Abstract
Leaf bases excised from in vitro derived shoots of pineapple (Ananas comosus LMerr. cv. Queen) were transformed with Agrobacterium tumefaciens strain EHA 105 harboring the pMSI168 plasmid with MSI-99, a magainin analogue, by co-cultivating for three days in the dark on Murashige and Skoog (MS) basal medium (CitationMurashige and Skoog, 1962). The leaf bases produced callus and/or multiple shoots upon transfer to MS medium supplemented with NAA (1.8 mg/l), IBA (2.0 mg/l), kinetin (2.0 mg/l), cefotaxime (400 mg/l) and kanamycin (50 mg/l) after six to eight weeks. Six percent of the leaf bases produced friable callus, and only two percent produced multiple shoots directly without intervening callus formation. Putatively transformed shoots (1–2 cm) were selected and multiplied on medium of the same composition, and elongated shoots (5 cm) were rooted on liquid CitationWhite's (1954) medium supplemented with NAA (0.1 mg/l ), IBA (0.4 mg/l), cefotaxime (400 mg/l), and kanamycin (100 mg/l). A few of the transgenic plants were grown to maturity in the greenhouse. The rooted plants were analyzed through PCR, Southern analysis of the PCR products, and reverse transcription (RT)-PCR. The results clearly confirmed the integration and expression of MSI-99. All the plants were morphologically normal and set fruits. To the best of our knowledge this is the first report on the transformation of pineapple with MSI-99, a magainin analogue.
INTRODUCTION
Pineapple is one of the economically important fruit crops of many tropical countries (CitationDuval et al., 2001). The pineapple fruit is parthenocarpic, seed set is poor and the plant is vegetatively propagated. Classical breeding is extremely laborious and time-consuming (CitationBotella et al., 2000). Unlike other fruits, pineapple fruit has no starch reserves of its own. The sugar that makes up 15% of the fruit accumulates before it is harvested and the harvested fruit does not have the resources to sweeten any further. As a practice, therefore, pineapples are picked when already ripe, unlike bananas or peaches, which ripen after harvest (CitationBeauman, 2005). Due to this practice, pineapple fruits are ridden with postharvest problems, such as spoilage due to fungal infections during handling, storage, and transport. Fusarium moniliforme var. subglutinins that causes fusariosis (gummosis), Chalara paradoxa (Thielaviopsis paradoxa) that causes ‘black rot’ (water blister), and Phytophthora cinnamomi that causes rot in green fruit on ratoons are some of the causes of fungal diseases infecting pineapple fruits.
Plants protect themselves against pathogens by various defense responses, including the production of antimicrobial peptides (CitationFeng et al., 2003). Antimicrobial proteins are short, cationic, amphiphilic peptides that form an important component of immune defenses (CitationHancock and Sahl, 2006). Magainin is one of the earliest reported antimicrobial peptides isolated from the skin secretions of the African clawed frog, Xenopus laevis. It is effective against gram-positive and gram-negative bacteria, fungi, and protozoa. Recently, a few reports have been made available on the expression of different analogues of magainin in tobacco (CitationLi et al., 2001), tomato (Alan et al., 2004), and potato (CitationO'Callaghan et al., 2004; CitationGanapathi et al., 2007) for obtaining enhanced disease resistance. A synthetic substitution analogue of magainin, MSI-99, was used in the transformation of tobacco and banana. The analysis of transgenic tobacco plants showed resistance to Sclerotinia sclerotiorum, Alternaria alternata, and Botrytis cinerea. Transgenic banana plants showed resistance to Fusarium oxysporum f.sp. cubense and Mycosphaerella musicola (CitationChakrabarti et al., 2003). In this communication, we report the successful expression of this synthetic peptide in pineapple and normal growth and fruiting of transgenic plants.
MATERIAL AND METHODS
Plant Material and Culture Media
In vitro cultures of pineapple (Ananas comosus L. Merr. cv. ‘Queen’) initiated from dormant axillary buds (from crowns) and multiplied on Pin-1 medium (CitationSoneji et al., 2002a) were used as stock cultures for leaf explants. Leaves (3–4 cm) were peeled and the base (0.5–1.0 cm) was excised and used for Agrobacterium-mediated transformation.
