Abstract
The effects of chitosan 1% and 1.5%, calcium chloride (CaCl2) 1% and 1.5%, chitosan 1% + gibberellic acid 100 ppm, chitosan 1.5% + gibberellic acid 100 ppm, jojoba wax, and glycerol (98%) coatings were evaluated on the shelf life and postharvest quality characteristics of banana fruits stored at 34 ± 1°C and 70–75% relative humidity, while uncoated fruits served as a control. The coatings of chitosan, chitosan + gibberellic acid, and jojoba wax delayed the changes in the weight loss percentage, decay percentage, total soluble solids, pH, titrable acidity, sugar accumulation, pigment degradation, and ascorbic acid compared to uncoated ones. Further, the least disease incidence was found to occur in the banana fruits treated with chitosan and chitosan + gibberellic acid. Hence, it can be concluded that coating with chitosan and chitosan + gibberellic acid has the potential to control decay percentage, prolong the shelf life, and preserve valuable attributes of banana. Calcium chloride and glycerol coatings neither showed any potential to control disease incidence nor prolong the storage life and preserve valuable attributes of banana fruit.
INTRODUCTION
Banana (Musa spp.) is the most widely cultivated and consumed fruit in the tropical and subtropical regions of the world where they constitute a major staple food crop for millions of people (CitationDeka and Choudhury, 2006). India is the largest producer of fruits in the world and banana tops the list with respect to production and area of cultivation (TIFAC Report cited in CitationSurendranathan et al., 2004). Bananas contain nutrients in more balanced proportion than many other fruits. They have nearly all the essential nutrients, including minerals and vitamins (CitationIslam et al., 2001). Bananas are unique due to their high calories and nutritive values. As compared to apples, they contain five times more vitamin A and iron, four times more protein, three times more phosphorus, twice the carbohydrates, and the other vitamins and minerals (CitationGasster, 1963). They are useful for patients with peptic ulcers, for treatment of infant diarrhea, celiac disease, and colitis (CitationRobinson, 1996). They are also ideal for patients suffering from gout, arthritis, kidney disorders, blood pressure, and heart problems (CitationStover and Simmonds, 1987).
In many cases, bananas are transported from localities of production great distances for marketing and consumption. Bananas, being a climacteric fruit, have a very short storage life. They are highly perishable and are, therefore, susceptible to several diseases resulting in extensive postharvest losses (CitationMaqbool et al., 2010). In turn, longer shelf life would enhance trade opportunities between nations by extending time constraints under which fresh produce must be delivered to more distant geographic markets or by allowing the use of slower and less expensive modes of transportation (CitationKader, 1986). But due to absence/non adoption of proper postharvest management practices, the postharvest loss of bananas is highest (22%) among all the fruits (CitationDeka and Choudhury, 2006). Reduction of postharvest losses reduces cost of production, trade and distribution, lowers the price for the consumer, and increases the farmers' income.
The extension of fruit shelf life is an important goal to be attained. Many storage techniques have been developed to extend the marketing distance and holding periods for commodities after harvest. Different preservation methodologies have been studied. One method of extending postharvest shelf life is the use of the edible coatings. Today, use of edible coatings is a common issue that is beneficial to protect nutrients of food, especially fruits and vegetables, and provide a long durability (CitationRaheleh et al., 2008). Edible coatings have long been known to protect perishable food products from deterioration by retarding dehydration, suppressing respiration, improving textural quality, helping retain volatile flavor compounds, and reducing microbial growth (CitationDebeaufort et al., 1998). The objective of this study was to elucidate the effects of edible coatings, such as chitosan [alone and in combination with gibberellic acid (GA3)], calcium chloride (CaCl2), jojoba wax, and glycerol on physiological and biochemical changes in harvested banana fruit with a view to optimization of this postharvest technology for green life extension.
MATERIALS AND METHODS
Fruit Materials
For the present study, hands of mature green bananas were obtained from a local farm in Vallabh Vidyanagar, Gujarat, India. Hands were cut into fingers, and dipped for 3 min in sodium-hypochlorite (500 ppm) fungicide solution to control postharvest diseases, and then were allowed to air dry. Fruits were selected for freedom from visual defects and uniformity of shape, color, and size. Subsequently, these banana fruits were grouped into 8 experimental sets with 20 fruits per set and a control set of 20 fruits. The postharvest treatments (edible coatings) viz. chitosan 1% (T1), chitosan 1.5% (T2), CaCl2 1% (T3), CaCl2 1.5% (T4), chitosan 1% + GA3 100 ppm (T5), chitosan 1.5% + GA3 100 ppm (T6), glycerol (T7), jojoba wax (T8), and (uncoated) control (T9) were given for 10 min. After the treatment, the fruits were air dried at an ambient temperature for 30 min in an attempt to reduce possible chemical injury.
