Abstract
In the present work the desorption isotherms of 2 cultivars of pears produced in Portugal were determined for the temperatures of 20, 30 and 40°C. This range of temperatures was selected because it covers the range of average daily temperatures in Portugal in the summer, when the solar drying of pears takes place. The data were obtained using an orthogonal distance regression algorithm (ODR) to five different models found in literature, namely Chen, Guggenheim-Anderson-de Boer (GAB), Halsey, Henderson, and Oswin. From the present work, it was possible to conclude that the 2 cultivars of pears studied show a similar behavior, and that at constant moisture content an increase in temperature enhances water activity. By comparing the different models, it was possible to conclude that the Oswin model is less appropriate for describing the drying process, and that neither the Chen nor the Halsey model is adequate. The Henderson, and particularly, the GAB models were very good at describing the different situations.
INTRODUCTION
All food materials are characterized by a particular sorption isotherm at a constant temperature. Equilibrium moisture data provided by sorption isotherms is very important for the determination of optimal storage conditions as well as predicting thermodynamic equilibrium models. When drying fruit, they assume an important role in properly choosing the end-point of the process, corresponding to the optimum residual moisture content of the dried food. Such isotherms also give valuable information on how strong the water is bound to the food matrix and is essential for predicting the drying and rehydration rates (CitationGuiné, 2002; CitationVázquez-Uña, 2001).
At the end of the drying process, the moisture content of the food reaches a state of equilibrium with the surrounding atmosphere. The moisture sorption isotherms are obtained when this equilibrium moisture content is plotted against the corresponding water activity of the food product for a constant temperature (CitationPark, 2001).
The final water content of the food determines the end-point of the drying process, which should correspond to the optimum residual moisture content of the final product. In fact, a high water content obtained with a short drying time leads to reduced stability, whereas drying the material to a moisture content below a given optimum value leads to energy waste with no apparent benefit.
The classification of adsorption isotherms in terms of the Van-der Walls adsorption of gases distinguishes five different types. For most foods, sorption isotherms are nonlinear, usually sigmoidal shape, however, for foods rich in soluble components such as sugars, the type that best seems to describe the sorption behavior is an exponential growth function (CitationVázquez-Uña, 2001; CitationRizvi, 1986).
Many different models can be found in the literature to describe the sorption behavior of foods (CitationPark, 2001, CitationViswanathan, 2003). Most of those models are represented by semiempirical equations that include a variable number of parameters.
In some cases one sorption model might not be enough to represent the sorption behavior over the entire water activity range, because water is associated with the food matrix in different ways for different activity regions.
In some of the more commonly used sorption isotherm models are listed. For the different models “We” represents the dry basis equilibrium moisture content, “Wm” is the dry basis monolayer moisture content, “aw” is the water activity, “R” is the gas constant (R = 8.31451 J.mol-1.K-1) and “T” is absolute temperature.
TABLE 1 Some of the Models Found in the Literature to Describe Sorption Isotherms
Two of the models presented show an explicit dependency on temperature (Halsey and Henderson), while some others include the temperature dependency on some parameters (Chen and GAB). The Oswin model does not take into consideration the influence of temperature.
Generally, higher temperatures result in a reduction of adsorbed molecules and therefore adsorption decreases with increasing temperature, although this dependency is usually small. However, this behavior is not universal as temperature affects many different phenomena and an increase in temperature can, in fact, increase the rates of adsorption, hydrolysis, and recrystallization processes (CitationRizvi, 1986). Foods rich in soluble solids, such as sugars, seem to exhibit antithetical temperature effects for higher values of ‘aw’ due to their increase solubility in water (CitationRizvi, 1986).
EXPERIMENTAL DESIGN
The pears used in this study (“Rocha” and “Rosa”) are of 2 different cultivars produced in Portugal.
The experimental data for the desorption isotherms were determined using circular slices of pear (30 mm in diameter and 3 mm thick), which were placed in net trays and dried in a ventilated chamber (WTB-Binder) at a constant temperature. Hourly, 3 different samples were randomly taken out of the chamber and were analyzed to determine their moisture content (with a Mettler Toledo HG53 Halogen Moisture Analyzer) and the corresponding water activity (measured by a Rotronic Hygroskop BT-RS1).
The sorption behavior of pears was investigated for constant drying temperatures of 20, 30, and 40°C, and the experimental data obtained allowed for the estimation of the parameters in the different models considered.
