Abstract
Tamarindus indica, commonly called tamarind, is a medium-sized evergreen tree that gives a high yield. The fruit is commonly used as a spice. Despite its commercial importance in the international market, it has been little explored. The genetic diversity and genetic relatedness of 36 tamarind genotypes were studied using amplified fragment length polymorphism (AFLP) markers. Twelve primer pairs were used for the screening of genotypes based on the number and quality of polymorphic fragments produced. These primer combinations produced 702 bands, 627 (89.3%) of which were polymorphic. The mean Nei's gene diversity index was 0.4868, and the Shannon's information index was 0.6802, indicating the abundant genetic diversity within the tamarind population. Among the tamarind genotypes, the dissimilarity coefficient ranged from 4.4 to 14. Cluster analysis grouped all tamarind genotypes into two major clusters (A and B) at 37 linkage distance and two minor clusters each. Cluster A included the genotypes predominantly with brown to dark brown fruit pulp and dark green leaves. However, the straight to semi-curved fruits were sub grouped in sub-cluster ‘A1’ and semi-curved to curved fruits in sub-cluster ‘A2’. Cluster B contained a collection of genotypes predominantly characterized with trees of orthotropic growth and semi-curved fruit shape. A good correlation of these amplified fragment length polymorphism-based groupings with their morphological traits was observed. We found moderate genetic diversity in these tamarind genotypes. The use of AFLP markers and the level of genetic variability detected within Southern Indian tamarind germplasm suggested that this is a reliable, efficient, and effective marker system that can be used for diversity analysis and subsequently in tree breeding programs.
INTRODUCTION
Tamarind (Tamarindus indica) is a monotypic genus belonging to the family Fabaceae (Leguminosae), popularly known as ‘Indian Date’ which originated in India (CitationMorton, 1987). It is a multipurpose tropical fruit tree used primarily for its fruits. Tamarind is a highly cross pollinated crop with a wide variation in the species, and the number of genotypes are estimated to be 19,327 (CitationLewis et al., 2005) with a chromosome number of 2n = 24 (CitationPurseglove, 1981). Primarily, tamarind is propagated through seeds; hence, a wide range of heterozygosity is exhibited for growth, yielding capacity, quantity, quality, size, and shape of fruits. Tamarind is now widely spread throughout semi-arid zones of Asia and Africa (CitationGamble, 1922). It is presently cultivated in home gardens, farmlands, on roadsides, on common lands, and frequently planted as a shade tree.
It grows up to 45 feet in height with a dense spreading crown and a clear trunk, and could be found in a wide range of agroclimatic conditions, as it is a highly tolerant to drought. The most valuable and useful part of the tree is the fruit. The pulp is a rich source of carbohydrates and vitamins (CitationDuke, 1981; CitationPurseglove, 1981; CitationIshola et al., 1990), which is slightly sweetish and more acidic in nature and is widely used as a spice in the preparation of chutneys, sauces, soups, and certain beverages besides its use in Ayurvedic medicines. Tamarind fruit pulp is very rich in ascorbic and tartaric acids, hence, it used as a preservative in the pickling industry (CitationTsuda et al., 1995). It is also commonly used for fodder (CitationKaitho et al., 1996) and timber because its heartwood is durable and termite resistant (CitationTimyan, 1996). Medicinally, the leaves and bark are used for the production of anti-inflammatory agents (CitationRimbau et al., 1999), against leucorrhoea (CitationSen and Behera, 2000), and for skin disorders (CitationPunjani and Kumar, 2002). The seed kernel powder is an important material used for sizing in textiles and paper industries (CitationEl-Siddig et al., 2006).
Even though the tamarind is an ancient domesticated tree, very little is known about its genetic improvement. Farmers have selected genotypes from natural populations based on desirable and observable characteristics, particularly based on fruit pulp. Variation in the vegetative growth characters, such as shoot length, root length, germination percentage, plant height, and pinnae per plant has also been recorded among cultivated genotypes (CitationChallapilli et al., 1995; CitationBennet et al., 1997; CitationShanthi, 2003). Diversity analysis using isozyme markers has been reported on tamarind genotypes (CitationShanthi, 2003). However, to our best knowledge, no attempts have been reported to analyze the genetic diversity among tamarind genotypes.
