounted to 30% and 9% for chlorophyll content and fluorescence, respectively ().
TABLE 2. Effect of B and NaCl concentration of the culture medium on the total chlorophyll (a + b) and chlorophyll fluorescence of raspberry explants (Rubus idaeus L. ‘Autumn Bliss’)
B and NaCl Effect on Ion Content
By increasing the B concentration from 0.1 mM to 0.5 mM in the growing medium the B concentration of the explants increased by 2.5× as a mean for the various NaCl treatments (). Under saline conditions, increasing B concentration in the medium increased the chloride and sodium content of the explants by 1.6× and 3×, respectively (). In addition, by increasing NaCl from 0 to 10 mM, chloride and sodium contents of the explants showed an increase at low B concentration only at 10 mM, although at high B concentrations the increase was obvious at 5 and 10 mM NaCl ().
TABLE 3. Effect of B and NaCl concentration of the culture medium on B, Cl, and Na concentrations of raspberry explants (Rubus idaeus L. ‘Autumn Bliss’)
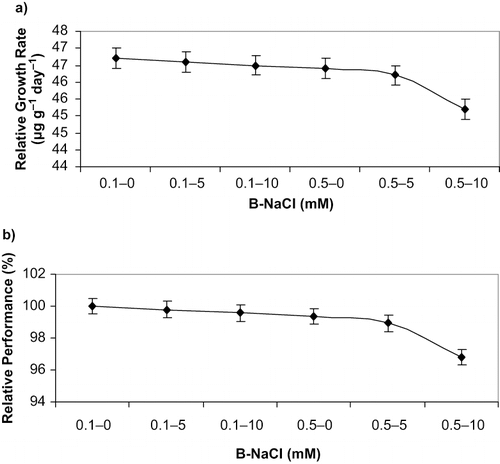
B and NaCl Effect on Relative Growth Rate and Relative Performance
The relative growth rate and the relative performance of the explants showed similar patterns for the various boron and salinity treatments (). Particularly, both showed a rapid decline when B and NaCl concentrations were highest in the medium ().
DISCUSSION
The raspberry explants did not show any visual toxicity symptoms. It thus appears that either shoots grown in vitro and intact plants differ in their sensitivity, like in kiwifruit shoot cultures (T.E. CitationSotiropoulos and Dimassi, 2004), or the experimental period was too short for the symptoms to become visible.
In the present study, the explants responded to high B and NaCl concentrations in the growing medium by exhibiting decreased growth. Reduced growth in response to salinity and boron has been reported and in other plants in vivo (CitationBastías et al., 2004; Laüchli, 2002; CitationSternberg et al., 2001) and in vitro (CitationSotiropoulos and Dimassi, 2004). Additionally, interactive effects of excess boron and salinity on growth and yield have been reported previously in wheat (CitationGrieve and Poss, 2000; CitationHolloway and Alston, 1992), tomato and cucumber (CitationAlpaslan and Gunes, 2001). Restriction of growth is mainly caused by salt-induced osmotic effects, ion toxicity, and mineral perturbations (CitationHasegawa et al., 2000). In the present study, B and NaCl application did not have a negative effect on the fresh-to-dry weight ratio. This may indicate that the restriction of growth in raspberry explants was not impaired with water limitations due to osmotic effects (CitationNeocleous and Vasilakis, 2007). Possibly, ion effects on the cytoplasm or chloroplast functions and the energy required to regulate inorganic ions may limit the potential for plant growth (CitationStavarek and Reins, 1984; CitationZhu, 2001).
The leaf chlorophyll content and fluorescence was not significantly affected by NaCl concentration when B concentration was low. However, at high B concentrations in the medium the leaf chlorophyll content and fluorescence were reduced in the presence of 5–10 mM NaCl. From these results, it could be suggested that the application of boron enhanced the negative effect of salinity on photochemical parameters and subsequent photosynthetic processes in our experiment (CitationBounfour et al., 2002). Similarly, in vitro chlorophyll reduction has been reported as a result of B toxicity in apple cultures (T. CitationSotiropoulos et al., 2004). Free radical–induced oxidative stress has been associated with several cellular toxic processes, including oxidation damage to chlorophylls (CitationCobb, 1992) and Cl ions at toxic levels can cause blockage in the electron transport within photosystem II (Feirabend et al., 1992). As a consequence, the combined effect of B and NaCl on raspberries in vitro possibly induces a toxic effect in photochemical parameters associated with limited metabolic energy for plant growth.