The treated banana fruits of experimental as well as control sets were subjected for the following physiological and biochemical analyses at the beginning of the experiment (i.e., 0 days) and after 5 and 10 days of storage in the laboratory with the average maximum and minimum temperature of it at 34 ± 1°C and relative humidity 70–75%.
Weight Loss Percentage (WLP)
The WLP of banana fruit samples was calculated by considering the differences between initial weight and final weight of currently tested banana fruits divided by their initial weight.
Decay or Rotting Percentage
The decay or rotting of the stored banana fruits was determined by their visual observations. Decayed fruits (physiological and microbial decay) were discarded in each sample and decay percent was recorded.
Shelf Life
The shelf life of these banana fruits was calculated by counting the days required for them to attain the last stage of ripening, but up to the stage when they remained still acceptable for marketing.
Total Soluble Solids (TSS), pH, and Titrable Acidity (TA)
The TSS content of the fruit was determined by using a refractrometer (Atago Co., Tokyo, Japan). A homogenous sample was prepared by blending the banana flesh in a blender. The sample was thoroughly mixed and a few drops were taken on the prism of the refractometer and a direct reading was taken by reading the scale in the meter as described in CitationAOAC (1994). The pH of the fruit samples was determined as per the method described by CitationAOAC (1994), while the titrable acidity (expressed as citric acid %) was determined by titrating 5 ml of juice with 0.1 N sodium hydroxide, using phenolphthalein as an indicator (CitationMazumdar and Majumder, 2003).
Biochemical Analysis
The total soluble sugars and starch content were determined by following the anthrone method, while the dinitrosalicylic acid method was followed for the reducing sugars and non reducing sugar content (CitationThimmaiah, 1999). The quantitative analyses of pigments, such as total chlorophylls (total chl.), chlorophyll ‘a’ (chl. ‘a’), chlorophyll ‘b’ (chl. ‘b’), and total carotenoids, were carried out as per the methods described by CitationWang et al. (2005). Ascorbic acid (vitamin C) content was determined by using the titrimetric method with the titration of filtrate against 2, 6-dichlorophenol indophenols, and the results of vitamin C content were expressed as mg.100 g−1 (CitationMazumdar and Majumder, 2003).
Statistical Analysis
All the performed analyses were carried out in triplicate and the standard deviation was calculated. The experimental design was a completely randomized design with three replicates. Analysis of variance (ANOVA) was used to detect treatment effect. Mean separation was performed by using least significance difference (LSD) at the P < 0.05 level. The data were analyzed using Duncan's multiple range test (CitationBliss, 1967).
RESULTS AND DISCUSSION
Weight Loss Percentage
Result shows the change of WLPs of coated and uncoated banana (control) during the storage period (). Coating process caused a significant decrease (P < 0.05) in WLPs as compared with the control samples. All the treatments, except T7, showed a reduction in WLPs compared to that of the control fruits. The control samples had significantly (P < 0.05) higher WLP (15.98%) after 10 days of storage, while banana samples coated with chitosan, chitosan + GA3, CaCl2, and jojoba wax had significantly (P < 0.05) lower WLP values. The weight loss in banana during ripening might be due to substrate loss by respiration and loss of water through various physiological mechanisms (CitationIslam et al., 2001). This reduction in weight loss was probably due to the effects of these coatings as a semi-permeable barrier against oxygen, carbon dioxide, moisture, and solute movement, thereby reducing respiration, water loss, and oxidation reaction rates (CitationBaldwin et al., 1999). The obtained results are in accordance with the findings of CitationGarcia et al. (1998) who reported that the chitosan film formed on the surface of the fruit delayed migration of moisture from the fruit into the environment, thus reducing weight loss during storage. The T2 (chitosan 1.5%) treatment was observed to prevent weight loss more than that of other tested treatments throughout the storage period but was less than that of the T5 (chitosan + GA3) treatment. The combined treatment (i.e., T5 and T6) was clearly effective in conferring a physical barrier to moisture loss and, therefore, retarding dehydration and fruit shriveling. In this experiment, the glycerol and CaCl2 coating was the least effective in reducing weight loss. CaCl2 treatments increased weight loss that was due to osmotic potential. Earlier studies by some investigators also indicated that postharvest application of Ca2+ accelerated ripening of ‘Cavendish’ bananas (CitationWillis et al., 1982a; CitationHuddar et al., 1991).
