Abstract
Effects of three preharvest sprays of calcium chloride (CaCl2) on fruit quality, yield, and mineral content in ‘Asgari’ table grape (Vitis vinifera) were studied. Calcium sprays were made at rates of 0%, 0.8%, 1.2%, 1.6%, and 2% w/v as T0, T1, T2, T3, and T4, respectively. Applications were made at fruit set, early pre-bunch closure, and late bunch closure. Fruit analyses showed that Ca partitioning in the various parts of the fruit was not proportional to the levels of Ca application. The rate of Ca accumulation in the berry during the berry development followed a sigmoid pattern in all treatments. The rate of Ca accumulation depended on both the growth phase and the level of Ca applied. The highest rate was recorded at 60 days after anthesis in the high Ca (T4) treatment. Calcium accumulation in the berry ceased after 80 days past anthesis (beginning of veraison) in all treatments, while Ca redistributed from flesh to skin during berry ripening. At the end of growing season, Ca content of rachis is almost 6 times greater than Ca content of berry in all treatments. Calcium accumulated mostly in the rachis and less in the flesh during grape berry development. Yield components including berry weight, cluster weight, and thus total yield per vine were not significantly influenced by Ca sprays, whereas berry drops and botrytis infection were reduced by Ca sprays. Quality components including juice-pH, SSC, and titratable acidity were not significantly affected, whereas berry firmness, berry color, and appearance improved at harvest time. A Ca-K antagonism was observed in this investigation. The vines in the high-Ca concentration (T4) treatment sometimes exhibited leaf injury (lesions), but this was not observed on cluster and berries.
INTRODUCTION
Monitoring Ca absorption and accumulation in the fruit tissue is an effective approach to manage and improve fruit quality (CitationFallahi et al., 1997; CitationShear, 1975). The Ca content of plant tissues can affect the severity of disease. Most fungi invade plant tissue by secreting extracellular pectolytic enzymes that dissolve the middle lamella, and calcium drastically inhibits the activity of these enzymes (CitationSalisbury and Ross, 1992). Calcium regulates the ripening of fruits and stimulates their coloring, ethylene production, and flesh firmness (CitationGerasopoulos and Richardson, 1999). Grapes with optimum Ca concentrations had improved fruit quality (CitationCupta et al., 1980), and increased resistance to Botrytis cinerea and delayed senescence (CitationChardonnet et al., 1997).
Calcium deficiency is common in all major fruit-growing regions. It is observed even on calcareous soil (CitationMalakouti et al., 1999). This deficiency causes many physiological disorders of fruits, such as internal breakdown, senescence breakdown, sunburn, fruit splitting, and spoilage (CitationSalisbury and Ross, 1992). Calcium deficiency is related to Ca uptake and its specific movement in the plant. Ions of Ca2+ are transported mainly into leaf tissues, whereas Ca accumulation into fruit tissues is strongly reduced due to the low amount and immobility of Ca2+ ions in the phloem sap (CitationMarschner, 1995), and it is difficult to accumulate enough in the fruit (CitationWhite, 2001).
In grapes, half of the seasonal requirements of Ca are absorbed from the end of bloom until veraison (CitationCreasy et al., 1993; CitationOllat and Gaudillere, 1996). Some studies indicate that Ca accumulates in grape berries throughout their development (CitationRogiers et al., 2000; CitationSchaller et al., 1992), whereas others indicate Ca accumulation stops after veraison (CitationCabanne and Doneche, 2001; CitationCabanne and Doneche, 2003; CitationChardonnet, 1994; CitationPossner and Kliewer, 1985). Therefore, plant Ca requirements must be continually obtained from external sources such as foliar sprays.
The grape cultivar ‘Asgari’ is one of the most popular seedless table grapes in Iran and neighboring countries. It is very susceptible to botrytis bunch rot (gray mold), splitting, and berry drop. In Iran, CaCl2 is a commonly used Ca material in most apple orchards, but it has never been applied to reduce diseases or to improve grape quality. Thus, the objective of this study was to investigate the impact of Ca management on fruit quality, yield, and mineral partitioning in different tissues of berry in ‘Asgari’ table grape.