DNA-based markers are useful tools for the identification of cultivars and varieties, and to eliminate genetically identical genotypes (CitationDuneman, 1994). DNA markers are considered to be the most suitable method to estimate genetic diversity because of their abundant polymorphism and independence of environment factors (CitationRenganayaki et al., 2001). Among the DNA-based markers, amplification fragment length polymorphism (AFLP; CitationZabeau and Vos, 1993) is considered to be a powerful, rapid, and reliable marker with potential application in genome mapping, fingerprinting, and marker-assisted breeding (CitationVos et al., 1995). AFLP is known to identify a large number of polymorphic loci than other Polymerase Chain Reaction (PCR) based markers without any knowledge of the species genome (CitationZhuang et al., 2009). Recently, AFLP has been successfully used to study the genetic diversity among perennial crops species, such as mango (CitationYamanaka et al., 2006), pistachio (CitationBasha et al., 2007), breadfruit (CitationSreekumar et al., 2007), marula (CitationMoganedi et al., 2007), apricot (CitationYuan et al., 2007), pomegranate (CitationYuan et al., 2007), grapes (CitationUpadhyay et al., 2007), jackfruit (CitationShyamalamma et al., 2008), pear (CitationBao et al., 2008), and fig (CitationBaraket et al., 2009). The present study evaluates the genetic diversity in a tamarind germplasm collection using polymorphisms revealed by 12 AFLP primers. In addition, we examined the relationships between morphological and molecular characters.
MATERIALS AND METHODS
Plant Material
Plant material from 36 tamarind genotypes was collected from the Department of Horticulture, University of Agricultural Sciences, Bangalore (). Approximately, 1 g of young leaves (<15 days old) was collected, washed using distilled water, and wiped with 70% (v/v) ethanol, prior to extraction of DNA. Morphological characterization of each genotype was recorded for tree growth habit and leaf and fruit characters.
TABLE 1 List of Origin and Accession Numbers of Tamarind Genotypes Used in Amplified Fragment Length Polymorphism Analysis
Genomic DNA Extraction
Genomic DNA was extracted from leaves of individual tamarind genotypes following the CTAB method (CitationNarayanaswamy et al., 2009). One g of fresh tamarind leaves was ground into fine powder using mortar and pestle. The powder was mixed with 6 ml extraction buffer, preheated to 65°C, containing 100 mM Tris-HCl, pH 8.0, 20 mM EDTA, 1.4 M NaCl, 3% (w/v) CTAB, 2% polyvinylpyrrolidone, and 1% (v/v) ¾β-mercaptoethanol, then incubated at 65°C for 1 hr with intermediate shaking. The mixture was cooled to room temperature, 8 ml cold 24:1 (v/v) chloroform:isoamylalcohol was added, and the contents were mixed well. After centrifugation at 9,000 × g for 10 min at 4°C, the supernatant was transferred to a fresh tube and the chloroform:isoamylalcohol step was repeated until a clear supernatant was obtained. NaCl (5 M) was added to the supernatant [0.5 (v/v)] and mixed gently, followed by the addition of 7 ml cold isopropanol to precipitate the DNA. The mixture was incubated at −20°C for 30 min and then centrifuged at 7,000 × g for 15 min. The resulting pellet was washed with 70% (v/v) ethanol, air-dried, and dissolved in TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0). Two μL RNase (10 mg/mL; bovine pancreatic ribonuclease; Bangalore Genei, Bangalore, India) was added to each sample, which was incubated for 1 hr at 37°C, mixed with an equal volume of 1:1 (v/v) phenol:chloroform, and centrifuged at 9,000 × g for 12 min at room temperature, rather than with chloroform alone. The DNA was precipitated by incubating at −20°C for 1 hr with 0.5 (v/v) 5 M NaCl and 1 volume of cold isopropanol. The resulting pellet obtained after centrifugation was dissolved in TE buffer, analyzed in an agarose gel, and quantified using a spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA).