High boron and saline conditions in the growing medium altered the nutrient status in explants tissues in our experiment. The concentration of boron, sodium, and chloride found in tissues indicated that under the levels of boron and NaCl applied in the medium uptake and accumulation was stimulated. Although B absorption is probably controlled by transpiration, under saline and excessive B conditions absorption may be passively diffused to the plants and the diffusion rates of B may be higher in salt sensitive plants (Danell et al., 2002). Recent papers have reported the induction of expression of the aquaporin isoform protein (PIP2-1) in salt-treated plants (CitationBastías et al., 2004) and considerable transport of B through plasma membrane aquaporin has been descripted (CitationDordas and Brown, 2001). In the present study, the accumulation of B in explant tissues was enhanced by increasing B in the medium, but uptake was not found to be stimulated under saline conditions. Similarly, other crops, like alfalfa, barley, and maize, responded independently to B and salinity conditions (CitationBastías et al., 2004). However, the application of B promoted the absorption of chloride and sodium from raspberry explants in the current study. An increase of membrane permeability caused by applied B under saline conditions has been reported in tomato. In contrast, membrane permeability was not found to differ in the presence of B applied under non-saline conditions (CitationAlpaslan and Gunes, 2001).
Finally, from these results and taking into consideration the relative growth rate and relative performance of the explants, it can be deduced that excessive B conditions enhanced the NaCl inhibitory effect on raspberries grown in vitro. The results obtained in the present study should be taken into account when cultivating raspberries in soils with high levels of salt and boron.
LITERATURE CITED
- Alpaslan , M. and Gunes , A. 2001 . Interactive effects of boron and salinity stress on the growth, membrane permeability and mineral composition of tomato and cumber plants . Plant Soil , 236 : 123 – 128 .
- Bastías , E.I. , González-Moro , M.B. and González-Murua , C. 2004 . Zea mays L. amylacea from the Lluta Valley (Arica-Chile) tolerates salinity stress when high levels of boron are available . Plant Soil , 267 : 73 – 84 .
- Bingham , F.T. , Strong , J.E. , Rhoades , J.D. and Keren , R. 1987 . Effect of salinity and varying boron concentration on boron uptake and growth of wheat . Plant Soil , 97 : 345 – 351 .
- Blackman , V.H. 1919 . The compound interest law and plant growth . Ann. Bot. , 33 : 353 – 360 .
- Bounfour , M. , Tanigoshi , K.L. , Chen , C. , Cameron , J.S. and Klauer , S. 2002 . Chlorophyll content and chlorophyll fluorescence in red raspberry leaves infested with Tetranychus urticae and Eotetranychus carpini borealis (Acari: Tetranychidae) . Environ. Entomol. , 31 : 215 – 220 .
- Cobb , A. 1992 . Herbicides and plant physiology , London : Chapman & Hall .
- Dannel , F. , Pfeffer , H. and Römheld , V. 2002 . Update o boron in higher plants—Uptake, primary translocation and compartmentation . Plant Biol. , 4 : 199 – 210 .
- Dordas , C. and Brown , P.H. 2001 . Evidence for channel mediated transport of boric acid in squash Cucurbita pepo . Plant Soil , 235 : 95 – 103 .
- Feierabend , J. , Schaan , C. and Hertwig , B. 1992 . Photoinactivation of catalase occurs under both high- and low-temperature stress conditions and accompanies photoinhibition of photosystem II . Plant Physiol. , 22 : 1554 – 1561 .
- Ferreyra , R.E. , Aljaro , A.U. , Ruiz , R.Sch. , Rojas , L. and Oster , J.D. 1997 . Behaviour of 42 crop species grown in saline soils with high boron concentrations . Agr. Water Mgt. , 34 : 111 – 124 .
- Grieve , C.M. and Poss , J.A. 2000 . Wheat response to interactive effects of boron salinity . J. Plant Nutr. , 23 : 1217 – 1226 .
- Hasegawa , P.M. , Bressan , R.A. , Zhu , J.K. and Bohnert , H.J. 2000 . Plant cellular and molecular responses to high salinity . Ann. Rev. Plant Physiol. Plant Mol. Biol. , 51 : 463 – 499 .