Fitting Procedure
The parameters of the models were estimated from the experimental data using an Orthogonal Distance Regression algorithm (ODR) with the derivatives approximated by central finite differences, where the unknown errors are taken into account in both dependent and independent variables (CitationGuiné, 2002).
If for a set of data (xi,yi), i = 1, … ,n, (where yi is supposed to be a non-linear function of xi and a set of parameters βk) both xi and yi contain unknown errors (δi and εi, respectively), then the observed value of yi satisfies (CitationBoggs, 1992):
As a result of unknown errors in both the dependent and independent variables, the ODR method finds the best solution of the estimation problem by minimizing the n orthogonal distance from the curve f(x;β) to all data points, i.e. (CitationBoggs, 1992):
The software package used to compute the parameters (ODRPACK) was developed by the Center for Computing and Applied Mathematics of the National Institute of Standards and Technology, USA (CitationBoggs, 1992).
In , the estimations for the different models are presented for the 2 cultivars of pears and the 3 temperatures studied. In each table the values of the parameters are presented together with the statistical information that characterizes the estimation: the number of observations (N), the sum of square deltas (Σ δ2—referring to the dependent variable), the sum of square epsilons (Σ ε2—referring to the independent variable), the sum of square errors (Σ e2), and the residual standard deviation (RSD). In all cases, the experimental data in the form of sets (aw and W) were treated separately for the 3 different temperatures studied, and the number of observations varied from 16–24.
TABLE 2 Results of the Parameter Estimation with Chen Model
TABLE 3 Results of the Parameter Estimation with GAB Model
TABLE 4 Results of the Parameter Estimation with Oswin Model
TABLE 5 Results of the Parameter Estimation with Halsey Model
TABLE 6 Results of the Parameter Estimation with Henderson Model
RESULTS AND DISCUSSION
Comparison of the 2 Cultivars Studied
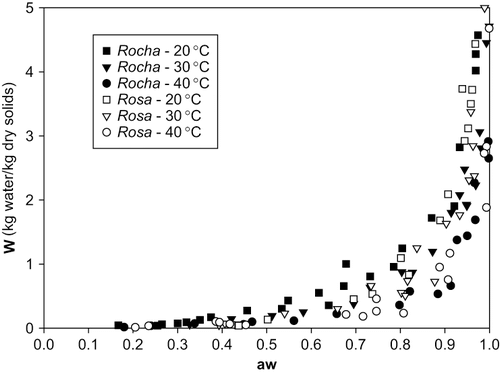
In , the experimental points obtained for the different temperatures for the 2 cultivars of pears are presented for comparative study. The 2 cultivars of pears under study, “Rocha” and “Rosa”, show a very similar desorption behavior when exposed to the 3 different temperatures tested: 20, 30 and 40°C.
Effect of Temperature and Aw on the Desorption Behaviour of the Pears
The range of pear moisture content (dry basis) analyzed varied from 0–5, corresponding to measured values of “aw” between approximately 0.15 and 1.0. From , it is possible to verify that for both types of pear, the equilibrium moisture content decreases with increasing temperature for the same “aw”, and decreases as “aw” diminishes for constant temperature. This behavior has been observed for many different food products, including tomato and onion (CitationKiranoudis, 1993, CitationLewicki, 1998; CitationViswanathan, 2003), tapioca (CitationSanni, 1997), potatoes (CitationMcLaughlin, 1998), maize kernels (CitationChen, 1998), and pears (CitationPark, 2001).
Evaluation of the Different Models Considered
compile the results of the estimation for each model, and include the values of the computed parameters as well as the corresponding statistical information. It is possible to see that, in general, the quality of the fits is quite acceptable regardless of a few cases in which the values of the sum of square errors (Σ e2) and residual standard deviation (RSD) are higher, which are the curves obtained with Oswin model for the 2 cultivars at 30 and 40°C, indicating that these fits are of poorer quality. The curves that best seem to fit the experimental data by presenting lower values of Σ e2 and RSD are the ones obtained with the Henderson model for the cultivar “Rocha” at all temperatures, and for the cultivar “Rosa” at 20°C. However, the quality of the fit of one particular model to the experimental data does not necessarily reveal the true nature of the process.
It is important to notice that there is a higher degree of uncertainty in the measurements of “aw” when compared to the values of “W”,expressed by the higher values of the errors associated to the variable “aw” than to “W”, with “aw” being either the dependent or independent variable, according to the form of the equation considered.
