Abstract
Pomegranate fruits were treated with 2% calcium chloride in combination with 2 mM of spermidine by either normal dip or vacuum infiltration for 4 min and then stored at 2°C for 4 months. After 0, 30, 60, 90, and 120 days, 12 fruits were taken from each treatment and stored at 20°C for an additional 3 days. Compared with control fruits, significantly higher activities in catalase and superoxide dismutase and a lower activity in peroxidase were noted in treated fruits. It was found that antioxidant enzymes are responsible for inducing chilling tolerance in pomegranate fruits and spermidine in combination with calcium chloride can improve these enzymatic activities.
INTRODUCTION
Pomegranate (Punica granatum L.) fruits have a short storage life at ambient temperatures and storing them at low temperatures causes chilling injury. Chilling injury (CI) is a physiological disorder that develops during the cold storage of many economically important crops native to the tropic and subtropic regions (CitationSaltveit and Morris, 1990). One of the main causes of chilling injury is the production of reactive oxygen species (ROS, such as O2 −, H2O2, and OH). To cope with low temperature-induced oxidative injury, plants have evolved mechanisms to scavenge these toxic and reactive species by antioxidant compounds and enzymes, such as SOD, CAT, and POX (CitationWise, 1995).
Pomegranates are susceptible to CI when stored for more than 2 months at temperatures below 5°C (CitationElyatem and Kader, 1984; CitationRamezanian et al., 2010). Symptoms of CI in pomegranate fruit include browning of the husk, pitting, husk scald, a higher sensitivity to decay, and internal seed discoloration and browning.
There is evidence that chilling may impose oxidative stress on plant tissues. For example, such stresses may be responsible for chilling associated with damage in cucumber fruit and pear fruit (CitationHariyadi and Parkin, 1991; CitationJu et al., 1994). ROS accumulation may cause oxidative damage to lipids, forming toxic products, such as malondialdehyde (MDA), a secondary end-product of polyunsaturated fatty acid oxidation. Accumulation of MDA is often taken into consideration as an indicator of CI, because degradation of polyunsaturated fatty acids produces peroxide ions and MDA production (CitationMacRae and Ferguson, 1985). Thus, MDA build-up is usually considered as an indicator of plant oxidative stress and also as the degree of damage to the structural integrity of cell membranes of plants exposed to low temperatures (CitationHodges et al., 1999; CitationPosmykt et al., 2005).
Plant cells are usually protected against reactive oxygen species by a complex antioxidant system. This involves both lipid-soluble antioxidants (α-tocopherol and β-carotene) and water soluble reductants (ascorbate and glutathione), and also enzymes, such as catalase (CAT), peroxidase (POX), and superoxide dismutase (SOD) (CitationZhang et al., 1995). SOD dismutates superoxide radicals to hydrogen peroxide and O2 in a reaction that is spontaneous and extremely rapid, thus protecting the cells against damage caused by superoxide radical reaction products. The products, which are potentially toxic compounds, are then reduced to water by a number of enzymes, mainly CAT and APX (CitationKochhar et al., 2003).
An increase in polyamine concentration (putrescine, put; spermidine, Spd; spermine, Spm) has been reported in plant tissues affected by environmental stress conditions (CitationBouchereau et al., 1999). In lemon and grapefruit flavedo, a negative correlation was reported between CI severity and the level of putrescine (CitationMcDonald and Kushad, 1986). It is assumed that polyamines have anti-senescence function (CitationZokaee Khosroshahi et al., 2007).
It has also been found that CaCl2 can induce chilling tolerance in some fruits, such as ‘Lisbon’ lemon (CitationSafizadeh et al., 2007).
Little information is available on how reactive oxygen species are involved in inducing chilling injury in pomegranate fruits and how the fruits' antioxidant systems cope with these ROS. Consequently, this research was undertaken to study the ameliorative effects of spermidine and calcium chloride on inducing chilling tolerance in pomegranate fruits and the effects of such treatments on fruits antioxidant enzymes.