TABLE 1 Effect of Edible Coatings and Their Combinations With Different Chemicals on Weight Loss Percentages (WLPS) and Decay Percentage of Banana Fruit During Storage at 34 ± 1°C
Decay Percentage
Data summarized in show the changes in decay percentage values of coated and uncoated bananas (control) during the storage period. Coatings significantly (P < 0.05) reduced the decay percentage as compared to that of control samples during the storage period. Decay percentage of control samples at the end of storage period was approximately four to five times higher than that of the banana fruits coated with chitosan, chitosan + GA3, and jojoba wax. Among all the presently tested treatments, T2 (i.e., 10.3%) and T5 (i.e., 9.9%) treatments were superior in controlling the decay percentage. Chitosan in combination with GA3 (T5) significantly reduced the decay level, i.e., 9.9% compared to all other treatments and control samples. This is consistent with the reports that chitosan has antifungal properties against several postharvest pathogens (CitationJiang and Li, 2001). CitationEl-Ghaouth et al. (1992a) suggested that chitosan induces chitinase, a defense enzyme, and catalyzes the hydrolysis of chitin, a common component of fungal cell walls, thus preventing the growth of fungi on the fruits. The results of the present study show that the banana fruits treated with jojoba wax had a lower decay percentage, i.e., 11.5% after 10 days of storage. Similar results were obtained by CitationAhmed et al. (2007) in orange fruit that was coated with jojoba wax and had lower decay percentage compared to the control fruits.
SHELF LIFE
The shelf life of banana fruit has been extended significantly (P < 0.05) with some of the treatments tested in the current study (). The banana fruits treated with the T6 treatment were found to extend their shelf life to the maximum duration of 17.2 days as compared to that of other presently tested treatments (). The treatment of 1% and 1.5% of chitosan caused the extension of shelf life of banana fruits tested in the current study by 15.3 and 16.2 days, respectively, as compared (i.e., 11.3 days) for fruits of the control set (). These results also support the view of CitationZhang and Quantick (1998) who reported that the application of a chitosan coating improved the quality and storability in strawberries and raspberries. The positive effect of a chitosan coating on storage life could probably be due to modifying the atmosphere within as in modified atmosphere (MA) storage. The depolymerization process of metabolism substrates is possibly influenced by the MA created inside fruits. The MA created can, therefore, delay ripening by delaying ethylene production and by reducing the level of internal oxygen and consequently prolonging the storage life of fruits (CitationEl-Ghaouth et al., 1992b). Among the tested postharvest treatments, the fruits treated with chitosan and GA3 (T6) exhibited longer shelf life and reduced spoilage (). CitationRao and Chundawat (1988) suggested that postharvest dipping of fruits in GA3 delayed the conversion of starch to sugars and reduced peroxidase activity and ethylene production. In the present study, calcium treatments showed a depressing impact on the shelf life. Studies carried out by other investigators also indicate that postharvest application of Ca2+ accelerated ripening of ‘Cavendish’ bananas (CitationWillis et al., 1982a; CitationHuddar et al., 1991). The hastened color development may be linked to faster achievement of senescence leading to shorter shelf life. A previous study on bananas showed a tendency towards a negative correlation of lesion diameter of anthracnose and fruit firmness (CitationPerera et al., 1999). These results appear to suggest that firmness reduction observed in Ca2+-treated bananas may develop a susceptibility to anthracnose and, hence, reduction of shelf life.
TABLE 2 Effect of Edible Coatings and Their Combination With Different Chemicals on Shelf Life (Days) and Total Soluble Solids (TSS) of Banana During Storage at 34 ± 1°C
Total Soluble Solids
Changes in the TSS of bananas over the storage time are shown in . The TSS of control fruit increased with storage time, while the banana fruits coated with chitosan, chitosan + GA3, and jojoba experienced a slower increase during the 10 days of storage. Data showed that control samples without coating treatments had significantly (P < 0.05) higher levels of TSS with 20.7% at 10 days of storage period. TSS values of banana treated with T1, T2, T5, T6, and T8 after 10 days of storage were approximately 1.3, 1.4, 1.4, 1.5, and 1.5 times lower, respectively, than the TSS values of control samples without coating. The concentrations of free sugars progressively increased with storage; this increase was quite markedly delayed by chitosan, chitosan + GA3, and jojoba coatings. This was probably due to the semi-permeable chitosan film, which formed on the surface of the fruits, causing the modification of internal atmosphere and the endogenous CO2 and O2 concentrations of the fruit, thus retarding ripening as shown by CitationBai et al. (1988). Other reports indicated a slow rise in TSS of mango and banana treated with chitosan (CitationKittur et al., 2001), which might be due to chitosan's effect on slowing down the ripening, respiration rise, and metabolism processes of the fruits. The combined treatment of chitosan and GA3 had a greater effect in reducing the TSS during storage compared to other tested treatments. During the 10 days of storage, the least amount of TSS (i.e., 14.0%) was seen in the T6- and T8-treated banana fruits.