MATERIALS AND METHODS
This research project was conducted with Vitis vinivera cultivar ‘Asgari’ in a commercial vineyard in Ehgleed, in the province of Fars, Iran, using 15-year-old grape vines during two growing seasons (2007 to 2008). ‘Asgari is a seedless table grape. The region Ehgleed is semiarid with an annual average precipitation of 350 mm (no summer rain). The soil composition was sand 65%; silt 16%; clay 19%; organic carbon <0.5%; soil pH 8.0; and carbonate content 27%. Grapevines in this experiment were planted at 2.0 m × 3.0 m spacing and rows were in a north-south orientation. Vines were unilateral cordon-trained, vertical shoot-positioned, and spur pruned to 25 buds per vine. Ten vines were used per experimental unit within the row, but only the central vines were used for determinations. The five replicates were arranged in a completely randomized design. All cultural practices (traditional flood irrigation system, winter and summer pruning, weed control, and plant protection) in this experimental vineyard were similar to those of commercial vineyards in the region.
Vines were sprayed with a solution containing CaCl2x6H2O, with point of deliquescence (POD: 33% w/v), and solubility 2790 (g/kg H2O) at different rates of 0%, o.8%, 1.2%, 1.6%, and 2% w/v CaCl2 (as: T0, T1, T2, T3, and T4) treatments. Sprays were applied in the morning with a volume of 800 L of water ha−1 by a handy sprayer until runoff. Spray was repeated three times every two weeks from fruit set to veraison. All vines also were fertigated with essential minerals based on the petiole mineral nutrient analyses.
Whole clusters were always sampled from the basal shoots of the cane. Samples were collected every two weeks after fruit set until harvest for each treatment. Fruit samples consisted of 50 to 60 berries per sample. To determine the Ca content of whole berries and of their component parts, berry and rachis samples were washed three times with distilled water, and they were separated into parts (skin, flesh, rachis). Homogeneity of samples was previously checked by berry size and density (CitationCabanne and Doneche, 2003). For each date, two lots of washed berries were immediately frozen at −30°C for later use. The first lot was used for analysis of whole berries and the second lot for analysis of different components of the berry (skin, flesh).
Samples were oven dried at 60°C for 48 hours and ground to pass through a 40-mesh screen and were analyzed only for Ca and K contents. Tissue samples were measured on the acid digested samples (H2SO4 + HNO3) using a spectrophotometer (Varian, AA-40, γ = 422.7 mm, air-acetylene flame). Calcium and K contents were reported in mg in 100 g of fresh mass (flesh). Also Ca content in different parts of fruit was expressed in mg of Ca per berry, and Ca-rachis was calculated in mg in 100g of fresh weight, and the berry K concentrations were expressed as ppm.
Fruits from all treatments were harvested in the end of August each year. Berry number per cluster and average weight of 100 berries (g) and total yield (kg vine−1) were recorded. Fifty berries were randomly sampled, and berry weight and berry quality attributes (soluble sold concentrations [SSC], pH, and titratable acidity [TA, as tartaric acid equivalent]) were determined. SSC was determined with a hand-held refractometer (Atago N1α). pH was measured with a pH meter (Horiba F-22) and titratable acidity was determined by titration with 0.1 N NaOH. Also, berries were rated on berry firmness, skin background color, incidence of botrytis, and berry drops. The flesh firmness (kg cm−2) was measured with an Effegi penetrometer (8 mm plunger) in fruit samples consisting of 40 berries per treatment at harvest. Berry drops were scored on a scale from 0 to 4: 0, <5%; 1, >5% <0%; 2, >10% <15%; 3, >15% < 20%; 4, >25% of 100 berries bunch−1 at harvest. Botrytis-disease severity was scored on a scale from 0 to 3: 0, no lesion; 1, small lesion; 2, medium lesion; 3, whole leaf diseased at harvest. Color index was scored on a scale from 0 to 4: 0, no change; 1, green; 2, bright; 3, yellow; 4, dark yellow at harvest. All data were subjected to analysis of variance (ANOVA) procedures and means were separated, using Duncan Multiple range test at P _ 0.05, using SAS-PC (ver. 6.12) (SAS Institute, 1990) software.
RESULTS
Since there was no significant interaction in Ca accumulation, yield, or berry quality attributes within two years, results of both seasons (2007–2008) were combined and only one mean value over the two years is presented in this article. Three applications of Ca from fruit set, early prebunch closure and late bunch closure significantly increased the Ca in the berry and its components (skin, flesh) and the rachis. There was not a linear increment of Ca accumulation in whole berries or berry components as a result of different levels of Ca application (). The rate of Ca accumulation during berry development followed a typical sigmoid pattern in the berries of all treatments (). The rate of Ca accumulation depended on both the growth phase and the amount of Ca applied. The highest rate was recorded at 60 days after anthesis in the high CaCl2 (T4) treatment (). Similar sigmoid patterns were observed for rates of Ca accumulation in the skin and rachis ( and ).
