Abstract
Twenty-one local pomegranate cultivars maintained in an ex-situ collection in Tunisia, were studied using 29 characteristics related to flowers, fruits, and seeds. Results showed an important phenotypic diversity. Some accessions were considered as particular cultivars (Garsi1, Zehri11, Tounsi2, and Jerbi1). Some others (Gabsi, Chelfi) were considered as multiclone varieties. Synonyms and homonyms could be detected among cultivars. These results emphasize the importance of morphological descriptors to identify pomegranate varieties and allowed to recommend cultivars for different uses (table fruit, juice, and derivatives). Nevertheless, these descriptors need to be completed by further studies as biochemical and molecular characterization.
INTRODUCTION
Steeped in history, mythology, folklore, and romance and almost in a class by itself, the pomegranate, Punica granatum, belongs to the family Punicaceae, which includes only one genus and two species (CitationEvreinoff, 1949; CitationZukovskij, 1950). The pomegranate is thought to have originated in Iran, but became quite common in Mediterranean Regions, the Middle East, and Asia. The fruit has been a symbol of fertility since ancient times. Pomegranates have been recently used in various ways, mainly for different industrial usage fields, such as fruit juice, conserve, vinegar (CitationChérif and Ayed, 1997; CitationKaya and Sözer, 2005; CitationMaestre et al., 2000), and for medicinal purposes (CitationLansky and Newman, 2007; CitationNeurath et al., 2005). This fact has consequently led to its prominent popularity in the world markets.
The preservation of pomegranate genetic resources is compulsory, and requires morphological, physiological, biochemical, genetic, and taxonomic investigations to expand our knowledge about this species' diversity. The significance of morphological traits and multivariate analysis for the characterization of pomegranate cultivars has been stressed in some studies (CitationMars and Marrakchi, 1999; CitationAl-Said et al., 2009; CitationMuradoglu et al., 2006). In Tunisia, characterization and identification of pomegranate germplasm is very important, since the same pomegranate cultivar may have several local names and the same local name may refer to different pomegranate cultivars. Characterization based on morphological markers is commonly used to solve duplication problems of germplasm. The objective of the present work was to identify and characterize 21 pomegranate accessions maintained at an ex-situ collection in Gabès, Tunisia, using morphological traits. This article aims also to elucidate relationships among the studied accessions. Obtained data are necessary to develop adequate phenotypic markers for commercial use and germplasm management.
MATERIALS AND METHODS
Plant Material
This research was carried out on 21 Tunisian pomegranate cultivars collected from 10 localities (). These cultivars represent nine local denominations and they are maintained in the same ex-situ collection at Gabès in south Tunisia (CitationMars and Marrakchi, 1998). The morphological variability concerned some attributes related to flowers, fruits, juice, and kernels.
TABLE 1 Cultivars Used in Pomegranate Characterization
Characteristics of Flowers
At full boom stage, twenty flowers were taken from an adult tree representing each cultivar. The length and the diameter of the flower were measured, as well as the width and the height of each petal. The number of petals was also recorded. The shapes of the flowers and the petal were determined using grading scales: 1 to 11 for flower shape and 1 to 3 for petal shape. Other grading scales were used to determine petal color and the stamen/pistil height ratio, four levels were considered () (CitationMars, 2001).
TABLE 2 Morphological Traits Used in Pomegranate Characterization
Characteristics of Fruits
At the harvest time, twenty fruit samples per cultivar were randomly collected from fruitful trees for morphological and chemical analysis. Many fruit attributes, such as fruit weight (g), fruit height (mm), fruit diameter (mm), skin color, skin thickness at the equatorial area (mm), weight of rind (g), and arils yield (%) (), were recorded using methods developed by CitationMars and Marrakchi (1999).
Characteristics of Juice
The percentage of juice was determined by pressing 100 g of arils. Juice color was assessed according to grading scale (). Its pH was measured on replicate samples per fruit using a digital pH meter. Titrable acidity, expressed as g/L of malic acid (CitationMars and Marrakchi, 1999), was determined using 10 ml of juice and titrated with 0.1 M NaOH to an end point of pH 8.2 as indicated by a phenolphthalein indicator (CitationAOAC, 2000). The total soluble solids content (°Brix) was determined on replicate juice samples per fruit using a temperature compensating hand-held Atago hand refractometer (ATAGO, Japan).