AFLP Reaction
The AFLP procedure was followed according to CitationVos et al. (1995) with minor modification. DNA was digested with EcoR1 and Mse1 at 37°C for 3 hr. Three μL of the digested DNA was run on a 1.4% agarose gel to verify the enzyme activity. EcoR I and Mse I adapters were ligated to each digested DNA sample for overnight (16 hr) to generate template DNA for amplification. Pre-amplification was carried out with primers (), each carrying one selective nucleotide (EcoR1+C, Mse1+T) in a thermocycler (Corbett Research Mortlake, New South Wales, Australia) set at 94°C denaturation (30 sec), 56°C annealing (1 min), and 72°C extension (1 min) for 20 cycles. The amplified products were diluted (1:5) with TE buffer and stored at −20°C. AFLP amplification was carried out with 12 primer pair combinations of EcoR1+3 (E-plus three nucleotides) and Mse1+3 (M-plus 3 nucleotides). The PCR amplifications were carried out as follows: one cycle at 94°C for 30 sec, 65°C for 30 sec, and 72°C for 1 min; followed by 12 cycles of touchdown PCR in which the annealing temperature was decreased by 0.7°C every cycle until a touchdown annealing temperature of 56°C was reached. Once reached, another 25 cycles were conducted as 94°C for 30 sec, 56°C for 30 sec, and 72°C for 1 min; 10°C for 30 min. The PCR products were mixed with 8 μl of formamide dye (98% formamide, 10 mM EDTA, bromophenol blue, xylene cyanol). Denatured by incubating at 94°C for 5 min and 10°C for 5 min, quickly cooled at −20°C. The denatured PCR products were separated on 4.5% denaturing polyacrylamide. The gel was run at 1,300 V until the xylene cyanol ran about two-thirds the length of the gel. Then the gel was separated carefully and developed by silver-staining.
TABLE 2 List of AFLP Primers and the Level of Polymorphism among Tamarind Genotypes
Data Analysis
Amplified fragments from each AFLP primer set were scored manually for their presence (1) or absence (0). The profiles of 36 genotypes of tamarind using 12 primers were assembled for statistical analysis. The sizes of the fragments were estimated using 50 bp standard DNA markers (Bangalore Genei Pvt. Ltd., Bangalore India), coelectrophoresized with the amplified products. A genetic dissimilarity matrix was developed using Euclidean Distances, which estimates all pairwise differences in the amplification products (CitationSokal and Sneath, 1973). A cluster analysis was based on Ward's method using a minimum variance algorithm (CitationWard, 1963). Estimates of dissimilarity were based on the simple matching coefficient (CitationSokal and Michener, 1958), Sij = a + d/a + b + c + d: where Sij is the similarity between two individuals i and j, a is the number of bands present in both i and j, b is the number of bands present in i and absent in j, c is the number of bands absent in i and present in j, and d is the number of bands absent in both i and j.
Genetic diversity analysis used the POPGENE 1.32 software (CitationFrancis and Yang, 2000). Genetic diversity parameters, such as the number and percentage of polymorphic bands, the average number of alleles per loci (Na), the effective number of alleles per loci (Ne), Nei's gene diversity (H), and Shannon's information index (I) were determined according to CitationNei (1973). The coefficient of gene differentiation among the genotypes was determined using Nei's gene diversity method (CitationNei, 1973) using the formula, GST = DST/HT, HT = HS + DST, where, HT is the total gene diversity, Hs is the gene diversity within the population, and DST is the gene diversity between populations.
RESULTS AND DISCUSSION
The objective of this study was to estimate the extent of the genetic diversity among tamarind genotypes using AFLP markers. Information on genetic diversity in cultivated plant species is important for efficient plant breeding and improvement (CitationNarayanaswamy et al., 2009). Genetic diversity among crop plant species is important for efficient utilization of plant genetic resources since geographical isolation of a population may cause its genome to drift away from other populations of the same species (CitationBiron et al., 2002). The traditional method of identifying species by morphological characters is now accompanied by DNA profiling that is more reliable (CitationNayak et al., 2003). However, evaluation of both molecular and morphological characters is highly relevant in organisms with a high tendency for morphological differentiation (CitationBagatini et al., 2005). Among the DNA markers, AFLP is a more reliable dominant marker and is able to detect polymorphisms between genotypes that were not distinguishable with other PCR-based techniques. Therefore, when investigating organisms with a high tendency for morphological differentiation, studies considering both molecular and morphological characters are highly relevant (CitationBartish et al., 2000).
The number of bands produced by the primer combinations is shown in . Twelve primer combinations were used to estimate the genetic diversity among 36 tamarind genotypes that produced 702 bands with an average of 58.50 fragments per primer. The primer combinations EcoR1+AGC/Mse1+GCG produced a maximum number of 73 bands while the primer combinations EcoR1+CTG/Mse1+TCA produced a minimum number of 43 bands. These primer combinations also showed a maximum (67) and minimum (23) polymorphic bands. On an average, 52.3 polymorphic bands (89.3%) were amplified for each primer pair. Similarly, a high level of polymorphism was shown using AFLP markers by CitationWang et al. (2007). The level of polymorphism observed in our study was high using the primer combinations. This high level of polymorphism could be explained due to the fact that these elite accessions were collected from different regions of Southern India and cultivated in the University germplasm collections and, hence, were diverse in origin. In addition, a large number of AFLP markers were scored in this study, and this might have contributed to a higher percentage of polymorphism than normally observed in intraspecific cultivars. We also observed that the level of polymorphism was higher in this study than many of the intraspecific AFLP marker-based studies: wheat cultivars adapted to the Pacific Northwest region (CitationBarrett and Kidwell, 1998), celery cultivars of California (CitationLi and Quiros, 2000), Japanese peach (CitationXu et al., 2006), and jackfruit cultivars of Southern India (CitationShyamalamma et al., 2008). In contrast, pummelo germplasm (CitationLiu et al., 2005), the ornamental cultivars of Ginkgo biloba (CitationWang et al., 2006), and Chinese pomegranate (CitationYuan et al., 2007) were 72%, 88%, and 73%, respectively, using AFLP markers. Our findings could also be attributed to the availability of a high number of tamarind cultivars (CitationLewis et al., 2005).