FIGURE 1 Comparison of Ca content (mg berry−1) in different parts of berry as influenced by 3 times of various levels of CaCl2 (W/V) applications on table seedless grape ‘Asgari’. T0 (control); T1 0.8%; T2 1.2%; T3 1.6%; T4 2% w/v CaCl2 concentration. Data presented are the mean of two years.
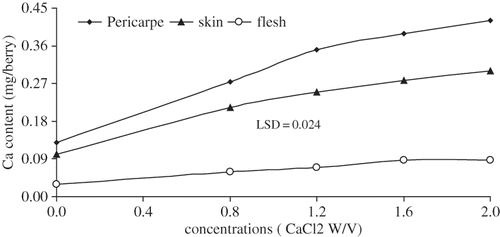
FIGURE 2 The rates of whole berry-Ca accumulation (mg berry−1) as influenced by 3 times of various levels of CaCl2 (W/V) applications on table seedless grape ‘Asgari’ during berry growth and development. T0 (control); T1 0.8%; T2 1.2%; T3 1.6%; T4 2% w/v CaCl2 concentration. Data presented are the mean of two years.
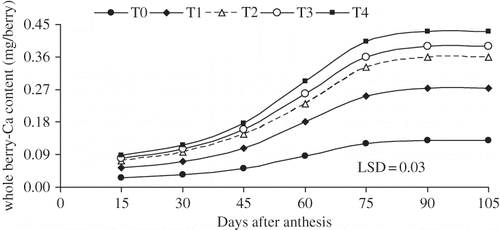
FIGURE 3 The rates of skin berry-Ca accumulation (mg berry−1) as influenced by 3 times of various levels of CaCl2 (W/V) applications on table seedless grape ‘Asgari’ during berry growth and development. T0 (control); T1 0.8%; T2 1.2%; T3 1.6%; T4 2% w/v CaCl2 concentration. Data presented are the mean of two years.
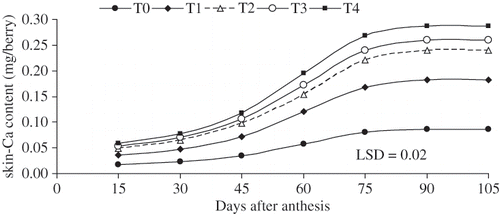
FIGURE 5 The rates of rachis-Ca accumulation (mg 100 g DM) as influenced by 3 times of various levels of CaCl2 (W/V) applications on table seedless grape ‘Asgari’ during berry growth and development. T0 (control); T1 0.8%; T2 1.2%; T3 1.6%; T4 2% w/v CaCl2 concentration. Data presented are the mean of two years.
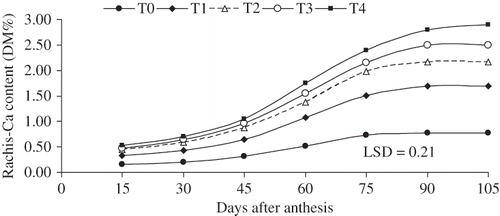
Calcium accumulation in the flesh was most intensive 60 days after anthesis in all treatments (including the control), than in the early or later parts of the season (). It was low at the beginning and it declined at the end of growth. Calcium accumulation in the berry stopped 80 days after anthesis (beginning of veraison) in all treatments, while Ca redistributed from flesh to skin during berry ripening ( and ). It appeared that the Ca contents in the berry flesh increased until veraison then decreased during ripening, whereas, skin Ca contents increased throughout berry development including ripening (). At the end of growing season, Ca content of rachis is almost 6 times greater than Ca content of berry in all treatments (). Treatments with higher CaC2 level (T4) had greater amount of Ca-accumulations in berry and its compartments ().
FIGURE 4 The rates of flesh-Ca accumulation (mg berry−1) as influenced by 3 times of various levels of CaCl2 (W/V) applications on table seedless grape ‘Asgari’ during berry growth and development. T0 0% Ca (control); T0, (control); T1, 0.8% Ca; T2, 1.2% Ca; T3, 1.6% Ca; T4 2% Ca concentration of foliar spray. (data presented are the mean of two years).