- Hatcher , J.T. and Wilcox , L.V. 1950 . Colorimetric determination of boron using carmine . Ann. Chem. , 22 : 567 – 569 .
- Holloway , R.E. and Alston , A.M. 1992 . The effect of salt and boron on growth of wheat . Austral. J. Agr. Res. , 43 : 987 – 1001 .
- Läuchli , A. 2002 . Functions of boron in higher plants: recent advances and open questions . Plant Biol. , 4 : 190 – 192 .
- Moran , R. 1982 . Formulae for determination of chlorophyllus pigments extracted with N,N-dimethyflormamine . Plant Physiol. , 69 : 1376 – 1381 .
- Murashige , T. and Skoog , F. 1962 . A revised medium for rapid growth and bioassays with tobacco tissue cultures . Physiol. Plant , 15 : 473 – 497 .
- Nable , R.O. , Banuelos , G.S. and Paull , J.G. 1997 . Boron toxicity . Plant Soil , 193 : 181 – 198 .
- Neocleous , D. and Minas , G. 2007 . An in-vitro test for salt (NaCl) tolerance of red raspberry Rubus idaeus L. cultures . Acta Hort. , 741 : 289 – 294 .
- Neocleous , D. , Papadopoulos , I. and Vasilakakis , M. 2005a . Investigating the possibility of fruiting primocane raspberries for off-season production in Cyprus . European J. Hort. Sci. , 70 : 89 – 95 .
- Neocleous , D. , Papadopoulos , I. and Vasilakakis , M. 2005b . Growing red raspberry in soilless culture under different chilling treatments for early summer production . Small Fruits Rev. , 4 ( 4 ) : 37 – 48 .
- Neocleous , D. and Vasilakakis , M. 2007 . Effects of NaCl stress on red raspberry (Rubus idaeus L. ‘Autumn Bliss’) . Sci. Hort. , 112 : 282 – 289 .
- Oliveira , P.B. , Oliveira , C.M. , Lopes-de-Fonseca , L. and Monteiro , A.A. 1996 . Off season production of primocane-fruiting red raspberry using summer pruning and polyethylene tunnels . HortScience , 31 : 805 – 807 .
- Oliveira , P.B. , Oliveira , C.M. , Monteiro , A.A. and Lopes-da-Fonseca , L. 1999 . Summer-pruning intensity affects on off-season production of primocane-fruiting red raspberries . Acta Hort. , 505 : 101 – 106 .
- Richards , L.A. 1954 . Diagnosis and improvement of saline and alkali soils . US Dept. Agr. Agr. Hdbk. , 60 : 160
- Ruiz , J.M. , Baghour , G. , Bretones , A. , Belakbir , A. and Romero , L. 1998 . Nitrogen metabolism in tobacco plants Nicotiana tabacum L.: Rile of boron as a possible regulatory factor . Intl. J. Plant Sci. , 159 : 121 – 126 .
- Sotiropoulos , T. , Mouchtaridou , G. , Dimassi , K. and Therios , I. The effect of boron concentration in the culture media on growth, element composition and chlorophyll content of in vitro apple rootstock MM 106 cultures . Paper presented at the 21st symposium of the Greek Society of Horticulture Science, Arta, 8–10 Oct. 2003 . 2004 .
- Sotiropoulos , T.E. and Dimassi , K.N. 2004 . Response to increasing rates of boron and NaCl on shoot proliferation and chemical composition of in vitro kiwifruit shoot cultures . Plant Cell Tissue Organ Cult. , 79 : 285 – 289 .
- Stavarek , S.J. and Rains , D.W. 1984 . The development of tolerance to mineral stress . HortScience , 19 : 13 – 18 .
- Stemberg , P.D. , Ulery , A.L. and Villa , C.M. 2001 . Salinity and boron effects on growth and yield of teraby and kidney beans . HortSci , 7 : 1269 – 1272 .
- Yermiyahu , U. , Keren , R. and Chen , Y. 2001 . Effect of compost organic matter on boron uptake by plants . Soil Sci. Soc. Amer. J. , 65 : 1436 – 1441 .
- Zhu , J.K. 2001 . Plant salt tolerance: Regulatory pathways, genetic improvement and model systems . Trends Plant Sci. , 6 : 66 – 71 .