A genetic dissimilarities matrix based on Euclidian distance revealed a moderate level of diversity within the accessions evaluated (). The genetic dissimilarity among 36 tamarind genotypes ranged from a low of 4.4 to a high of 14 with an average similarity among this group of genotypes of 9.74. The NCBS-2 and NCBS-3 genotypes were found to be the most genetically similar and originated from the same geographical region. Both the genotypes showed orthotropic tree growth character, but differed in semi-curved and straight fruit shape, respectively. The genotypes NB1, with orthotropic tree growth, semi-curved fruit shape, dark brown fruit pulp, and dark green leaves showed the highest dissimilarity with the genotypes H4, H5, PKM1, PKM2, BT4, PG1, PG2, MG1, MG2, MG3, NCBS1, NCBS2, and NCBS3. Although, each of these genotypes was collected from different geographical locations in the southern part of India, the majority of them had morphologically similar attributes in terms of tree growth character, fruit shape, fruit pulp color, and leaf color, but differed in one or two characters. Similar results were obtained in our previous study investigating the use of AFLP markers in jackfruit (CitationShyamalamma et al., 2008). This may be due to the fact that most of these genotypes were selected based on their morphological traits despite diverse origins. This was also observed by CitationAzad et al. (2007), suggesting that these morphological characteristics correlated poorly with environmental factors. Our results show a moderate amount of genetic diversity to exist among the tamarind genotypes. Therefore, this germplasm could play an important role in the preservation of genetic diversity and future development of tamarind cultivars.
TABLE 3 Genetic Dissimilarity Matrix of 36 Tamarind Genotypes Based on Polymorphism of AFLP Markers
Variance analysis among the tamarind genotypes is shown in . The Nei's gene diversity index (H) and the Shannon's information index (I) were highest in the NJ57 and NB15 genotypes (H = 0.5000; I = 0.6931), and was lowest in N30 genotype (H = 0.4523; I = 0.6446). The observed number of alleles (Na) was 2.0000 among all of the genotypes analyzed and the genotypes NJ57 and NB15 had the highest, and genotype NB30 was the lowest in the effective number of alleles (Ne) in the population. The AFLP markers analysis, using Nei's genetic diversity analysis and Shannon's diversity index, demonstrated that a large proportion of variation existed among populations. A high level of population differentiation may be explained by the type of species' breeding system (CitationHogbin and Peakall, 1999) and sexual propagation (seed) in tamarind. Also, perennial species generally maintain relatively higher levels of variation than annuals and short-lived perennials (CitationLedig, 1986; CitationVrijenhoek, 1985) and it is proposed that a high genetic diversity is due to the ability of the species to adapt to changing environmental conditions. The effective allele number is another observation index to determine the genetic variations within a population, which shows the interaction between the alleles per loci. Our study showed that the observed number of alleles was larger than the effective number of alleles. In the study, the effective allele number was 1.8257−1.9998 among the genotypes (). As the number of effective alleles is closer to the number of alleles distributed in a population, the alleles exist evenly in the population.