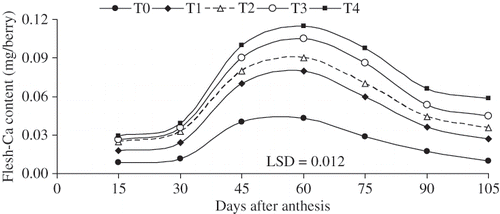
TABLE 1 Effects of Various Levels of CaCl2 Sprays on Berry Weight, Juice Soluble Solids Concentrations (SSC), Juice K, pH, and Titratable Acidity in ‘Asgari’ Table Grape in the Province of Faras, IranFootnote a
TABLE 2 Effects of Various Levels of CaCl2 Sprays on Berry Weight, Juice Soluble Solids Concentrations (SSC), Juice K, pH, and Titratable Acidity in ‘Asgari’ Table Grape in the Province of Faras, IranFootnote a
Yield was not influenced by Ca sprays (), whereas berry drop was reduced as the Ca content of the berry increased (). Berry botrytis infection was reduced, and berry firmness and skin yellow color were increased (). Juice pH, SSC, SSC/TA were not affected by CaCl2 sprays (). A Ca-K antagonism was observed in this investigation, as the levels of Ca application increased the K/Ca ratio decreased (). The vines treated with the highest CaCl2 concentration (T4) sometimes showed leaf injury (lesions; data not shown), but this was not observed on rachis and berries.
TABLE 3 Effects of Various Levels of CaCl2 Sprays on Skin Ca, Berry Firmness, Botrytis Infection, Berry Drop, and Skin Color of ‘Asgari’ Table Grape in the Province of Faras, IranFootnote a
DISCUSSION
Increasing Ca accumulation in the berry tissues is important for many aspects of table grape management. Application of CaCl2 increased the Ca content of the berry, with the majority of Ca being found in the cell wall fraction of the skin. The cation Ca2+ promotes the stability of the cell wall by chelating the free carboxylic groups of galacturic units. This results in an increase in berry firmness, and the prolonging of fruit shelf life (CitationChardonnet et al., 1997), and perhaps less berry drop and bunch rot, which was the case in our experiment. Applying CaCl2 causes a thickening of the fungal cell wall, a consequence of the high osmotic pressure developed (CitationSalisbury and Ross, 1992). This could retard the elongation phase of the hyphae and result in a loss of cell wall elasticity. CitationRosenquist and Morrinson (1989) have shown that the most resistant grape cultivars to splitting have a thicker skin, and this can be enhanced by Ca application.
The application of CaCl2, decreases in berry K levels. A Ca-K antagonism was observed in this investigation. The high CaCl2 (T4) foliar spray treatment resulted in significantly decreased K/Ca (), which can directly and indirectly affect fruit quality.
Calcium sprays significantly increased Ca accumulation in the berry, but the rates of increase were not proportional to the rates of Ca application (). Calcium levels in the rachis were several times greater than in the fleshy pericarp (pulp). The Ca accumulation in the plant tissues (skin, flesh, rachis) changed during the growinng season. The highest rates occurred during 60 to 75 days after anthesis, however, they rose as the level of CaCl2 application increased (, , , and ). These results are agreement with previous reports (CitationCabanne and Doneche, 2003; CitationCupta et al., 1980; CitationFindlay et al., 1987; CitationRogiers et al., 2000). Calcium content in the rachis is more than 6 times greater than that in the pericarp almost in all treatments ().
The differences in Ca accumulation among different tissues may be attributed to the redistribution of Ca2+ ions and differences in cell structure. Compared to pericarp cells, rachis cells have thicker walls and a greater capacity to accumulate Ca. Similarly, skin cells have thicker walls and more cytoplasm to accumulate Ca as compared to flesh cells (CitationConradie and Myburgh, 2000; CitationNii and Coombe, 1983).
In plants, Ca is primarily transported through the xylem system. At veraison, a rupture of the xylem vessels occurs in the pericarp, and this is probably responsible for the halt of Ca accumulation in this compartment (CitationDorino et al., 1987). The balance of Ca supply between flesh and whole berry is then shifted entirely toward the skin (CitationDorino et al., 1987). The accumulation of Ca in the skin thus continues through ripening. It is possible that part of the Ca accumulating in the skin during ripening is translocated from the pericarp to the skin. In our experiment, calcium was accumulated in the flesh until veraison and then gradually decreased until it reaches a plateau around maturity (). It can be inferred that while Ca accumulation ceases at the onset of ripening in the pericarp, it persists in the skin (CitationCabanne and Donbche, 2001; CitationCabanne and Doneche, 2003; CitationChardonnet, 1994). However, Ca accumulation in the skin was slightly lower than the entire Ca loss in the flesh (comparison of , , and ). Calcium accumulation in the berries does not always cease after veraison (CitationOllat and Gaudillère, 1996; CitationRogiers et al., 2000), and water movement from vine to a berry with its pedicel girdled (i.e., phloem interrupted) still occurs (CitationRogiers et al., 2001).