In , the experimental points are plotted together with the curves predicted with each model for the 3 temperatures studied. By observing these figures, the similarity between the GAB and Henderson models is evident ( and , respectively), which seem to describe quite adequately the desorption behavior of both types of pears for the temperatures studied. These results are in agreement with the lower values of Σ e2 and RSD found for these fits. However, the Henderson model is slightly less capable of evidencing the differences in the curves for the 3 temperatures that are observed with the GAB model in the range of “aw” between 0.5 and 0.8, for the pears of the cultivar “Rosa.”
FIGURE 2 Representation of sorption isotherms for 20, 30 and 40°C with GAB model: (a) cultivar Rocha (b) cultivar Rosa.
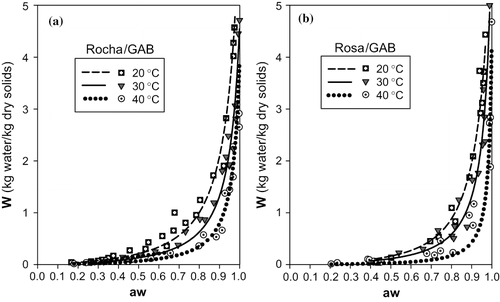
FIGURE 3 Representation of sorption isotherms for 20, 30 and 40°C with Henderson model: (a) cultivar Rocha (b) cultivar Rosa.
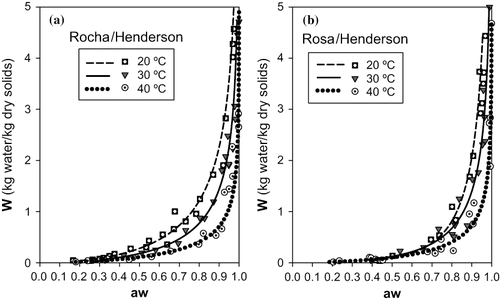
FIGURE 4 Representation of sorption isotherms for 20, 30 and 40°C with Oswin model: (a) cultivar Rocha (b) cultivar Rosa.
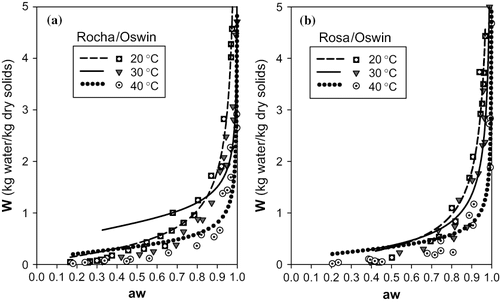
FIGURE 5 Representation of sorption isotherms for 20, 30 and 40°C with Chen model: (a) cultivar Rocha (b) cultivar Rosa.
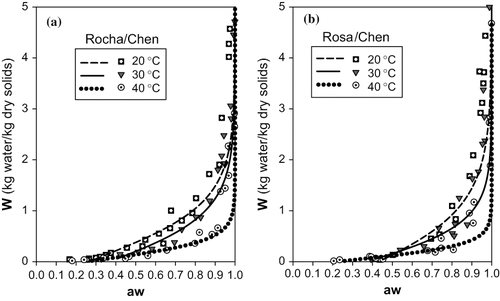
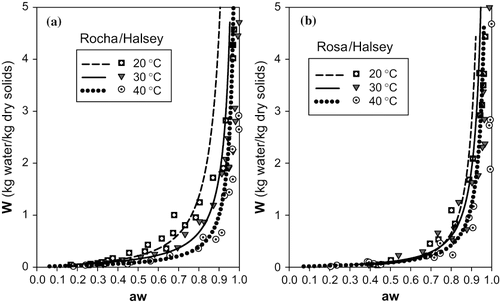
FIGURE 6 Representation of sorption isotherms for 20, 30 and 40°C with Halsey model: (a) cultivar Rocha (b) cultivar Rosa.
The Oswin model () is the one that shows a poorer performance, particularly for higher temperatures (30 and 40°C), although it is apparently good at describing the desorption behavior of both types of pear at 20°C.
The fits obtained with the Chen model () are relatively good for the 3 temperatures, taking into consideration that the values of the parameters that characterize the quality of the fit (Σ e2 and RSD) are quite low. However, in , the good fit of this model to the experimental data is visible for lower temperatures (20 and 30°C), but it is not so evident for the temperature of 40°C, when compared to other models, and in particular the GAB model.