TABLE 4 Summary of Genetic Variation for All Loci
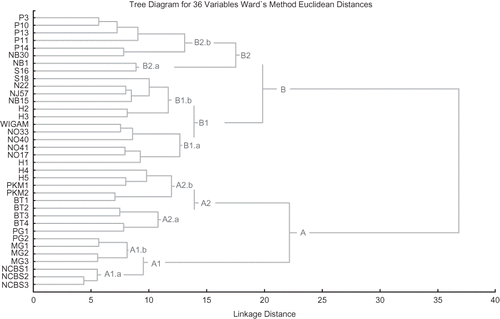
In the dendrogram (), the genotypes were divided into two major clusters ‘A’ and ‘B’ at 37 linkage distance and 16 and 20 genotypes, respectively. Cluster ‘A’ was segregated into two sub-clusters ‘A1’ and ‘A2’, at 22 linkage distances with two minor clusters each. The minor cluster ‘A1a’ with three genotypes (NCBS1, NCBS2, NCBS3) was characterized by trees with orthotropic growth, straight fruits with brown pulp and light green colored leaves. However, NCBS1 was characterized with plageotropic growth and NCBS2 with semi-curved fruit shape and dark green leaves. Genotypes ‘NCBS2’ and ‘NCBS3’ were closely linked together at 4 map distances. In contrast, the four genotypes PG2, MG1, MG2, and MG3 in cluster ‘Alb’ were characterized by trees with semi-curved fruits with dark brown pulp and dark green colored leaves. All the genotypes grouped under sub-cluster ‘A1’ were characterized with orthotropic growth and straight or semi-curved fruit shape.
Sub-cluster ‘A2’ consisted of 9 genotypes and was segregated into two minor clusters ‘A2a’ and ‘A2b’ at 14 linkage distance. The four genotypes (BT2, BT3, BT4, and PG1) of minor cluster ‘A2a’ showed orthotropic growth, semi-curved fruit shape and pulp color was light to dark brown, except for PG1 with plageotropic growth. However, five genotypes (H4, H5, PKM1, PKM2, and BT1) of the ‘A2b’ minor cluster, showed plageotropic growth, curved fruits, and dark green leaves, except for PKM1 with orthotropic growth. In general, the genotypes in minor cluster ‘A2a’ and A2b were segregated as orthotropic and plageotropic growth, and semi-curved and curved fruit, respectively. However, all the genotypes of sub-cluster ‘A2’ predominantly showed dark green colored leaves. In general, the majority of genotypes in cluster ‘A’ showed brown to dark brown fruit pulp and dark green leaves. However, characteristic straight to semi-curved fruits were observed in sub-cluster ‘A1’ and semi-curved to curved fruits were observed in sub-cluster ‘A2’.
The genotypes of major cluster ‘B’ were segregated into two sub-clusters, viz., ‘B1’ and ‘B2’ at 19.8 linkage distances. Sub-cluster ‘B1’ with 12 genotypes was segregated into two minor clusters ‘B1a’ and ‘B1b’ at 13.5 linkage distance, with six genotypes each. The genotypes (WINGAM, NO33, NO40, NO41, NO17, and H1) of minor group ‘B1a’ were characterized by an orthotropic tree growth pattern with straight to semi-curved fruit shape, and light brown pulp, except for NO33 with brown pulp and NO17 with dark brown pulp. The six genotypes (S18, N22, NJ57, NB15, H2, and H3) of the minor cluster ‘B1b’ were characterized by semi-curved fruits with light brown to dark brown colored pulp and dark green leaves. All genotypes of the group B1 predominantly showed orthotropic growth character, straight to semi-curved fruits and light green leaves in group B1a and dark green leaves in group B1b.
The genotypes at sub-cluster ‘B2’ were divided into two minor clusters ‘B2a’ and ‘B2b’ at 17.5 linkage map distance. The two genotypes (NB1 and S16) of minor cluster ‘B2a’ linked at 8.5 linkage distance. Both the genotypes shared orthotropic growth and semi-curved fruit shape, but genotype NB1 had dark brown pulp and dark green leaves and genotype S16 had brown pulp and light green leaves. The six genotypes (P3, P10, P13, P11, P14, and NB30) of minor cluster ‘B2b’ were grouped together at 13 linkage distances. All the genotypes of this group shared orthotropic growth and semi-curved fruit shape, except for genotype P11 with straight fruit shape. The pulp color in the cluster ‘B2b’ varied from light brown to dark brown and the leaf color varied from light green to dark green. In general, the genotypes of the major cluster ‘B’ was predominantly characterized by trees having orthotropic growth and semi-curved fruit shape.
All the tamarind genotypes analyzed in the present studies were collected from southern India with variable growth and fruit morphologies. AFLP analysis revealed a high level of polymorphism (89.3%), proving their wider origin and higher cross pollination. Since tamarind is a perennial tree crop, the ex situ collection could accommodate only a limited number of accessions. Based on the AFLP analysis, genetically closely associated genotypes could be identified, such as P3 and P10, PG2 and MG1, MG2 and MG3, and NCBS2 and NCBS3, which could be avoided for further breeding programs, thus proving the potential of DNA-based markers to determine the genetic relationship among genotypes and could have a practical application in breeding hybrids (CitationJain et al., 1999). In summary, the use of AFLP markers is a useful tool for germplasm analysis and for detection of genetic relationships within tamarind genotypes. Knowledge on genetic diversity will help in the efficient management of tamarind germplasm and future hybridization programs.