MATERIALS AND METHODS
In preliminary experiments, different concentrations of calcium chloride and spermidine were evaluated and the best treatment was adopted for use in this research (data not shown).
Pomegranate (Punica granatum L. cv. Malas-e-Yazdi) fruits were picked manually in mid-September 2008 at the Agricultural Research Center of Yazd Province (central part of Iran). This cultivar ripens early with tasty low acid arils and soft stones. Fruits were harvested by hand when fully mature and were immediately transported to the laboratory. Defective fruits (sunburns, cracks, bruises, and cuts in the husk) were discarded. The remaining fruits were randomized and divided into 10 lots of 60 fruits each for the following treatments in quadruplicate (each replicate contained 12 individual fruits): Control (distilled water at 25°C for 4 min), Ca2S2N (normal dip in 2% CaCl2 + 2 mM spermidine for 4 min) and Ca2S2V (vacuum infiltration of 2% CaCl2 + 2mM spermidine for 4 min). For each treatment, fruits were placed in 20 L of solution, containing tween-20 (2 g L−1) as non-ionic polysorbate surfactant. For vacuum infiltration, a reduced pressure of 247.52 mmHg was used in a 15 L capacity vacuum desiccator (Model B-1834-Gallenkamp, GallenKamp Company, England, UK). Spermidine was prepared from Sigma Company UK.
Treated fruits were then placed on desiccant paper and allowed to dry at room temperature before being stored at 2°C. At days 0, 30, 60, 90, and 120, 12 fruits were taken from each treatment (three from each replicate) and stored at 20°C for a further 3 days (shelf life).
After this period, the fruit husks were carefully cut and samples of 1 g of husk were frozen in liquid nitrogen and were stored at −70°C.
The damage caused by CI, was assessed by observing the extent of husk browning, pitting, scald, and internal seed discoloration and necrosis, recorded as percentage of affected zones where 1 = no injury, 2 = slight (up to 25%), 3 = moderate (25–50%), 4 = moderately severe (50–75%), and 5 = severe (75–100%).
Lipid peroxidation in fruit husk was determined by estimating the MDA content (CitationHeath and Packer, 1968) The supernatant prepared for POX enzyme assay was used. The concentration of MDA was calculated by using the extinction coefficient of 155 mM.cm−1 expressed as mM MDA g−1 fresh weight (FW).
CAT was extracted from 1 g of husk using 5 ml solution, containing 200 mM potassium phosphate buffer (pH 7.0) and 10 μL Triton X-100. The homogenates were centrifuged at 12,000 × g, 4°C for 20 min. The activity of CAT was determined as a decrease in absorbance at 240 nm for 1.5 min following the decomposition of H2O2 (CitationAebi, 1984). The reaction mixture (3 ml) contained 200 mM of phosphate buffer (pH 7.0), 30 mM of H2O2, and 100 μL of enzyme extract.
One gram of husk tissue was homogenized in 10 ml 0.2 M phosphate buffer (pH 5.8) containing 0.1 mM ethylenediamine tetraacetic acid and 1% polyvinyl polypyrolidone at 4°C. The homogenate was centrifuged at 27,000 × g for 15 min at 4°C twice and the supernatant was used for POX (CitationSiegel and Galston, 1967). POX activity was determined by spectrophotometer reading the oxidation of guaiacol in the presence of H2O2 (extinction coefficient 26.6 mM cm−1) at 470 nm. One unit of POX activity represents the amount of enzyme catalyzing the oxidation of 1 mM of guaiacol per minute at 25°C.
One gram of husk was homogenized in 1 ml of 50 mM potassium phosphate buffer (pH 7.8) containing 1.33 mM diethyltriamine pentaacetic acid at 4°C and then centrifuged twice at 27 000 × g for 15 min at 4°C (CitationSala and Lafuente, 2000). The supernatant was used for SOD assay (CitationBeauchamp and Fridovich, 1971). The unit of SOD activity was defined as that amount of enzyme which caused 50% inhibition of the initial rate of reaction in the absence of enzyme. Specific activities of all enzymes were expressed as units per mg protein.