Seasonal uptake of Ca has been studied by CitationConradie (1981) on ‘Chenin blanc’ / ‘99R’. The study showed that little Ca was accumulated by the vine prior to budburst nor in the 22 days after budburst, though Ca reserves decreased in the roots as new growth accumulated Ca in this period.
Calcium movement occurs mainly in xylem (CitationMengel and Kirkby, 1987). In grape berries, the xylem seems to be a major route of Ca entry. Rates of berry transpiration also increase during berry growth and development while berry Ca increases. The increase in berry transpiration is probably due to the increase in temperature and stomatal opening frequency (CitationBlanke and Leyhe, 1987). Calcium is phloem immobile and is translocated only in xylem (CitationHanger, 1979). Changes in the K/Ca ratio in berries have been used as an indicator of changes in the relative berry K influx via xylem and phloem (CitationHrazdina et al., 1984; CitationOllat and Gaudillère, 1996; CitationRogiers et al., 2001; CitationRogiers et al., 2005). While K accumulation occurs throughout all growth stages, the accumulation of Ca ceases at veraison in ‘de Chaunac’ (CitationHrazdina et al., 1984) or continues at rates much lower than K in ‘Cabernet Sauvignon’ (CitationOllat and Gaudillère, 1996) and ‘Shiraz’ (CitationRogiers et al., 2001).
CONCLUSIONS
Manipulation of berry Ca accumulation requires an understanding of the function of Ca in the berries. Calcium in grape berries improves the fruit quality of ‘Asgari’ table grapes by increasing the berry firmness and reducing berry drop and fungi diseases. During development, the berry skin is a strong sink for Ca, especially after veraison. High Ca (2%) sprays did not proportionally increased Ca accumulation in the berry flesh and skin and caused some leaf damage. Application of Ca at 1.2% w/v increased Ca absorption and accumulation in the berry, and thus, this rate is considered as the optimum rate for Ca spray. A Ca-K antagonism was observed in this investigation.
Special thanks to the Institute of water and soil research of Fars for generous helps in mineral analysis in this research project. The technical assistance in many aspects of this experiment from the personnel of the University of Idaho Pomology Program in Idaho, USA is greatly appreciated.
LITERAURE CITED
- Blanke , M.M. and Leyhe , A. 1987 . Stomatal activity of the grape berry cv. Riesling, Müller Thurgau and Ehrenfelser . J. Plant Physiol. , 127 : 451 – 460 .
- Cabanne , C. and Doneche , B. 2001 . Changes in polygalacturonase activity and calcium content during ripening of grape berries . Amer. J. Enol. Viticult. , 52 : 331 – 335 .
- Cabanne , C. and Doneche , B. 2003 . Calcium accumulation and redistribution during the development of grape berry . Vitis. , 42 ( 1 ) : 19 – 21 .
- Chardonnet , C.O. 1994 . Le calcium de la baie de raisin—Relation entre la cohesion des parois cellulaires et la sensibilite a Bolrytis cinerea , France : These de Doctoral. Universite Victor Segalen Bordeaux 2 .
- Chardonnet , C.O. , Lhyvernay , A. and Donech , B. 1997 . Effect of calcium treatment prior to Botrytis cinerea infection on the changes in pectic omposition of grape berry . Plant Pathol. , 50 : 213 – 218 .
- Conradie , W.J. 1981 . Seasonal uptake of nutrients by Chenin blanc in sand culture II. Phosphorous, potassium, calcium and magnesium . South African J. Enol. Viticult. , 2 ( 1 ) : 7 – 13 .
- Conradie , W.J. and Myburgh , P.A. 2000 . Fertigation of Vitis vinifera L. cv. Bukettruaube / 110 Richter on a sandy soil . South African J. Enol. Viticult. , 21 ( 1 ) : 40 – 47 .
- Creasy , G.L. , Price , S.F. and Lombard , P.B. 1993 . Evidence for xylem discontinuity in Pinot noir and Merlot grapes: Dye uptake and mineral composition during berry maturation . Amer. J. Enol. Viticult. , 44 : 187 – 193 .