pH and Titrable Acidity
Uncoated (control) fruit was observed to have higher pH values compared with other coated fruits (). Chitosan 1.5% and chitosan (1.5%) + GA3 (100 ppm) coating proved to be significantly better (P < 0.05) in maintaining low pH, compared to uncoated fruits and CaCl2-coated fruits. The combined treatment of chitosan and GA3 showed the changes in pH effectively, delaying fruit ripening and senescence. All the coated fruits have shown lower pH compared to that of uncoated fruits. The pH in both coated and uncoated fruits increased, while their titrable acidity decreased significantly (P < 0.05) along with increased storage time (). These results are in accordance with those reported by CitationGarcia et al. (1998) that the decrease of acidity during storage demonstrated fruit senescence. The change in pH is associated with a number of reasons; it might be due to the effect of coating on the biochemical condition of the fruit and slower rate of respiration and metabolic activity (CitationJitareerat et al., 2007). Coating slowed the changes of pH and TA by effectively delaying fruit senescence. The higher level of TA in the fruits coated with chitosan and the combination with GA3 () may be due to a protective O2 barrier or reduction of O2 supply to the fruit surface that inhibited the respiration rate (CitationJiang and Li, 2000). Increased activity of citric acid during ripening or reduction in acidity may be due to their conversion into sugars and their further utilization in the metabolic processes of the fruit. The chitosan coating treatment [i.e., T1 (4.4%) and T2 (4.2%)] showed significantly (P < 0.05) higher amounts of TA than that of chitosan + GA3 [i.e., T5 (3.3%) and T6 (3.2%)] during 10 days of storage. Slowing down the banana ripening by means of coating (chitosan and jojoba) and GA3 causing inhibition of enzyme activity could be the reason for delay in the use of organic acids in the enzymatic reactions of respiration. This result was consistent with reports of CitationGarcia et al. (1998) and CitationZhang and Quantick (1998) who reported that TA of chitosan or starch-based coated strawberries kept under cold storage decreased with time, but to a lesser extent than that of uncoated fruit.
TABLE 3 Effect of Edible Coatings and Their Combination With Different Chemicals on pH and Titrable Acidity (TA) of Banana During Storage at 34 ± 1°C
Sugars
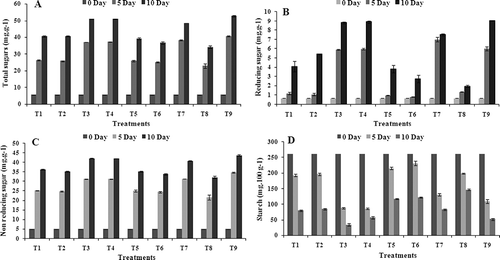
Gradual increase was seen in the content of total sugars, reducing sugars, and non reducing sugars in coated and uncoated banana fruits, but the accumulation of the sugars in coated fruits was found to be less (, B, C). It is clear from the results that at the time of harvest the sugars were very low, but with the passage of time, total sugars increased with ripening. An increase in reducing sugar might be attributed to enzymatic conversion of starch to reducing sugar and also to conversion of some non reducing sugar to reducing sugar through the process of inversion. The coatings of chitosan, chitosan + GA3, and jojoba wax were found to lower the total sugars, reducing sugars, and non reducing sugars compared to fruits of the control set. Among all the coated fruits, the fruits of T8 (34.2 mg.g−1), T6 (36.6 mg.g−1), and T5 (39.2 mg.g−1) sets showed significantly (P < 0.05) lower amounts of total sugar content compared to that of control fruits (52.8 mg.g−1) (, B, C). This view is supported by CitationHoa and Ducamp (2008) who studied the effects of different coatings on biochemical changes of ‘cat Hoa loc’ mangoes in storage and observed that the content of reducing sugars and total sugars were lower with respect to that of control fruits. A maximum amount of reducing sugars (9.1 mg.g−1) in control fruits () might be due to rapid conversion of starch to sugars as a result of moisture loss and decrease in acidity by physiological changes during storage.