Protein in extracts was determined by the method of Bradford using bovine serum albumin as standard (CitationBradford, 1976).
Data are presented as standard error of mean using at least three replicates. Analysis of variance was performed by SAS/STAT GLM procedure, using the Duncan multiple range test at 5% level for means comparison.
RESULTS
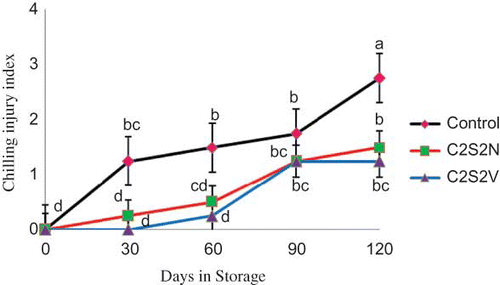
Fruits of ‘Malas-e-Yazdi’ pomegranate trees are chilling sensitive when stored below 5°C. The present study revealed that the combination of calcium chloride and spermidine reduce the external and internal browning, which are the typical chilling injury symptoms in pomegranate fruits (). Chilling injury was detected from the first sampling date (30 days) in control fruits (CI index = 1.25) and was increased drastically thereafter ().
FIGURE 1 Symptoms of chilling injury on the control (A), normal dipped fruits in 2% CaCl2 in combination with 2 mM spermidine (C2S2N) (B), and fruits treated by vacuum infiltration of 2% CaCl2 in combination with 2 mM spermidine (C2S2V) (C), after 4 months at 2°C and then kept at 20°C for 3 days (color figure available online).

FIGURE 2 Effect of 2% CaCl2 in combination with 2 mM spermidine by normal dip and vacuum infiltration methods on changes in chilling injury index of pomegranate fruit during storage at 2°C. Each value is the mean of three replicate samples ±S.E. Values labeled with the same letters are not different at the 5% level (color figure available online).
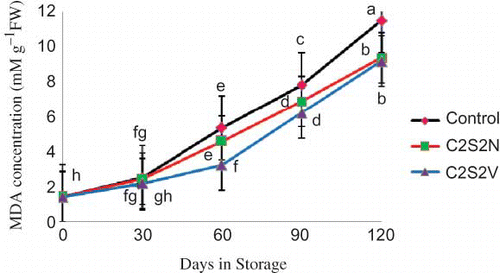
The amounts of MDA were determined in the peel of pomegranate fruits stored at 2°C at different times (). In the fruit peel, the amount of MDA at harvest time was 1.43 nM/g FW. When fruits were stored at 2°C, MDA levels gradually increased until the first month of storage (43, 41.86, and 35% in control, C2S2N, and C2S2V, respectively).
FIGURE 3 Effect of 2% CaCl2 in combination with 2 mM spermidine by normal dip and vacuum infiltration methods on changes in MDA concentration in pomegranate fruit during storage at 2°C. Each value is the mean of three replicate samples ±S.E. Values labeled with the same letters are not different at the 5% level (color figure available online).
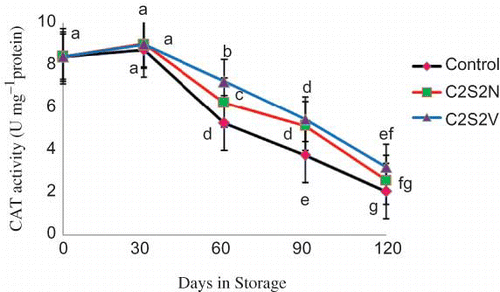
The differences in CAT activity between treated and untreated fruits was detected from the first sampling date (30 days in storage) (). Henceforth, a drastic decrease in CAT activity was found in control fruits. The activity of CAT in fruits treated with C2S2V was higher than that with C2S2N at the second sampling date. However, the differences disappeared thereafter.