Total phenolics content was determined with the Folin Ciocalteu (SCHARLAU CHEMIE SA, Sentmenat, Spain) reagent (CitationSingleton et al., 1999) and expressed as mg gallic acid 100 ml−1. The assay was performed using an automated UV/visible spectrophotometer. The calcium concentration in juice was determined by complexometry using EDTA solution (CitationAFNOR, 1996) and expressed as mg Ca/100 g.
Characteristics of Kernels
The pulp was removed from the seeds and then these were dried. From each cultivar, 25 of the woody part (kernel) were randomly chosen from a homogenous sample; the following characteristics were studied: weight, length, width, and thickness measured by a digital calliper and the shape using a grading scale ().
The content of cellulose was determined using the Weende method coupled to a Fibertec apparatus “Hot Extractor” (FOSS, Sweden) (CitationGerbaud et al., 2001). After grinding the woody part, 1 g of each sample underwent two hydrolyses: one acid within sulphuric acid H2SO4 (0.128 M) and another alkaline with potassium hydroxide KOH (O.223 M). The sample was then dried at 100°C for 6 hr and finally burned at 500°C for 3 hr. The weighted residue constitutes cellulose that was expressed as percent of seed weight.
Statistical Analysis
For all parameters, ANOVA was used to determine differences between cultivars. Comparison of the mean values of all was made using the LSD test at 95% confidence interval. With the purpose to examine relationships between characters, correlation analyses were also presented. Multivariate relationships among cultivars were revealed through a principal component analysis (PCA) using a correlation matrix derived from the significant characters. The squared Euclidean distance was used to perform cluster analysis. Statistical analyses were computed using SPSS 13.0 statistical software.
RESULTS
Flower Characteristics
Flower shape observation allowed distinguishing two main categories representing vase (1 to 7) and bell (8 to 11) types. The majority of cultivars have vase-shaped flowers. Regarding flower length, the cultivar TN2 presented the lowest average (26.7 mm) while JB2 showed the highest average (34.3 mm). Considering flower diameter and number of petals, the cultivar GS1 showed the lowest values. Petal shape varied from oval (RF1, JR1, CH8-1, and TN2) to orbicular (GB19) and petal length varied from 19.1 mm for TN15 to 26.03 mm for CH8-2. The largest petal belonged to GB19 cultivar and the smallest width was observed on RF1. Considering the size of pistil in relation to the stamen length, most of the cultivars showed relatively short pistils (level 2). JB4 had the longest pistil (2.64) and ZH11 had the shortest one (). Differences between cultivars were significant for all tested parameters.
TABLE 3 Mean Values, Standard Deviation, and Significance Degree of Differences Between Pomegranate Cultivars for Flower Morphometric Characters
Fruit Characteristics
The cultivar GS1 showed the smallest values for fruit characteristics except skin thickness and calyx diameter (). Fruit weight ranged from 87.1 g (GS1) to 590.8 g (CH8-2). Except for GS1, ZH4, ZH5, and ZH11, all other cultivars exceeded 300 g per fruit (). Skin weight showed significant differences between cultivars. The cultivar CH8-2 presented the heaviest skin while the three cultivars Zehri (ZH4, ZH5, and ZH11) and GS1 had the lowest averages (). Skin thickness differed also among cultivars. JB2 had the thickest skin (2.8 mm) while RF1 had the thinnest one (2.2 mm).Interestingly, RF1 and TN9-2 contained a significantly higher yield of arils per fruit compared to other cultivars (). The cultivars also differed significantly in fruit size; only the cultivars CH8-2, GB19, and KD1 had an average height over 90 mm. These cultivars also gave the largest fruits. All cultivars exhibited green yellowish, red pink, and red skin colors when ripe, but ZH4 differed from others and showed a dark red purple color. Differences between cultivars were noted also for calyx diameter, with the highest average for CH8-2 (33.2 mm) and the lowest average for ZH4 (17.7 mm).