FIGURE 1 Effect of edible coatings on content of total sugars (A), reducing sugar (B), non reducing sugar (C), and starch (D) of banana fruit during storage at 34 ± 1°C. Vertical bars represent ±SE of means for three replicates. T1—chitosan (1%); T2—chitosan (1.5%); T3—calcium chloride (1%); T4—calcium chloride (1.5%); T5—chitosan (1%) + gibberellic acid (100 ppm); T6—chitosan (1.5%) + gibberellic acid (100 ppm); T7—glycerol (98%); T8—jojoba oil; T9—control (uncoated).
Starch
Starch is bulk polysaccharides in banana fruit and its degradation results in pronounced softening (CitationPrasanna et al., 2007; CitationZhang et al., 2010). Significant (P < 0.05) variation was found in respect to starch content in the coated banana fruits during storage period (). However, degradation of starch in coated fruit was slow as compared with control fruit and was accompanied by a delay in the increase of soluble sugar content. The result of the present study further reveals that the amount of starch content was very high at the beginning of their storage period and thereafter started to decline. A decrease in starch content during banana ripening was also observed by CitationChacon et al. (1987). At the storage of 10 days interval, the fruits coated with T8 and T6 have shown the maximum amount of starch content, i.e., 146.6 mg.g−1 and 122 mg.g−1, respectively. Thus, the results of the present study indicate that the least value of starch content (i.e., 34.4 mg.g−1 and 52.2 mg.g−1) was perceived in the T3 and T9 (control) treated fruits, respectively (), and these are in line with that obtained by CitationIslam et al. (2001) who reported that the banana fruits coated with waxol had a higher amount of starch compared to the control fruits.
Pigments
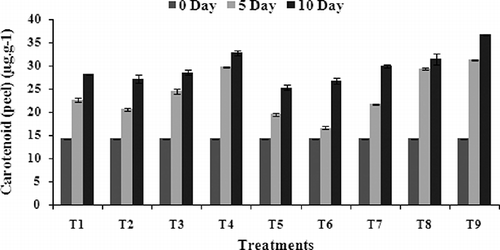
The data obtained pertaining to the total chlorophyl (chl.) and total carotenoids as affected by the coatings tested under the current study are presented in and . The quantitative analysis of photosynthetic pigments indicates that they occur more in the coated fruits in both pulp and peel. In contrast, control fruits exhibit weaker stimulation on total chl. accumulation. All the treatments, except T7, showed significantly (P < 0.05) more amounts of total chl. in peel as compared to that of the control set (). During the end of the storage period, the fruits coated with chitosan, chitosan + GA3, and jojoba coating have shown a higher impact on the accumulation of photosynthetic pigments (chl. ‘a’, chl. ‘b’, and total chl.) in peel as well as pulp. The retardation of color development in the banana fruit coated with chitosan and chitosan + GA3 in this investigation can be attributed to the modified internal fruit atmosphere, which caused slowing down of the ripening process indicating by lower change of peel color of fruits. The coating reduced the fruit respiration and ethylene production (CitationEryani Raqeeb et al., 2009). The slow respiration and reduced ethylene synthesis, in turn, delayed ripening and senescence, resulting in less change in the greenish yellow color of fruits (CitationKader et al., 1989). Higher amounts of chl. ‘a’ (37.1 μg.g−1), chl. ‘b’ (12.9 μg.g−1), and total chl. (48.0 μg.g−1) in the peel was seen in T6, T8, and T6, respectively, at the 10 days of storage period (, B, C). In the case of pulp, the fruits treated with T6 treatment have revealed a greater amount of total chl., i.e., 6.0 μg.g−1 (). The control fruits have shown higher impact on their chlorophyll degradation. The least amount of total chl. was seen in the uncoated fruits in peel as well as pulp, i.e., 9.2 μg.g−1and 1.3 μg.g−1, respectively (, D), compared to the fruits coated with chitosan, chitosan + GA3, and jojoba wax. The loss of green color was the most obvious change in bananas, which was probably due to the physicochemical changes by degradation of the chlorophyll structure and increase in carotenoid pigments during storage. The principal agents responsible for this degradation might be the oxidative system, pH change, and enzymes, such as chlorophyllase (CitationWillis et al., 1982b). The data of the present study suggested that the level of carotenoid content progressively increased in both the coated as well as uncoated fruits during their storage periods (). The increase in carotenoid content during storage might be due to a series of physicochemical changes, such as the breakdown of chlorophyll and increase in carotenoid pigments of the peel caused by the enzymatic oxidation and photo degradation. The delay in ripening and degradation of chlorophyll and retention of green color for a longer period also depend on the types of coating (CitationKittur et al., 2001).