ACKNOWLEDGMENT
The authors acknowledge Kirk House Trust for supporting the study.
LITERATURE CITED
- Azad , A.K. , Jones , J.G. and Haq , N. 2007 . Assessing morphological and isozyme variation of jackfruit (Artocarpus heterophyllus Lam.) in Bangladesh . Agrofores. Sys. , 71 : 109 – 125 .
- Bagatini , Y.M. , Panarari , R.S. , Higuti , J. , Cecilio , E.B. , Prioli , A.J. and Prioli , S.M.A.P. 2005 . Morphological and molecular characterization of Corbicula (Mollusca, bivalvia) at Rosana Reservoir, Brazil . Acta. Sci. Biol. Sci. , 27 : 397 – 404 .
- Bao , L. , Chen , K. , Zhang , D. , Li , X. and Teng , Y. 2008 . An assessment of genetic variability and relationships within Asian pears based on AFLP (amplified fragment length polymorphism) markers . Sci. Hort. , 116 : 374 – 380 .
- Baraket , G. , Chatti , K. , Saddoud , O. , Mars , M. , Marrakchi , M. , Trifi , M. and Salhi-Hannachi , A. 2009 . Genetic analysis of Tunisian fig (Ficus carica L.) cultivars using amplified fragment length polymorphism (AFLP) markers . Sci. Hort. , 120 : 487 – 492 .
- Barrett , B.A. and Kidwell , K.K. 1998 . AFLP-based genetic diversity assessment among wheat cultivars from the Pacific Northwest . Crop Sci. , 38 : 1261 – 1271 .
- Bartish , I.V. , Rumpunen , K. and Nybom , H. 2000 . Combined analyses of RAPDs, cpDNA and morphology demonstrate spontaneous hybridization in the plant genus Chaenomeles . Heredity , 85 : 383 – 392 .
- Basha , A.I. , Padulosi , S. , Chabane , K. , Hadj-Hassan , A. , Dulloo , E. , Pagnotta , M.A. and Porceddu , E. 2007 . Genetic diversity of Syrian pistachio (Pistacia vera L.) varieties evaluated by AFLP markers . Gen. Res. Crop Evol. , 54 : 1807 – 1816 .
- Bennet , S.S.R. , Durai , A. , Nicodemus , A. , Gireesan , K. , Nagarajan , B. and Varghees , M. 1997 . “ Genetic improvement of Tamarindus indica L. for increased productivity ” . In Proc. Natl. Symp. Tamarindus indica Edited by: Tirupati , L. 47 – 50 . Andhra Pradesh, , India
- Biron , D.G. , Landry , B.S. , Nenon , J.P. , Coderre , D. and Boivin , G. 2002 . Geographical origin of an introduced pest species, Delia radicum (Diptera: Anthomyiidae), determined by RAPD analysis and egg micromorphology . Bull. Entomol. Res. , 90 : 23 – 32 .
- Challapilli , A.P. , Chimmad , V.P. and Hulamini , N.C. 1995 . Studies on correlation of some fruit characters in tamarind fruits . Karnataka J. Agr. Sci. , 8 ( 1 ) : 114 – 115 .
- Duke , J.A. 1981 . Hand book of legumes of world economic importance , 228 – 230 . New York : Plenum Press .
- Duneman , F. 1994 . “ Molecular classification of Malus with RAPD markers ” . In Progress in temperate fruit breeding , Edited by: Schmidt , H. and Kellerhals , M. 295 – 300 . Dordrecht, , the Netherlands : Kluwer Academic Publishers .
- El-Siddig , K. , Williams , J.T. , Gunasena , H.P.M. , Prasad , B.A. , Pushpakumara , D.K.N.G. , Ramana , K.V.R. and Vijayanand , P. 2006 . Tamarind fruits for the future. International Centre for Underutilised Crops , 188 Southampton, , UK : University of Southampton .
- Francis, C.Y. and R.C. Yang. 2000. Popgene version 1.32. July 2008 http://www.ualberta.ca/_fyeh/index.htm (http://www.ualberta.ca/_fyeh/index.htm)
- Gamble , J.S. 1922 . A manual of Indian timbers , 866 London : Sampson Low, Marston & Co .