TABLE 4 Mean Values, Standard Deviation, and Significance Degree of Differences Between Pomegranate Cultivars for Fruit
Juice Characteristics
Significant differences were noted in the volume of juice between studied cultivars, with JR1 having the greatest content and GS1 having the least (64.1%). The most colored juice occurred in JB8 while ZH11 had the brightest one. This latter showed also the greatest TSS content (15.9 °Brix) and the highest phenolics amount (194.1 mg/100 ml). The lowest pH value (3.1) was obtained for GS1 and the highest (4.5) for CH8-1 (). The titrable acidity of pomegranate juice was particularly high in GS1 (18.7 g/l) and low in ZH5 (2.0 g/l). The rest of the cultivars showed acidity levels ranging from 2 to 4 g/l. Calcium content varied from 7.0 mg/100 g for RF1 to 12.1 mg/100 g for JR1 ().
TABLE 5 Mean Values, Standard Deviation, and Significance Degree of Differences Between Pomegranate Cultivars for Juice Characteristics
Kernel Characteristics
There were significant differences between cultivars for all parameters. ZH11 provided the lightest and the highest kernels (). CH8-1, CH8-2, and KD1 had heavier kernels over 100 mg. Cultivar RF1 produced small and short kernels of 6.2 mm average length, while GS1 and GB11 had the largest and the thickest kernels (). Most of the cultivars exhibited prismatic and triangular kernel shape. Nevertheless, the three cultivars Zehri (ZH4, ZH5, and ZH11) showed stretched kernels while CH4 had oval kernels. Regarding cellulose content, GS1 seeds had the highest content (60.14%), while those of JR2 and RF1 were poorer with 36.7 and 36.8%, respectively ().
TABLE 6 Mean Values, Standard Deviation, and Significance Degree of Differences Between Pomegranate Cultivars for Kernel Morphometric Characters
Correlation Between Characters
Some significant correlations were observed between characters measured on different parts of the pomegranate. Regularity of shapes was proved by positive correlations between weight and diameter for fruits (0.93**), between height and diameter for flower (0.58**), and between length and width for petals (0.21**). Significant and positive correlations were found between fruit weight and kernel weight (0.61**) and kernel width and fruit diameter (0.52**). Correlation between flower length and pistil length (0.62**) on one hand and between flower shape and pistil length (−0.50**) on the other, may indicate that long flowers with large ovaries had developed pistils. A negative correlation was noted between flower diameter and calyx diameter (−0.32*).
Principal Components Analysis
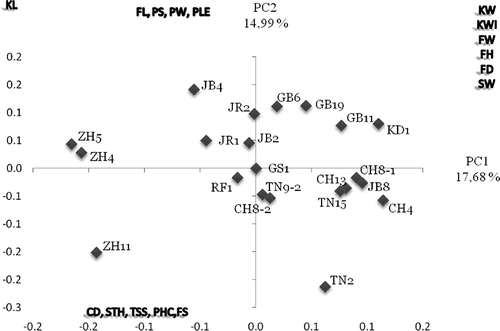
The Eigenvalues obtained by PCA indicate that the first three components provide a good summary of the data. They explained 42.2% of the total variability. For the definition of principal components, we considered important characters to be those with coefficients greater than half (CitationRanjbar et al., 2007). The first component, PC1, had large positive loadings for kernel weight, kernel width, fruit and skin weight, and fruit size (height and diameter) and negative loadings for kernel length. It represented 7.7% of the total variation (). These descriptors are very important in differentiating between cultivars. PC2, consisting mainly of calyx diameter, skin thickness, total soluble solids, phenolics content, flower shape and length, petal shape and width, and pistil length, accounted for 15.0% of the total variation, while PC3 correlated to external fruit color, arils yield, and juice yield explained 9.5% of the total variation (). When PC1 was plotted against PC2 and PC3, some groups and some isolated cultivars were clearly defined (). CH8-1, CH4, CH13, JB8, and TN15 had large positive scores on the PC1 axis. They were opposed to ZH4 and ZH5, which had large negative scores. The first cultivars were characterized by high mean values for fruit and skin weight, fruit size, and kernel weight and width, while Zehri 4 and Zehri 5 harbored low mean values for the same traits (). Two cultivars were isolated on the second axis: Tounsi 2 and Zehri 11 (). The first one was characterized by small flowers and small petals, colored and sweet juice, and fiber-rich seeds. Zehri 11 provided fruits with tight calyx, a very sweet juice, and a high amount of polyphenols. Also, its flowers and pistils were short (). PC2 can also group some other cultivars, such as GB6, GB11, and GB19, which had common characteristics.