Agrobacterium tumefaciens-Mediated Transformation and Regeneration of Transgenic Plants
Leaf bases of pineapple were used for transformation. The leaf bases were co-cultivated with A. tumefaciens strain EHA105 (CitationHood et al., 1993) harboring pMSI168. The plasmid map and the construction of the expression vector are described previously (CitationChakrabarti et al., 2003). A single Agrobacterium colony was grown in liquid YENB medium essentially as described (CitationShekhawat et al., 2008). The leaf bases were co-cultivated in the presence of acetosyringone (100 μM) for 20 min. Subsequently, the leaf bases were pat-dried thoroughly on sterilized tissue paper sheets and co-cultivated on Pin-1 medium (CitationSoneji et al., 2002a) solidified with gelrite 0.2% (Sigma-Aldrich) for three days (50 explants per petri plate). After co-cultivation, leaf bases were transferred to Pin-1 medium supplemented with cefotaxime (400 mg/l) for three days. Induction of multiple shoots was achieved upon transfer to Pin-1 medium containing cefotaxime (400 mg/l) and kanamycin (50 mg/l) after six to eight weeks of repeated (weekly) subculture. Rooting of shoots (5 cm) was achieved on filter paper bridges in liquid RM medium (CitationSoneji et al., 2002a) containing cefotaxime (400 mg/l) and kanamycin (100 mg/l) after six weeks. Hardening of transgenic plants was carried out in the greenhouse by transferring to paper cups filled with autoclaved horticultural soil. Eleven three-month-old hardened plants were randomly chosen for molecular analysis.
Analysis of Pineapple Transgenic Plants by Polymerase Chain Reaction (PCR)
Total nucleic acids from the leaves of eleven hardened transformed plants and untransformed control plants were isolated using the method of CitationDellaporta and others (1983). The nucleic acids were treated with RNAse (final concentration 100 μg/ml) at 37°C for 1 hr, followed by phenol-chloroform extraction and alcohol precipitation. The upstream primer used for PCR was specific to the secretory signal sequence (P168), and the 3′-end primer was specific to the 5′ region of the nos terminator (Pnos). The primer sequences used are same as described previously (Chakraborty et al., 2003). A 50 ng sample of total DNA was used for PCR analysis in a reaction volume of 50 μl containing 1x Taq polymerase buffer, 100 μM each dNTP, 1 μM each primer, and 1 unit of Taq polymerase (Bangalore Genei, India). The PCR conditions used were initial melting at 94°C for 4 min, followed by 30 cycles of amplification consisting of 94°C for 1 min, 58°C for 1 min, 72°C for 1 min, followed by final extension at 72°C for 10 min. The products were analyzed on a 2% agarose gel using 1x TAE buffer.
Southern Analysis of PCR Products
The PCR products after gel separation were blotted onto nylon membranes (Hybond N+; Amersham-Pharmacia). The 500bp EcoRI fragment from pSAN168 containing the MSI-99 gene and nos terminator was radioactively labeled with α-[32P]dCTP using the Random Primer Kit from BRIT (India) according to the manufacturer's instructions for hybridization. The blotting and subsequent hybridization were carried out according to CitationSambrook and others (1989).
Reverse Transcription (RT)-PCR of Transgenic Pineapple Plants
Total nucleic acids were isolated from the leaves of hardened transformed and untransformed control plants as described above, and then treated with RNAse-free DNAse (Amersham-Pharmacia) at final concentration of 5 units/μg of total nucleic acid for 1 hr at 37°C. Total RNA was alcohol-precipitated after phenol-chloroform extraction. A 4 μg aliquot of total RNA was used for cDNA synthesis in a final volume of 15 μl using oligo-dT primer (first strand cDNA synthesis kit; Amersham-Pharmacia) according to the manufacturer's instructions. A 4 μl sample of the first-strand reaction mix was used for PCR amplification using conditions as above, except the initial melting. The products were analyzed as described earlier.
RESULTS AND DISCUSSION
Regeneration of Transgenic Pineapple Plants from Leaf Bases
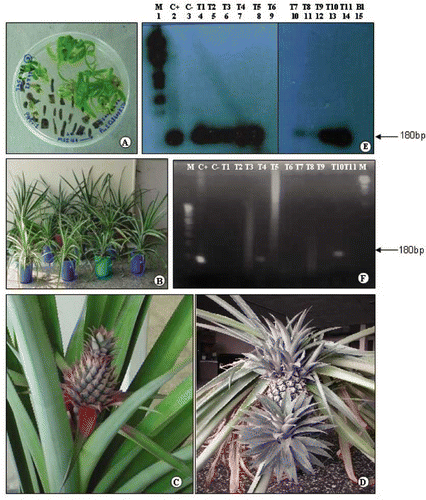
The leaf bases co-cultivated with Agrobacterium showed initial swelling at the cut edges and started proliferation in four to six weeks time in 6% of the cultures. Some of the explants produced callus (6%), and 2% produced shoots directly without any intervening callus (). The developed callus was isolated and upon subculture produced multiple shoots (7–8 shoots per 1 cm piece of callus). The directly differentiated shoots (without callus formation) were multiplied on Pin-1 media containing cefotaxime (400 mg/l) and kanamycin (50 mg/l). Only directly differentiated shoots were used for analysis. The luxuriantly growing, dark green colored shoots were excised individually and transferred to rooting medium (RM) in which they produced 2–4 roots/shoot. Complete plants with several roots were obtained in about six months after the co-cultivation of leaf bases with Agrobacterium. Putative transformants were hardened in the greenhouse (), and eleven were used for molecular analysis. The transgenic lines were further grown to maturity. There were no morphological changes, and flowering and fruit development was observed to be normal ( and ).