FIGURE 4 Effect of 2% CaCl2 in combination with 2 mM spermidine by normal dip and vacuum infiltration methods on changes in CAT activity of pomegranate fruit during storage at 2°C. Each value is the mean of three replicate samples ±S.E. Values labeled with the same letters are not different at the 5% level (color figure available online).
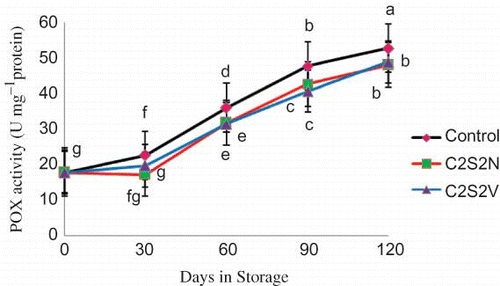
In control fruits, at the beginning of storage, POX activity was 17.93 U.mg−1 protein and at the end, was increased to 52.63 U.mg−1 protein (). In treated fruits, POX activity increased with time but there was a significant difference between treated and untreated fruits at each sampling date. However, no significant difference was observed in POX activity between C2S2N and C2S2V treatments.
FIGURE 5 Effect of 2% CaCl2 in combination with 2 mM spermidine by normal dip and vacuum infiltration methods on changes in POX activity of pomegranate fruit during storage at 2°C. Each value is the mean of three replicate samples ±S.E. Values labeled with the same letters are not different at the 5% level (color figure available online).
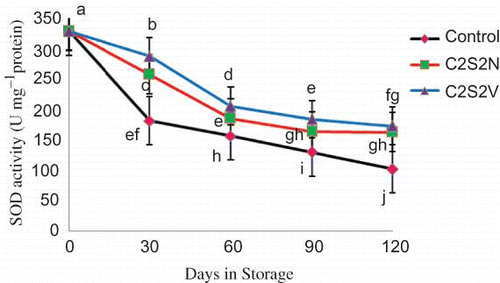
SOD activity in both control and treated fruits, decreased with storage time. Pre-treated fruits maintained significantly higher SOD activity throughout the storage (). SOD activity at the beginning of storage was 328.09 U.mg−1 protein and was decreased drastically by the first sampling date (181.45 U.mg−1 protein). Afterwards, the rates of decrease in SOD activity in both treated and untreated fruits were reduced. C2S2N and C2S2V treatments increased SOD activity in comparison with control.
FIGURE 6 Effect of 2% CaCl2 in combination with 2 mM spermidine by normal dip and vacuum infiltration methods on changes in SOD activity of pomegranate fruit during storage at 2°C. Each value is the mean of three replicate samples ±S.E. Values labeled with the same letters are not different at the 5% level (color figure available online).
DISCUSSION
In this study, C2S2N and C2S2V both reduced chilling injury, however, the effects of C2S2V treatment was more pronounced than that of C2S2N. The severity of chilling injury is related to the storage temperature and duration (CitationCohen and Schiffman-Nadel, 1978). Lipid peroxidation can be shown by measuring the content of tissue MDA, because MDA is a secondary end-product of polyunsaturated fatty acid oxidation. The degradation of the polyunsaturated fatty acids by their peroxidation produced MDA and at the same time induced membrane rigidification and cell death (CitationPosmykt et al., 2005).The magnitude of lipid peroxidation depends on the severity of cold stress and is correlated with the extent of CI (CitationLukatkin, 2002). Free radical-induced lipid peroxidation is one of the main causes of cell membrane deterioration. Many free radicals are highly reactive chemically and can induce the oxidative breakdown of double bonds in the fatty acids of membrane lipids. The level of lipid peroxidation is dependent on the balance between ROS production by the respiratory chain and ROS consumption by antioxidant systems (CitationImahori et al., 2008). Results of this study showed that postharvest treatment of pomegranate fruits with 2% calcium chloride in combination with 2 mM spermidine can enhance chilling tolerance by increasing the activity of several antioxidant enzymes. Increasing evidence is suggesting that oxidative stress caused by an excess production of ROS, such as O− 2, H2O2, and hydroxyl radical, can contribute to the development of chilling injury and antioxidant enzymes, such as