- Cupta , O.P. , Jinal , P.C. and Singh , S.P. 1980 . Effect of pre harvest spray of calcium-nitrate on the on the storage, behavior of grape. cv. “Perlette” . Agr. J. , 10 : 102 – 109 .
- Dorino , H. , Lano , A. and Oogionni , F. 1987 . Patterns of water flow in Riesling berries in relation to developmental changes in their xylem morphology . Vitis , 26 : 123 – 131 .
- Fallahi , E. , Conway , W. , Hickey , K.D. and Sams , C.E. 1997 . The role of calcium and nitrogen in postharvest quality and diseases resistance of apple . HortScience , 32 : 831 – 835 .
- Findlay , N. , Oliver , K.J. , Nil , N. and Coombe , B.G. 1987 . Solute accumulation by grape pericarp cells. IV, Perfusion of pericarp apoplast via the pedicel and evidence for xylem malfunction in ripening berries . J. Expt. Bot. , 38 : 668 – 679 .
- Gerasopoulos , D. and Richardson , D.G. 1999 . Storage temperature and fruit calcium alter the sequence of ripening events of “de’ Anjou” pears . HortScience , 34 ( 2 ) : 316 – 318 .
- Hanger , B.C. 1979 . Movement of calcium in plants. Commun . Soil Sci. Plant Anal. , 10 : 171 – 193 .
- Hrazdina , G. , Parsons , G.F. and Mattick , L.R. 1984 . Physiological and biochemical events during development and maturation of grape berries . Amer. J. Enol. Viticult. , 35 : 220 – 227 .
- Malakouti , M.J. , Tabatabaei , S.J. , Shahabi , A. and Fallahi , E. 1999 . Effects of calcium chloride on apple fruit quality of trees grown in calcareous soil . J. Plant Nutr. , 22 : 1451 – 1454 .
- Marschner , H. 1995 . Mineral nutrition of higher plants , 2nd , 889 San Diego, CA : Academic Press Inc .
- Mengel , K. and Kirkby , E. 1987 . Principles of plant nutrition , 4th , Worblaufen-Bern, , Switzerland : International Potash Institute .
- Nii , N. and Coombe , B.G. 1983 . Structure and development of the berry and pedicel of the grape Vitis vinifera . L. Acta Hort. , 139 : 129 – 140 .
- Ollat , N. and Gaudillère , J.P. 1996 . Investigation of assimilate import mechanisms in berries of Vitis vinifera var. ‘Cabernet Sauvignon’ . Acta Hort. , 427 : 141 – 149 .
- Possner , D.R.E. and Kliewer , M.W. 1985 . The localisation of acids, sugars, potassium and calcium in developing grape berries . Vitis , 24 : 229 – 240 .
- Rogiers , S.Y. , Greer , D.H. , Hatfield , J.M. , Orchard , B.A. and Keller , M. 2005 . Mineral sinks within ripening grape berries ( Vitis vinifera L.) . Vitis , 45 ( 3 ) : 115 – 123 .
- Rogiers , S.Y. , Keller , M. , Holzapfel , B. P. and Viroona , J.M. 2000 . Accumulation of potassium and calcium by ripening berries on field vines of Vitis vinifera (L) cv . Shiraz. Australian J. Grape Wine Res. , 6 : 240 – 243 .
- Rogiers , S.Y. , Smith , J.A. , White , R. , Keller , M. , Holzapfel , B.P. and Viroona , J.M. 2001 . Vascular reaction in berries of Vitis vinifera cv . Shiraz. Australian J. Grape Wine Res. , 7 : 46 – 51 .
- Rosenquist , J.K. and Morrinson , J.C. 1989 . Some factors affecting cuticle and wax accumulation on grape berries . Amer. J. Enol. Viticult. , 40 : 241 – 244 .
- Salisbury , F.B. and Ross , C.W. 1992 . “ Plant physiology ” . In , 4th , Belmont, CA, , USA : Wadsworth, Inc .
- SAS software version 6.12.2005 , Cary, NC : SAS Institute, Inc .
- Schaller , K. , Loiinebtz , O. and Ciiikkasubbanna , V. 1992 . Calcium absorption by the grape berries of different cultivars during growth and development . Wein-Wiss. , 47 : 62 – 65 .
- Shear , C.B. 1975 . Calcium related disorders of fruits and vegetables . HortScience , 10 : 361 – 365 .
- White , P.J. 2001 . The pathways of calcium movement to the xylem . J. Expt. Bot. , 52 : 891 – 899 .