TABLE 7 Proportions of Variability for the First Three Principal Components and Eigenvalues of Different Characters Evaluated in the Pomegranate Cultivars
Cluster Analysis
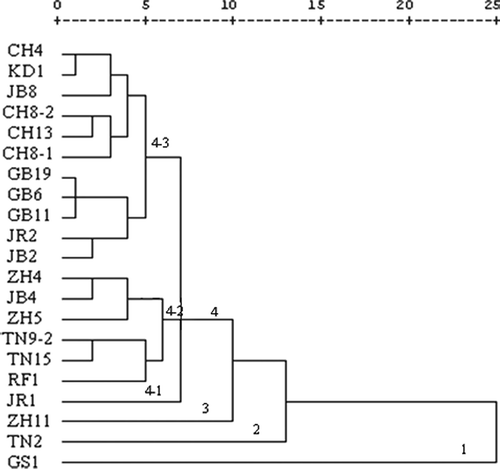
Morphological analysis based on different characters showed high polymorphism with 21 pomegranate accessions. The dendrogram based on squared Euclidian distance clustered accessions into four major groups (). The first cluster constituted by one cultivar Garsi 1 (GS 1) was detached at a distance d = 25. It had no similarities with other cultivars. It's a very particular sour cultivar with very small fruits, heavy kernels, and small flowers. The cultivar Tounsi 2 formed the second monophyletic group at d = 13. This cultivar had the smallest flowers with relatively short pistils and little petals. Also, the third group, detached at d = 10, was constituted by the unique accession Zehri 11. The rest of the cultivars clustered in a quite heterogeneous group having three subgroups 4-1, 4-2, and 4-3 (). This large group was isolated at d = 7. Most of the cultivars were closely related and grouped in the same branches with genetic dissimilarity ranging from 1 to 5 indicating relatively lower diversity within this group. Among the three subgroups of the major group 4, subgroup 4-1 consisted of the juiciest accession JR1. The second subgroup 4-2 contained six cultivars: two cultivars Tounsi (TN9-2 and TN15) clustered together with Rafrafi 1 (RF1), ZH5, JB4, and ZH4. The third subgroup was the largest one with eleven cultivars (GB6, GB11, GB19, CH4, KD1, JR2, JB2, JB8, CH8-1, CH8-2, CH13). The closest genotypes in this subgroup were three cultivars named Gabsi, KD1, and CH4 with the highest similarity level. This was followed by two pairs of cultivars (JB2 and JR2) and (CH8-2 and CH13). Although three accessions (JB2, JB4, and JB8) were clustered in the main group 4, these three cultivars showed considerable variations mainly related to flower characters. Lowest distances were noted between three Gabsi cultivars (GB6, GB11, and GB19). Also, three Chelfi cultivars (CH8-1, CH8-2, and CH13) were grouped together.
DISCUSSION
The diversity that occurs in pomegranate genetic resources originated through hybridization associated with vegetative and sexual propagation (CitationMars, 2000) and has resulted in a variety of cultivars. In Tunisia, over 60 cultivars have been inventoried (CitationMars and Marrakchi, 1998).
In this study, the results of the principal component analysis support those of the cluster analysis. The first three components accounted for 42.2% of the total variation; this is relatively low compared to those reported by authors. CitationMars and Marrakchi (1999) reported a total variability of 59.9% explained by the three first principal components using characteristics of fruits and juice of 30 other Tunisian pomegranate accessions. PCA analysis revealed that the most important discriminating traits were flower length, petal width, pistil length, fruit and skin weight, fruit size, calyx diameter, aril yield, juice yield, and kernel weight and width. Our results confirmed those reported previously by several researchers on pomegranate germplasm. CitationMars and Marrakchi (1999) found that the discriminating characters were fruit size, color, and juice characteristics. The same traits were found to be very important by CitationDrogoudi et al. (2005) for characterization of 20 Greek pomegranate cultivars. CitationDurgaç et al. (2008) also found that fruit weight, aril number/fruit, peel color, and soluble solids/acidity ratio are important traits for discriminating six Turkish cultivars.