CAT, POX, and SOD, which play important roles in scavenging ROS and alleviating chilling injury (CitationHariyadi and Parkin, 1991; CitationSala, 1998; CitationZheng et al., 2008). Therefore, protection against oxidative injury is crucial to cell survival under chilling stress and is thought to be the major mechanism of resistance to chilling stress. It has been reported that the improvement of chilling tolerance in harvested horticultural crops is associated with the enhancement in the activities of antioxidant enzymes (CitationCao et al., 2009). CAT is one of the enzymes that protect cells against ROS by catalyzing the decomposition of hydrogen peroxide to form oxygen and water. In this study, CAT activity decreased during storage. Used treatments increased CAT activity compared with untreated fruits during storage. However, in long-term storage, the differences between two application methods disappeared. Reactions leading to fruit browning can be catalyzed by POX. In this study, used treatments decreased POX activity significantly; however, there were no differences between two application methods. SOD may play a protective role by detoxifying free radicals. The superoxide radicals produced during chilling stress might be more efficiently dismutated to H2O2 and molecular oxygen in the treated fruits.
In the present work in all treatments, the rates of CAT and SOD activity ( and ) decreased, while those of POX increased (). The present results indicated that some enzymatic reactions contribute to the chilling tolerance of pomegranate fruits and the efficiency of this system can be improved by calcium chloride in combination with spermidine.
ACKNOWLEDGMENTS
The authors are grateful to Shiraz University for financial support and to the Agricultural Research Centre of Yazd Province for providing the pomegranate fruits.
LITERATURE CITED
- Aebi , H. 1984 . Catalase . In Vitro Methods in Enzymology , 105 : 121 – 126 .
- Beauchamp , C. and Fridovich , I. 1971 . Superoxide dismutase: Improved assay and assay applicable to PAGE . Ann. Biochem. , 44 : 276 – 287 .
- Bouchereau , A. , Aziz , A. , Larhe , F. and Martin-Tanguy , J. 1999 . Polyamines and environmental challenges: Recent developments . Plant Sci. , 140 : 103 – 125 .
- Bradford , M.M. 1976 . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding . Ann. Biochem. , 72 : 248 – 254 .
- Cao , S. , Zheng , Y. , Wang , P. Jin and Huijing , R. 2009 . Methyl jasmonate reduces chilling injury and enhances antioxidant enzyme activity in postharvest loquat fruit . Food Chem. , 115 : 1458 – 1463 .
- Cohen , E. and Schiffman-Nadel , M. 1978 . Storage capability at different temperatures of lemons grown in Israel . Sci. Hort. , 9 : 251 – 257 .
- Elyatem , S.M. and Kader , A. 1984 . Post-harvest physiology and storage behaviour of pomegranate fruits . Sci. Hort. , 24 : 287 – 298 .
- Hariyadi , P. and Parkin , K.L. 1991 . Chilling induced oxidative stress in cucumber fruits. Postharvest Biol . Technol. , 1 : 33 – 35 .
- Heath , R.I. and Packer , L. 1968 . Photoperoxidation in isolated chloroplasts. 1. Kinetics and stiochiometry of fatty acid peroxidation . Arch. Biochem. Biophys. , 125 : 189 – 198 .
- Hodges , D.M. , Delong , J.M. , Forney , C.F. and Prange , R.K. 1999 . “ Improving the thiobarbituric acid reactive substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds ” . In Planta Vol. 207 , 604 – 611 .
- Imahori , Y. , Takemura , M. and Bai , J. 2008 . Chilling-induced oxidative stress and antioxidant responses in mume (Prunus mume) fruit during low temperature storage . Postharvest Biol. Technol. , 49 : 54 – 60 .