FIGURE 1 Regeneration of transgenic pineapple plants and their molecular analysis. A. Direct regeneration of shoots from transformed leaf bases; B. Hardened transgenic plants growing in the greenhouse; C., D. Fruiting of transgenic plant in the greenhouse; E. PCR-Southern analysis of eleven transgenic plants: Lanes 4–14, transformed plants (T1–T11), lane 1 marker (M), lanes 2 and 3 +vector and −vector control (C+ and C−), lane 15 blank. Note the hybridization of the probe with 180bp band corresponding to MSI–99 gene. Plants T1 to T5 and T8 to T11–transformed; T6 and T7–untransformed; F. RT-PCR analysis of eleven hardened transformed plants. Note the 180bp band corresponding to MSI–99 in T4 and T10 plants and positive control.
Molecular Analysis of Transgenic Plants
PCR analysis was carried out using upstream primers specific to MSI-99 gene and the down stream primer specific to 5′end of the nos terminator. The plant expression vector pMSI168 was used as a positive control and untransformed pineapple plants of similar age were used as negative control. As expected, a 180bp band was amplified in the positive control and some of the transformed plants, while it was absent in the control (untransformed plants). To further confirm the identity of the MSI-99-specific amplified bands in the positive control and transformed plants, PCR Southern analysis was carried out using a radioactively-labeled MSI-99 gene along with nos terminator. Only the amplified products of expected size (180bp) in the positive control and transgenic samples hybridized with the probe and were visualized, confirming the identity of the amplification product and the transgenic nature of the plants ().
To confirm the expression of MSI-99 in transgenic plants, RT-PCR was done using total RNA isolated from control and transgenic plants. Synthesis of c-DNA, using oligo-dT primers and PCR, was carried out using the same primers as mentioned earlier. Two transformed lines (T4 and T10) clearly showed the amplification fragment of 180bp, while it was absent in control untransformed plants (). PCR and RT-PCR analysis of shoots raised from callus also showed similar results (data not included).
Most early studies with pineapple propagation were limited to regeneration in axillary buds, which were not amenable to genetic transformation. The main objective of this investigation, therefore, was to establish an efficient Agrobacterium-mediated transformation system in pineapple, using the most suitable explant. Our earlier studies with regeneration in untransformed pineapple leaf bases had successfully yielded an almost similar number of multiple shoots per explant (CitationSoneji et al., 2002b) as was obtained in the present study after transformation with a magainin analogue (MSI-99) coding for broad spectrum antimicrobial activity. This indicates that the transformation event does not interfere with the regeneration potential of pineapple leaf bases isolated from in vitro raised shoots, and is a good explant for genetic manipulation studies.
Very few reports are available on genetic transformation of pineapple, but most of them have used biolistic mediated transformation (CitationFiroozabady et al., 1997; CitationNan and Nagai 1998; CitationKo et al., 2000; CitationBotella et al., 2000; CitationBotella and Fairbairn, 2005). Recently, CitationSripaoraya and others (2006) reported pineapple plants transformed with the bar gene for bialaphos resistance and studied transgene stability, gene expression, and herbicide tolerance under field conditions. In their study, the bar gene was stable and expressed throughout the duration of the trial. Fruit characteristics and yield were not affected by the introduction and expression of the transgene. Agrobacterium-mediated transformation has been reported by CitationEspinosa and others (2002), wherein pineapple calli (1.5 mm to 2 mm) were co-cultivated with different strains of Agrobacterium tumefaciens. After 24 hrs co-cultivation, 40% of the calli were observed to be GUS-positive, and the use of temporary immersion bioreactors resulted in the recovery of 6.6% transgenic plants. Further, it is stated that the use of phosphinothricin and hygromycin is better for selection compared to kanamycin. In our studies, leaf bases from in vitro shoots were used for co-cultivation with Agrobacterium, and the frequency of transgenic plant recovery was 8% (2% explants giving rise to shoots directly, and 6% producing callus and subsequently producing transformed shoots). The use of leaf bases for transformation as in the present study is advantageous compared to use of callus, as directly regenerated shoots minimize the undesirable problem of somaclonal variation in the transgenic plants.