FIGURE 2 Effect of edible coatings on content of total chlorophylls (peel) (A), chlorophyll ‘a’ (peel) (B), chlorophyll ‘b’ (peel) (C), and total chlorophylls (pulp) (D) of banana fruit during storage at 34 ± 1°C. Vertical bars represent ±SE of means for three replicates. T1—chitosan (1%); T2—chitosan (1.5%); T3—calcium chloride (1%); T4—calcium chloride (1.5%); T5—chitosan (1%) + gibberellic acid (100 ppm); T6—chitosan (1.5%) + gibberellic acid (100 ppm); T7—glycerol (98%); T8—jojoba oil; T9—control (uncoated).
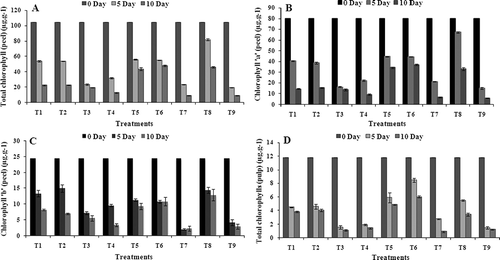
FIGURE 3 Effect of edible coatings on carotenoid content of banana peel during storage at 34 ± 1°C. Vertical bars represent ±SE of means for three replicates. T1—chitosan (1%); T2—chitosan (1.5%); T3—calcium chloride (1%); T4—calcium chloride (1.5%); T5—chitosan (1%) + gibberellic acid (100 ppm); T6—chitosan (1.5%) + gibberellic acid (100 ppm); T7—glycerol (98%); T8—jojoba oil; T9—control (uncoated).
Ascorbic Acid
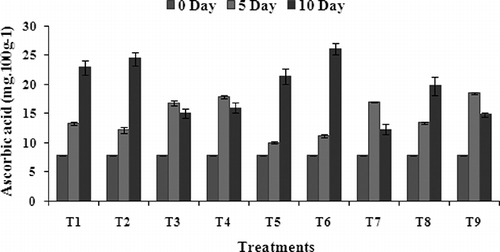
Ascorbic acid is one of the most abundant antioxidants present in fruits. Ascorbic acid was found to maintain with postharvest application of chitosan, chitosan + GA3, and jojoba coating. Chitosan treatment with GA3 (T6) had the highest content (26.2 mg.100 g−1) of ascorbic acid compared with other coatings and control (14.9 mg.100 g−1) fruits during 10 days of their storage periods (). On the other hand, CaCl2-coated banana fruits have not shown any significant differences with control sets. Chitosan coating at 1 and 1.5% maintained the loss of ascorbic acid compared to the uncoated ones. This study has demonstrated that the combined treatment (chitosan + GA3) delayed the increase of ascorbic acid and the loss at the ripening stage of the banana. After 10 days of storage, all the coatings, except CaCl2 and glycerol, were much more effective in reducing the ascorbic acid loss of banana fruits than the uncoated (control) fruit. The present study showed that vitamin C content of chitosan and chitosan+GA3-coated bananas were highest among all the treatments at the end of the storage. This may be due to the effect of chitosan coating on retarding ripening and slowing down the metabolism activity, which causes oxidation of ascorbic acid. The reason for high vitamin C content in coated fruit can be attributed to the slow ripening rate of chitosan-treated fruit. This view is also shared by CitationAbbasi et al. (2009), who reported that the ascorbic acid contents in irradiated Crab chitosan-coated mango fruits were higher than unirradiated chitosan-coated fruits at the end of the storage. Oxidation of ascorbic acid may be caused by several factors, including exposure to oxygen, metals, light, heat, and alkaline pH (CitationAbbasi et al., 2009). As CitationAyranci and Tunc (2003) stated, slowing down of vitamin loss was attributed to the low oxygen permeability of the coating.
FIGURE 4 Effect of edible coatings on ascorbic acid content of banana fruit during storage at 34 ± 1°C. Vertical bars represent ±SE of means for three replicates. T1—chitosan (1%); T2—chitosan (1.5%); T3—calcium chloride (1%); T4—calcium chloride (1.5%); T5—chitosan (1%) + gibberellic acid (100 ppm); T6—chitosan (1.5%) + gibberellic acid (100 ppm); T7—glycerol (98%); T8—jojoba oil; T9—control (uncoated).