- Hogbin , P.M. and Peakall , R. 1999 . Evaluation of the conservation of genetic research to the management of endangered plant Zieria prostrata . Conservation Biol. , 13 : 514 – 522 .
- Ishola , M.M. , Agqbaji , E.B. and Agabaji , A.S. 1990 . A chemical study as Tamarindus indica fruits grown in Nigeria . J. Sci. Food Agr. , 51 : 141 – 143 .
- Jain , A. , Apparanda , C. and Bhalla , P.L. 1999 . Evaluation of genetic diversity and genome fingerprinting of Pandorea (Bignoniaceae) by RAPD and inter-SSR PCR . Genome , 42 : 714 – 719 .
- Kaitho , R.J. , Umunna , N.N. , Hsahlai , I.V. , Tamminga , S. , Vanbruchem , J. , Hanson , J. and Vandewouw , M. 1996 . Palatability of multipurpose tree species—Effect of species and length of study on intake and relative palatability by sheep . Agroforestry Sys. , 33 : 249 – 261 .
- Ledig , F.T. 1986 . “ Heterozygosity, heterosis, and fitness in outbreeding plants ” . In Conservation biology , Edited by: Soule , M. E. 77 – 104 . Sunderland, MA : Sinauer .
- Lewis , G. , Schrire , B. , Mackinder , B. and Lock , M. 2005 . Legumes of the world , 577 Kew, Surrey, , UK : Royal Botanic Gardens .
- Li , G. and Quiros , C.F. 2000 . Use of amplified fragment length polymorphism markers for celery cultivar identification . HortScience , 35 : 726 – 728 .
- Liu , Y. , Sun , Z.H. , Liu , D.C. , Wu , B. and Tao , J.J. 2005 . Assessment of the genetic diversity of pummelo germplasms using AFLP and SSR markers . Sci. Agr. Sinica. , 38 : 2308 – 2315 .
- Moganedi , K.L.M. , Colpaert , N. , Breyne , P. , Sibara , M.M. and Goyvaerts , E.M.A. 2007 . Determination of genetic stability of grafted marula trees using AFLP markers . Sci. Hort. , 111 : 293 – 299 .
- Morton , J. 1987 . Tamarind , 115 – 121 . Miami, FL : Fruits of Warm Climates, Florida Flair Books .
- Narayanaswamy , P. , Rao , V. , Murthy , B.N.S. and Simon , L. 2009 . Microsatellite-based genetic diversity assessment in Grape (Vitis vinifera L) germplasm and its relationship with agronomic traits . Intl. J. Fruit Sci. , 9 : 92 – 105 .
- Nayak , S. , Rout , G.R. and Das , P. 2003 . Evaluation of the genetic variability in bamboo using RAPD markers . Plant Soil Environ. , 49 : 24 – 28 .
- Nei , M. 1973 . Analysis of gene diversity in subdivided populations . Proc. Natl. Acad. Sci. USA. , 70 : 3321 – 3323 .
- Punjani , B.L. and Kumar , V. 2002 . Folk medicinal plants used for skin disorders in the tribal pockets of Sabarkantha district, Gujarat . J. Nat. Remed. , 2 : 84 – 87 .
- Purseglove , L.W. 1981 . Tropical crops—Dicotytedons , Vol. 1 , 204 – 206 . Trinidad : Longmans Green and Co. Ltd .
- Renganayaki , K. , Read , J.C. and Fritz , A.K. 2001 . Genetic diversity among Texas bluegrass genotypes (Poa arachnifera Torr.) revealed by AFLP and RAPD markers . Theoret. Appl. Genet. , 102 : 1037 – 1045 .
- Rimbau , V. , Cerdan , C. , Vila , R. and Iglesias , J. 1999 . Anti-inflammatory activity of some extracts from plants used in the traditional medicine of North-African countries . Phytother. Res. , 13 : 128 – 132 .
- Sen , S.K. and Behera , L.M. 2000 . Ethnomedicinal plants used against lecorrhoea at Bargarh district in Orissa (India) . Neo. Botanica. , 8 : 19 – 22 .
- Shanthi , A. 2003 . Studies on variations and association in selected populations, plantations and clones in tamarind (Tamarindus indica Linn.) , Coimbatore, , India : Ph.D. Thesis. Bharathiar University .
- Shyamalamma , S. , Chandra , S.B.C. , Hegde , M. and Narayanswamy , P. 2008 . Evaluation of genetic diversity in jackfruit (Artocarpus heterophyllus Lam.) based on amplified fragment length polymorphism markers . Genet. Mol. Res. , 7 : 645 – 656 .