The curves presented in were obtained with the Halsey model, and show a relative adequacy to the desorption processes at study. However, for the curve of the cultivar “Rocha” at 20°C we observed a significant departure from the experimental data for values of “aw” higher than 0.8. The curves for the cultivar “Rosa” obtained with this model do not seem to evidence the true differences observed experimentally for the 3 temperatures. In fact, in the range of “aw” <0.8 the curves predicted with the Halsey model for the different temperatures are practically coincident, when other models can differentiate them, particularly the Chen and GAB models.
CONCLUSIONS
From the present work, it was possible to conclude that the 2 cultivars of pears studied show a similar desorption behavior in the range of temperatures evaluated, and that at constant moisture content, an increase in temperature enhances water activity following the same pattern found for other food products.
From comparing the different models, it was possible to conclude that the Oswin model is less appropriate for describing the desorption processes studied, and that the Chen and Halsey models, although showing some adequacy in some of the cases, are also not very good in representing the behavior of both types of pears in the range of temperatures evaluated. The Henderson and GAB models, and this last in particular, were revealed to be very good at describing the different situations in the study for the 2 cultivars of pears and the 3 temperatures.
LITERATURE CITED
- Anderson , R.B. 1946 . Modifications of the BET equation . J. Am. Chem. Soc. , 68 : 686 – 691 .
- de Boer , J.H. 1953 . The dynamical character of adsorption , Oxford : Clarendon Press .
- Boggs , P.T. 1992 . User's reference guide for ODRPACK version 2.10. Software for Weighted Orthogonal Distance Regression , Gaithersburg, MD, , USA : National Institute of Standards and Technology .
- Chen , C.S. and Clayton , J.T. 1971 . The effect of temperature on sorption isotherms of biological materials . T. ASAE , 14 : 927 – 929 .
- Chen , C. and Jayas , D.S. 1998 . Dynamic equilibrium moisture content for grain drying. Can . Agr. Eng. , 40 ( 4 ) : 299 – 303 .
- Guggenheim , E.A. 1966 . Applications of satistical mechanics , Oxford : Clarendon Press .
- Guiné , R.P.F. and Castro , J.A.A.M. 2002 . Experimental determination and computer fitting of desorption isotherms of d. joaquina pears . Food and Bioprod. Proc.: T. IchemE, part C , 80 ( C3 ) : 149 – 154 .
- Halsey , G. 1948 . Physical adsorption on non-uniform surfaces . J. Chem. Phys. , 16 : 931 – 937 .
- Henderson , S.M. 1952 . A basic concept of equilibrium moisture . Agr. Eng. , 33 : 29 – 32 .
- Kiranoudis , C.T. , Maroulis , Z.B. , Tsami , E. and Marinos-Kouris , D. 1993 . Equilibrium moisture content and heat desorption of some vegetables . J. Food Eng. , 20 ( 1 ) : 55 – 74 .
- Lewicki , P.P. 1998 . A three parameter equation for food moisture isotherms . J. Food Proc. Eng. , 21 ( 2 ) : 127 – 144 .
- McLaughlin , C.P. and Magee , T.R.A. 1998 . The determination of sorption isotherms and the isosteric heats of sorption for potatoes . J. Food Eng. , 35 ( 3 ) : 267 – 280 .
- Oswin , C.R. 1946 . The kinetics of package life. III. The isotherm . J. Soc. Chem. Ind. , 65 : 419 – 423 .
- Park , K.J. and Bin , A. 2001 . Obtenção de isotermas de sorção e modelagem matemática para a pêra Bartlett (Pyrus sp.) com e sem desidratação osmótica. Ciênc . Tecnol. Aliment , 21 ( 1 ) : 73 – 77 .
- Rizvi , S.S.H. 1986 . Thermodynamic properties of foods in dehydration in engineering properties of foods , New York : Marcel Dekker .
- Sanni , L.O. , Atere , C. and Kuye , A. 1997 . Moisture sorption isotherms of fufu and tapioca at different temperatures . J. Food Eng. , 34 ( 2 ) : 203 – 212 .
- Vázquez-Uña , G. , Chenlo-Romero , F. and Moreire-Martínez , R. 2001 . “ Adsorption and desorption isotherms of lupin (Lupius albus L.) at several tempeatures ” . In CHEMPOR: 8th International Conference of Chemical Engineers , 1071 – 1076 . Portugal : Universidade de Aveiro .
- Viswanathan , R. , Jayas , D.S. and Hulasare , R.B. 2003 . Sorption isotherms of tomato slices and onion shreds . Biosystems Eng. , 86 ( 4 ) : 465 – 472 .