PCA as well as cluster results allowed singling out a few cultivars with particular distinctive characters, such as GS1, ZH11, TN2, and JR1. But, on the whole, some similarities among the cultivars could be found. Moreover, cultivars have been named in different zones resulting in numerous synonyms and homonyms (CitationMars, 2001). In fact, the majority of cultivars with same names from different agro-ecological zones were found to be morphologically identical and clustered together indicating the possible presence of duplicates within cultivars Gabsi (GB6, GB11, and GB19), Chelfi (CH8-1, CH13, and CH8-2), and Tounsi (TN9-2 and TN15). It's interesting to indicate that cultivars' grouping was not correlated to their geographical origin and this could be due to gene flow between regions (CitationMars and Marrakchi, 2004). Nevertheless, many cultivars, such as KD1 and CH4, had different names in different agro-ecological zones but showed close resemblance and grouped into the major group suggesting possible mislabeling. In fact, identification of pomegranate cultivars is traditionally based on morphological criteria. However, close genetic relationships as well as morphological changes due to environment are major constraints for the correct identification of cultivars (CitationKaemmer et al., 1992).
Although TN9-2 and RF1 didn't exhibited higher fruit size, they yielded more arils per fruit, which is a highly desirable property in the food processing industry (CitationMaskan, 2006). Whereas cultivars with big size fruit (CH8-2, KD1, JR1, etc.) are more suitable for fresh consumption. Skin color is also an important quality attribute in pomegranate marketing (CitationElyatem and Kader, 1984). Thus, cultivars, such as ZH4, ZH5, ZH11, and JB8, with deep red fruit coloration tend to have greater consumer demand in the local and international market. The significant variation in pomegranate fruit and juice color indicates that these attributes may be used as maturity indices for harvest management (CitationKader, 2004). On the basis of kernel size and fiber content, cultivars, such as RF1, ZH4, and TN9-2, may be considered more suitable for fresh consumption, while GS1, CH8-1, CH8-2, and KD1 would be more suited for juice extraction due to their kernels big size and hardness. The acid taste and sugar content are important quality attributes of pomegranate juice, which contribute to its valuable use in the food and beverage industry. Because of their large variability in fruit and juice attributes (taste, color, etc.), the sour cultivar GS1 as well as some sweet ones, such as GB6, CH4, and ZH5, may be recommended for formulation of a wide range of beverages and derivatives. But, true values of different parameters of promising genotypes should be revealed with replicated researches.
CONCLUSIONS
Morphometric and pomological analyses of Tunisian pomegranate cultivars showed a large diversity within this local germplasm. These analyses are very useful for its collection, management, and use in future breeding programs. Nevertheless, morphological descriptors, which are environmentally influenced, are not enough to identify pomegranate cultivars because the differences among them are often ambiguous. Biochemical (CitationAl-Said et al., 2009) as well as molecular (CitationJbir et al., 2008) markers are required to complete this study in order to evaluate and better estimate diversity among Punica granatum genetic resources.
LITERATURE CITED
- AOAC (Association of Official Analytical Chemists) . 2000 . Official methods of analysis , 17th Arlington, Virginia, Gaithersburg, MD, , USA
- AFNOR . 1996 . Report of French Standards of fruit juices and vegetables: specification and analytical methods . Reports from French , 3 ( 13 ) : 100
- Al-Said , F.A. , Opara , L.U. and Al-Yahyia , R.A. 2009 . Physico-chemical and textural quality attributes of pomegranate cultivars (Punica granatum L.) grown in the Sultanate of Oman. J . Food Eng. , 90 ( 1 ) : 129 – 134 .
- Chérif , J.K. and Ayed , N. 1997 . Grenadine and syrups from pomegranate fruit: revelation method of natural syrups authenticity . Fruits , 52 : 99 – 110 .
- Drogoudi , P.D. , Tsipouridis , C. and Michailidis , Z. 2005 . Physical and chemical characteristics of pomegranates . HortScience , 40 ( 5 ) : 1200 – 1203 .
- Durgaç , C. , Özgen , M. , Simsek , O. , Aka , K.Y. , Kiyga , Y. , Çelebi , S. , Gündüz , K. and Serçe , S. 2008 . Molecular and pomological diversity among pomegranate (Punica granatum L.) cultivars in Eastern Mediterranean region of Turkey . African J. Biotechnol. , 7 ( 9 ) : 1294 – 1301 .