- Sokal , R.R. and Michener , C.D. 1958 . A statistical method for evaluating systematic relationships . Univ. Kansas Sci. Bul. , 38 : 1409 – 1438 .
- Sokal , R.R. and Sneath , P.H.A. 1973 . Principles of numerical taxonomy , 359 San Francisco, CA : W.H. Freeman and Co .
- Sreekumar , V.B. , Binoy , A.M. and George , S.T. 2007 . Genetic and morphological variation in breadfruit (Artocarpus altilis (Park.) Fosberg) in the Western Ghats of India using AFLP markers . Gen. Res. Crop Evol. , 54 : 1659 – 1665 .
- Timyan , J. 1996 . Important trees in Haiti , Washington, DC : Southeast Consortium for International Development .
- Tsuda , T. , Mizuno , K. , Ohshima , K. , Kawakishi , S. and Osawa , T. 1995 . Super critical carbon dioxide extraction of antioxidant components of Tamarind (Tamarindus indica L.) seed coat . J. Agr. Food Chem. , 43 : 2803 – 2806 .
- Upadhyay , A. , Saboji , M.D. , Reddy , S. , Deokar , K. and Karibasappa , G.S. 2007 . AFLP and SSR marker analysis of grape rootstocks in Indian grape germplasm . Sci. Hort. , 112 : 176 – 183 .
- Vos , P. , Hogers , R. , Bleeker , M. , Reijans , M. , van Derlee , T. , Hornes , M. , Frijters , A. , Pot , J. , Peleman , J. , Kuiper , M. and Zabeau , M. 1995 . AFLP: A new technique for DNA fingerprinting . Nucl. Acids Res. , 23 : 4407 – 4414 .
- Vrijenhoek , R.C. 1985 . “ Animal population genetics and disturbance: The effects of local extinctions and recolonizations on heterozygosity and fitness ” . In The ecology of natural disturbance and path dynamics , Edited by: Pickett , S.T.A. and White , P.S. 265 – 285 . London : Academic Press .
- Wang , L. , Xing , S.Y. , Yang , K.Q. , Wang , Z.H. and Guo , Y.Y. 2006 . Genetic relationships of ornamental cultivars of Ginkgo biloba analyzed by AFLP techniques . Acta. Genet. Sin. , 33 : 1020 – 1026 .
- Wang , W. , Chen , L. , Yang , P. , Hou , L. , He , C. , Gu , Z. and Liu , Z. 2007 . Assessing genetic diversity of populations of topmouth culter (Culter alburnus) in China using AFLP markers . Biochem. Systematic Ecol. , 35 : 662 – 669 .
- Ward , J.H. 1963 . Hierarchic grouping to optimize an objective function . J. Amer. Stat. Assoc. , 58 : 236 – 239 .
- Xu , D.H. , Wahyuni , S. , Sato , Y. , Yamaguchi , M. , Tsunematsu , H. and Ban , T. 2006 . Genetic diversity and relationships of Japanese peach (Prunus persica L.) cultivars revealed by AFLP and pedigree tracing . Genet. Resour. Crop Evol , 53 : 883 – 889 .
- Yamanaka , N. , Hasran , M. , Xu , D.H. , Tsunematsu , H. , Idris , S. and Ban , T. 2006 . Genetic relationship and diversity of four Mangifera species revealed through AFLP analysis . Genet. Resour. Crop Evol. , 53 : 949 – 954 .
- Yuan , Z. , Chen , X. , He , T. , Feng , J. , Feng , T. and Zhang , C. 2007 . Population genetic structure in apricot (Prunus armeniaca L.) cultivars revealed by fluorescent-AFLP markers in southern Xinjiang, China . J. Genet. Genomics , 34 : 1037 – 1047 .
- Yuan , Z. , Yin , Y. , Qu , J. , Zhu , L. and Li , Y. 2007 . Population genetic diversity in Chinese pomegranate (Punica granatum L.) cultivars revealed by fluorescent-AFLP markers . J. Genet. Genomics , 34 : 1061 – 1071 .
- Zabeau , M. and Vos , P. Selective restriction fragment amplification: A general method for DNA fingerprinting . European patent application no. 92402629.7 . 1993 .
- Zhuang , Q. , Dan-wei , M.A. , Wang , W.G. , Wang , S.H. and Chen , F. 2009 . Genetic diversity of Pogonatherum paniceum (Lam.) Hack. in Southwest China revealed by AFLP markers . Afr. J. Biotechnol. , 8 : 1226 – 1232 .