The plants have been engineered to express antimicrobial peptides for the incorporation of disease resistance (Osusky et al., 2000). These are small, cationic, pore-forming peptides having a wide occurrence across the genera and even in plants. The broad spectrum of antimicrobial activity of these peptides (at concentrations relatively non-toxic to eukaryotic cells) and rapid mode of synthesis with minimum input with respect to energy and biomass, means these are being explored as attractive choices for genetically engineered resistance in crop plants. Among the different antimicrobial peptides investigated, magainin is one of the earliest reported peptides (CitationZasloff, 1987). It is effective against a wide spectrum of biological agents such as both gram-positive and gram-negative bacteria, fungi, protozoa and even mycoplasma (CitationSmith et al., 2001). A few magainin analogues have been expressed in transgenic plants for conferring resistance to phytopathogenic bacteria and fungi (CitationLi et al., 2001; CitationDeGray et al., 2001). In our studies, we have used MSI-99, which has three substitutions in the original magainin sequence, making it more positively charged and more effective than the parent peptide (CitationChakrabarti et al., 2003). As pineapple is affected by several phytopathogens, the generation of transgenic plants expressing this peptide may enhance resistance. The results of our investigations revealed that the MSI-99 gene had been stably integrated into pineapple genome, and RT-PCR analysis showed its expression. However, bioassay studies need to be carried out to confirm the acquired resistance in the transgenic plants. Further studies are underway in this direction.
The authors thank Dr. John Sanford, Sanford Scientific Inc., Waterloo, NY, USA for providing pSAN168 synthetic gene construct.
LITERATURE CITED
- Allan , A.R. , Blowers , A. and Earle , E.D. 2004 . Expression of a magainin-type antimicrobial peptide gene (MSI-99) in tomato enhances resistance to bacterial speck disease . Plant Cell Rpt. , 22 : 388 – 396 .
- Beauman , F. 2005 . The pineapple: King of fruits. p , 336 London : Chatto & Windus .
- Botella , J.R. and Fairbairn , D.J. 2005 . Present and future potential of pineapple biotechnology . Acta Hort. , 622 : 23 – 28 .
- Botella , J.R. , Cavallaro , A.S. and Cazzonelli , C.I. 2000 . “ Towards the production of transgenic pineapple to control flowering and ripening. p. 115–122 ” . In Proc. .3rd Intl. Pineapple Symp. Acta Hort Edited by: Subhadrabandhu , S. and Chairidchai , P. Vol. 529 , 115 – 122 .
- Chakrabarti , A. , Ganapathi , T.R. , Mukherjee , P.K. and Bapat , V.A. 2003 . MSI-99, a magainin analogue, imparts enhanced disease resistance in transgenic tobacco and banana . Planta , 216 : 587 – 596 .
- DeGray , G , Rajasekaran , K. , Smith , F. , Sanford , J. and Daniell , H. 2001 . Expression of an antimicrobial peptide via the chloroplast genome to control phytopathogenic bacteria and fungi . Plant Physiol. , 127 : 852 – 862 .
- Dellaporta , S.L. , Wood , J. and Hicks , J.B. 1983 . A plant DNA minipreparation: Version II . Plant Molec. Biol. Rpt. , 1 : 19 – 27 .
- Duval , M.F. , Noyer , J.L. , Perrier , X. , Coppens d’ Eeckenbrugge , G. and Hamon , P. 2001 . Molecular diversity in pineapple assessed by RFLP markers . Theor. Appl. Genet , 102 : 83 – 90 .
- Espinosa , P. , Lorenzo , J.C. , Iglesias Yabor , A.L. , Menendez , E. , Borroto , J. , Hernandez , L. and Arencibia , A.D. 2002 . Production of pineapple transgenic plants assisted by temporary immersion bioreactors . Plant Cell Rpt. , 21 : 136 – 140 .
- Feng , J , Yuan , F. , Gao , Y. , Liang , C. , Xu , J. , Zhang , C. and He , L. 2003 . A novel antimicrobial protein isolated from potato (Solanum tuberosum) shares homology with an acid phosphatase . Biochem. J. , 376 : 481 – 487 .