CONCLUSIONS
The results of the current investigation indicate that banana fruit coated with chitosan alone, chitosan in combination with gibberellic acid, and jojoba coatings cause a significant delay in the change of weight loss, decay percentage, total soluble solids, pH, titrable acidity, sugar accumulation, pigment degradation, and ascorbic acid content compared to uncoated ones. Chitosan 1.5% and chitosan 1.5% + gibberellic acid 100 ppm was more effective as a protective coating on banana fruits by maintaining the quality characteristics, prolonging the shelf life, and preserving the valuable attributes of banana fruits during the storage period. Hence, these coatings, being environmentally friendly, provide good handling procedures to enhance banana export to long distances with minimum losses.
ACKNOWLEDGMENT
Neeta B. Gol is grateful to the University Grant Commission (UGC), New Delhi for financial support under the program of Meritorious Fellowship.
LITERATURE CITED
- Abbasi , N.A. , Iqbal , Z. , Maqbool , M. and Hafiz , I.A. 2009 . Postharvest quality of mango (Mangifera indica L.) fruits as affected by chitosan coating . Pakistan J. Bot. , 41 : 343 – 357 .
- Ahmed , D.M. , El Shami , S.M. and El Mallah , M.H. 2007 . Jojoba oil as a novel coating for exported Valencia orange fruit part 1: The use of trans (isomerized) jojoba oil . Amer. Eur. J. Agr. Environ. , 2 : 173 – 181 .
- AOAC (Association of Official Analytical Chemists) . 1994 . Official methods of analysis , 16th Virginia, , USA
- Ayranci , E. and Tunc , S. 2003 . A method for the measurement of oxygen permeability and development of edible films to reduce the rate of oxidative reactions in fresh foods . Food Chem. , 80 : 423 – 431 .
- Bai , R.K. , Huang , M.Y. and Jiang , Y.T. 1988 . Selective permeabilities of chitosan acetic acid complex membrane and chitosan polymer complex membrane for oxygen and carbon dioxide . Polym. Bul. , 20 : 83 – 88 .
- Baldwin , E.A. , Burns , J.K. , Kazokas , W. , Brecht , J.K. , Hagernmaier , R.D. , Bender , R.J. and Pesis , E. 1999 . Effect of two edible coatings with different permeability characteristics on mango (Mangifera indica L.) ripening during storage . Postharvest Biol. Technol. , 17 : 215 – 226 .
- Bliss , C.I. 1967 . “ Statistical methods for research in the natural sciences ” . In Statistics in biology , Vol. 1 , 558 New York : McGraw Hill .
- Chacon , S.I. , Viquez , F. and Chacon , G. 1987 . Physico-chemical banana ripening scale . Fruits , 42 : 95 – 102 .
- Debeaufort , F. , Quezada Gallo , J.A. and Voilley , A. 1998 . Edible films and coatings: Tomorrow's packagings: A review . Critical Rev. Food Sci. , 38 : 299 – 313 .
- Deka , B.C. and Choudhury , S. 2006 . Postharvest treatments for shelf life extension of banana under different storages . Acta Hort. , 723 : 948 – 950 .
- El Ghaouth , A. , Arul , J. , Asselin , A. and Benhamou , N. 1992a . Antifungal activity of chitosan on postharvest pathogens induction of morphological and cytological alterations in Rhizopus stolonifer . Mycol. Res. , 96 : 769 – 779 .
- El Ghaouth , A. , Ponnampalam , R. , Castigne , F. and Arul , J. 1992b . Chitosan coating to extend the storage life of tomatoes . HortScience , 27 : 1016 – 1018 .
- Eryani Raqeeb , A.A. , Mahmud , T.M.M. , Sayed Omar , S.R. , Zaki , A.R.M. and Al Eryani , A.R. 2009 . Effects of calcium and chitosan treatments on controlling anthracnose and postharvest quality of Papaya (Carica papaya L.) . Intl. J. Agr. Res. , 4 : 53 – 68 .
- Garcia , M.A. , Martino , M.N. and Zaritzky , N.E. 1998 . Plasticized starch based coatings to improve strawberry (Fragaria ananassa) quality and stability . J. Agr. Food Chem. , 46 : 3758 – 3767 .
- Gasster , M. 1963 . The banana in geriatric and low-caloric diets . Geriatrics , 18 : 782 – 786 .
- Hoa , T.T. and Ducamp , M. 2008 . Effects of different coatings on biochemical changes of ‘cat Hoa loc’ mangoes in storage . Postharvest Biol. Technol. , 48 : 150 – 152 .
- Huddar , A.G. , Chandramonti , H.D. and Nachegowda , V. 1991 . Effect of postharvest application of calcium on ripening of bananas cv . Robusta. Haryana J. Hort. Sci. , 20 : 60 – 64 .
- Islam , M. S. , Islam , M. S. and Kabir , A.H.M.F. 2001 . Effect of postharvest treatments with some coating materials on the shelf life and quality of banana . Pakistan J. Biol. Sci. , 4 : 1149 – 1152 .