- Elyatem , S.M. and Kader , A.A. 1984 . Post-harvest physiology and storage behaviour of pomegranate fruits . Sci. Hort. , 24 : 287 – 298 .
- Evreinoff , V.A. 1949 . The pomegranate . Overseas fruits , 4 ( 5 ) : 161 – 170 .
- Gerbaud , E. , Dutoit , T. , Barroit , A. and Toussaint , B. 2001 . Mineral contents of forage stubble and their weeds: the example of a farm in southeast of France (Vaucluse) . Animal Res. , 50 : 495 – 505 .
- Jbir , R. , Hasnaoui , N. , Mars , M. , Marrakchi , M. and Trifi , M. 2008 . Characterization of Tunisian pomegranate (Punica granatum L.) cultivars using amplified fragment length polymorphism analysis . Sci. Hort. , 115 : 231 – 237 .
- Kader , A.A. 2004 . Postharvest technology of horticultural crops , Davis, , CA : University of California .
- Kaemmer , D. , Afza , R. , Weising , K. , Kahl , G. and Novak , F.J. 1992 . Oligonucleotide and amplification fingerprinting of wild species and cultivars of banana (Musa spp) . Biotechnology , 10 : 1030 – 1035 .
- Kaya , A. and Sözer , N. 2005 . Rheological behaviour of sour pomegranate juice concentrates (Punica granatum L.) . Intl. J. Food Sci. & Technology , 40 ( 2 ) : 223 – 227 .
- Lansky , E.P. and Newman , R.A. 2007 . Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer . J. Ethnopharmacology , 109 : 177 – 206 .
- Maestre , J. , Melgarejo , P. , Tomás-Barberán , F.A. and Garcia-Viguera , C. 2000 . New food products derived from pomegranate . Options Méditerranéennes, Série A , 42 : 243 – 245 .
- Mars , M. 2000 . Pomegranate plant material: Genetic resources and breeding, a review . Options Méditerranéennes, Série A , 42 : 55 – 62 .
- Mars , M. 2001 . Genetic resources of pomegranate (Punica granatum L.) in Tunisia: Prospection, conservation and diversity analysis , 200 Ph.D. Thesis in Natural Sciences. Tunis El Manar University . (+annexes)
- Mars , M. and Marrakchi , M. 1998 . Conservation and valorization of genetic resources of pomegranate (Punica granatum L.) in Tunisia . Plant Genetic Resources Newsletter , 114 : 36 – 39 .
- Mars , M. and Marrakchi , M. 1999 . Diversity of pomegranate (Punica granatum L.) germplasm in Tunisia . Genetic Resources and Crop Evolution , 46 : 461 – 467 .
- Mars , M. and Marrakchi , M. 2004 . Analysis of genetic diversity and clonal selection in the pomegranate (Punica granatum L.) . Journal of Arid Regions. NS , : 116 – 122 .
- Maskan , M. 2006 . Production of pomegranate (Punica granatum L.) juice concentrate by various heating methods: Colour degradation and kinetics . J. Food Eng. , 72 : 218 – 224 .
- Muradoglu , F. , Fikret Balta , M. and Ozrenk , K. 2006 . Pomegranate (Punica granatum L.) genetic resources from Hakkari, Turkey . Res. J. Agr. Biol. Sci. , 2 ( 6 ) : 520 – 525 .
- Neurath , A.R. , Strick , N. , Li , Y. and Debnath , A.K. 2005 . Punica granatum (Pomegranate) juice provides an HIV-1 entry inhibitor and candidate topical microbicide . Ann. New York Academy Sci. , 1056 : 311 – 327 .
- Ranjbar , M. , Naghavi , M.R. , Zali , A. and Aghaei , M.J. 2007 . Multivariate analysis of morphological variation in accessions of Aegilops crassa from Iran . Pakistan J. Biol. Sci. , 10 ( 7 ) : 1126 – 1129 .
- Singleton , V.L. , Orthofer , R. and Lamuela-Raventos , R.M. 1999 . Analysis of total phenols and other oxidation substrates and antioxidant by means of Folin-Ciocalteu . Methods in Enzymology , 299 : 152 – 178 .
- Zukovskij , P.M. 1950 . “ Punica ” . In Cultivated plants and their wild relatives , 60 – 61 . Moscow : State Publishing House Soviet Science .