- Ju , Z. , Yuan , Y. , Liou , C. , Zhan , S. and Xiu , S. 1994 . “ Effects of low temperature on H2O2 and heart browning of Chili and Yali pear (Pyrus bretscheideri, R.) ” . In Sci. Agr. Sin Vol. 27 , 77 – 81 .
- Kochhar , S. , Watkin , C.B. , Conklin , P.L. and Brown , S.K. 2003 . A quanitative and qualitative analysis of antioxidant enzymes in relation to susceptibility of apples to superficial scald . J. Amer. Soc. Hort. Sci. , 128 : 910 – 916 .
- Lukatkin , A.S. 2002 . Contribution of oxidative stress to the development of cold-induced damage to leaves of chilling-sensitive plants: 2. The activity of antioxidant enzymes during plant chilling . Russian J. Plant Physiol. , 49 : 782 – 788 .
- MacRae , E.A. and Ferguson , I.B. 1985 . Changes in catalase activity and hydrogen peroxide concentration in plants in response to low temperature . Physiol. Plant. , 65 : 51 – 56 .
- McDonald , R.E. and Kushad , M.M. 1986 . Accumulation of putrescine during chilling injury of fruit . Plant Physiol. , 82 : 324 – 326 .
- Posmykt , M.M. , Baily , C. , Szafrańska , K. , Janas , K.M. and Corbineau , F. 2005 . Antioxidant enzymes and isoflavonoids in chilled soybean (Glycine max (L.) Merr.) seedlings . J. Plant Physiol. , 162 : 403 – 412 .
- Ramezanian , A. , Rahemi , M. , Maftoun , M. , Kholdebarin , B. , Eshghi , S. , Safizadeh , M.R. and Tavallali , V. 2010 . The ameliorative effects of spermidine and calcium chloride on chilling injury in pomegranate fruits after long-term storage . Fruits , 65 : 169 – 177 .
- Safizadeh , M.R. , Rahemi , M. and Aminlari , M. 2007 . Effect of postharvest calcium and hot-water dip treatments on catalase, peroxidase and superoxide dismutase in chilled Lisbon lemon fruit . Intl. J. Agr. Res. , 2 : 440 – 449 .
- Sala , J.M. and Lafuente , M.T. 2000 . Catalase enzyme activity is related to tolerance of mandarin fruits to chilling. Postharvest Biol . Technol. , 20 : 81 – 89 .
- Sala , J.M. 1998 . Involvement of oxidative stress in chilling injury in cold-stored mandarin fruits . Postharvest Biol. Technol. , 13 : 255 – 261 .
- Saltveit , M.E. and Morris , L.L. 1990 . “ Overview of chilling injury in horticultural crops ” . In Chilling injury of horticultural crops , Edited by: Wang , C.Y. Boca Raton, FL : CRC Press .
- Siegel , B.Z. and Galston , A.W. 1967 . The isoperoxidases of Pisum sativum . Plant Physiol. , 42 : 221 – 226 .
- Wise , R.R. 1995 . Chilling-enhanced photooxidation: The production, action and study of reactive oxygen species produced during chilling in the light . Photosyn. Res. , 45 : 79 – 97 .
- Zhang , J. , Cui , S. , Li , J. , Wei , J. and Kirkham , M.B. 1995 . Protoplasmic factors, antioxidant responses, and chilling resistance in maize . Plant Physiol. Biochem. , 33 : 567 – 575 .
- Zheng , Y.H. , Reymond , W.M.F. , Wang , S.Y. and Wang , C.Y. 2008 . Transcript levels of antioxidative genes and oxygen radical scavenging enzyme activities in chilled zucchini squash in response to superatmospheric oxygen . Postharvest Biol. Technol. , 47 : 151 – 158 .
- Zokaee Khosroshahi , M.R. , Esna-Ashari , M. and Ershadi , A. 2007 . Effect of exogenous putrescine on post-harvest life of strawberry (Fragaria ananasa Duch.) fruit, cultivar selva . Sci. Hort. , 114 : 27 – 32 .