- Firoozabady , E. , Heckert , M. , Oeller , P. and Gutterson , N. 1997 . “ Transformation and regeneration of transgenic pineapple plants ” . In 5th Intl. Congr. Plant Molec. Biol Singapore (Abstract No. 1358)
- Ganapathi , T.R. , Ghosh , S.B. , Laxmi , N.H.S. and Bapat , V.A. 2007 . Expression of an antimicrobial peptide (MSI-99) confers enhanced resistance to Aspergillus niger in transgenic potato . Indian J. Biotechnol. , 6 : 63 – 67 .
- Hancock , R.E.W. and Sahl , H.G. 2006 . Antimicrobial and host defense peptides as new anti-infective therapeutic strategies . Nat. Biotech. , 24 : 1551 – 1557 .
- Hood , E.E. , Gelvin , S.B. , Melchers , L.S. and Hoekama , A. 1993 . New Agrobacterium helper plasmid for gene transfer to plants . Transgenic Res. , 2 : 208 – 218 .
- Ko , H.L. , Graham , M.W. , Hardy , V.G. , Jobin , M. , ’Hare , T.J. O and Smith , M.K. 2000 . “ Transformation of pineapple using biolistics ” . In 6th Intl. Congr. Plant Molec. Biol Quebec, , Canada (Abstract no. S03–64)
- Li , Q. , Lawrence , C.B. , Xing , H.Y. , Babbit , R.A. , Bass , W.T. , Maiti , I.B. and Everett , N.P. 2001 . Enhanced disease resistance conferred by expression of an antimicrobial magainin analog in transgenic tobacco . Planta. , 212 : 635 – 639 .
- Murashige , T. and Skoog , F. 1962 . A revised medium for rapid growth and bioassays with tobacco tissue cultures . Physiol. Plant. 15 , : 473 – 497 .
- Nan , G.L. and Nagai , C. 1998 . Genetic transformation of pineapple (Ananas comosus) via particle bombardment . In Vitro Cell. Dev. Biol. Plant. , 34 : 54 – A .
- O'Callaghan , M. , Gerard , E.M. , Waipara , N.W. , Young , S.D. , Glare , T.R. , Barrell , P.J. and Conner , A.J. 2004 . Microbial communities of Solanum tuberosum and magainin producing transgenic lines . Plant and Soil. , 266 : 47 – 56 .
- Osusky , M. , Osuska , L. , Kay , W. and Misra , S. 2005 . Genetic modification of potato against microbial disease in vitro and in planta activity of a dermaseptin B1 derivative, MsrA2 . Theor. Appl. Genet. , 111 : 711 – 722 .
- Sambrook , J. , Fritsch , E.F. and Maniatis , T. 1989 . Molecular cloning: A laboratory manual , New York : Cold Spring Harbor Laboratory Press .
- Shekhawat , U.K.S. , Ganapathi , T.R. , Srinivas , L. , Bapat , V.A. and Rathore , T.S. 2008 . Agrobacterium-mediated genetic transformation of embryogenic cell suspension cultures of Santalum album . L. Plant Cell Tissue Organ Cult. , 92 : 261 – 271 .
- Smith , F. , Blowers , A.D. , Van Eck , J. and Sanford , J. Expression of magainin and PGL classes of antimicrobial peptide genes in plants, and their use in creating resistance to multiple plant pathogens. U.S. Patent no. 6235973 . 2001 .
- Soneji , J.R. , Rao , P.S. and Mhatre , M. 2002a . Somaclonal variation in micropropagated dormant axillary buds of pineapple (Ananas comosus L. Merr.) . J. Hort. Sci. Biotechnol. , 77 : 28 – 32 .
- Soneji , J.R. , Rao , P.S. and Mhatre , M. 2002b . In vitro regeneration from leaf explants of pineapple (Ananas comosus L. Merr.) . J. Plant Biochem. Biotechnol. , 11 : 117 – 119 .
- Sripaoraya , S. , Keawsompong , S. , Insupa , P. , Power , J.B. , Davey , M.R. and Srinives , P. 2006 . Genetically manipulated pineapple: Transgene stability, gene expression and herbicide tolerance under filed conditions . Plant Breeding , 125 : 411 – 413 .
- White , P.R. 1954 . The cultivation of animal and plant cells , 237 New York : Ronald Press .
- Zasloff , M. 1987 . Magainins, a class of antimicrobial peptides from Xenopus skin: Isolation, characterization of two active forms and partial cDNA sequence of a precursor . Proc. Natl. Acad. Sci. Washington, D.C. , 84 : 5449 – 5453 .