- Jiang , Y.M. and Li , Y.B. 2000 . Effect of chitosan coating on postharvest life and quality of longan fruit . Food Chem. , 73 : 139 – 143 .
- Jiang , Y.M. and Li , Y.B. 2001 . Effect of chitosan coating on postharvest life and quality of longan fruit . J. Food Chem. , 73 : 139 – 143 .
- Jitareerat , P. , Paumchai , S. and Kanlayanarat , S. 2007 . Effect of chitosan on ripening enzymatic activity, and disease development in mango (Mangifera indica L.) fruit . N. Z. J. Crop Hort. Sci. , 35 : 211 – 218 .
- Kader , A.A. 1986 . Potential applications of ionizing radiation in postharvest handling of fresh fruits and vegetables . Food Technol. , 40 : 117 – 121 .
- Kader , A.A. , Zagory , D. and Kerbel , E.L. 1989 . Modified atmosphere packaging of fruits and vegetables . Crit. Rev. Food Sci. Nutr. , 28 : 1 – 30 .
- Kittur , F. , Saroja , N. , Habibunnisa and Tharanathan , R. 2001 . Polysaccharide based composite coating formulations for shelf extension of fresh banana and mango . Eur. Food Res. Technol. , 206 : 44 – 47 .
- Maqbool , M. , Ali , A. and Alderson , P.G. 2010 . Effect of cinnamon oil on incidence of anthracnose disease and postharvest quality of bananas during storage . Intl. J. Agr. Biol. , 12 : 516 – 520 .
- Mazumdar , B.C. and Majumder , K. 2003 . Methods on physico-chemical analysis of fruits , 112 – 116 . Delhi, , India : Daya Publ. House .
- Perera , O.D.A.N. , Basnayake , B.M.K.M.K. and Karunaratne , A.M. 1999 . Physicochemical characteristics, popularity and susceptibility to anthracnose of some local banana cultivars . J. Nat. Sci. Foundation , 27 : 119 – 130 .
- Prasanna , V. , Prabha , T.N. and Tharanathan , R.N. 2007 . Fruit ripening phenomena—An overview . Critical Rev. Food Sci. , 47 : 1 – 19 .
- Raheleh , G. , Ahmad , K. and Hamid , B.G. 2008 . Application of edible coating for improvement of quality and shelf-life of raisins . World Applied Sci. J. , 3 : 82 – 87 .
- Rao , D.V.R. and Chundawat , B.S. 1988 . Ripening changes in Sapota Cv. Kalipati at ambient temperature . Indian J. Plant Physiol. , 31 : 205 – 208 .
- Robinson , J.C. 1996 . “ Morphological characteristics and plant development ” . In Bananas and plantains , 8 – 33 . Wallingford : CABI .
- Stover , R.H. and Simmonds , N.W. 1987 . Bananas , 3rd , 468 – 470 . London : Longman .
- Surendranathan , K.K. , Ramaswamy , N.K. and Pendharkar , M.B. 2004 . Role of phosphorylase and amylase in accelerated ripening of mutant banana ‘Basrai-10Gy’ . Indian J. Biotechnol. , 3 : 382 – 387 .
- Thimmaiah , S.K. 1999 . Standard methods of biochemical analysis , 51 – 59 . New Delhi, , India : Kalyani Publication .
- Wang , Z.F. , Ying , T.J. , Bao , B.L. and Huang , X.D. 2005 . Characteristics of fruit ripening in tomato mutant epi . J. Zhejiang Univ. Sci. , 6 : 502 – 507 .
- Willis , R.B.H. , Tirmazi , S.I. and Scott , K.J. 1982a . Effect of postharvest application of calcium on ripening rates of pears and bananas . J. Hort. Sci. , 57 : 431 – 435 .
- Willis , R.B. , Lee , T.H. , Graham , D. , Glasson , W.B. and Hall , E.G. 1982b . Postharvest: An introduction to the physiology and handling of fruits and vegetables , 34 – 35 . Westport : AVI Publishing Co .
- Zhang , D.L. and Quantick , P.C. 1998 . Antifungal effects of chitosan coating on fresh strawberries and raspberries during storage . J. Hort. Sci. Biotechnol. , 73 : 763 – 767 .
- Zhang , H. , Yang , S. , Joyce , D.C. , Jiang , Y. , Qua , H. and Duan , X. 2010 . Physiology and quality response of harvested banana fruit to cold shock . Postharvest Biol. Technol. , 55 : 154 – 159 .