Abstract
Ericoid mycorrhizae are interfaces for nutrient exchange between specialized fungi and roots of blueberries and other ericaceous plants. Dark septate endophytes also associate with roots of the Ericaceae and many other plant families, but the nature of the interaction is not well understood. We measured ericoid mycorrhizae and dark septate endophyte colonization in organic and conventional blueberry fields matched by soil series and, if possible, cultivar and field age. The percentage of hair root cells with ericoid mycorrhizae was generally higher in organic than conventional fields, while dark septate endophyte colonization was higher in sand than muck soils. Across both muck and sandy soil types, ericoid mycorrhizal colonization was negatively correlated with field age, and dark septate endophyte colonization was negatively correlated with soil carbon, total soil nitrogen, and soil ammonium levels. When only sandy soils were included in the analysis, ericoid mycorrhizal colonization was positively correlated with soil pH and hair-root diameter, and negatively correlated with field age, soil ammonium levels, and total soil nitrogen. On sandy soils, dark septate endophyte colonization was not correlated with any measured variable. The functions of ericoid mycorrhizae and dark septate endophytes in commercial blueberry fields require further investigation.
INTRODUCTION
Ericoid mycorrhizae (ERM) are symbioses between specialized soil fungi and roots of many ericaceous plants, including highbush blueberries (Vaccinium corymbosum L.). Ericoid mycorrhizae are characterized by the formation of intracellular hyphal coils in the epidermis of hair roots and hyphal extension of up to 1 cm from the root surface (CitationRead, 1984). ERM fungi mobilize nutrients from organic matter by releasing extracellular enzymes that break down simple and complex organic polymers (CitationRead et al., 2004), and enzyme production continues in symbiosis with roots (CitationYang et al., 2006). ERM act as a conduit for soil nutrients that would otherwise be less available to non-mycorrhizal plants, including nitrate (CitationKosola et al., 2007), organic nitrogen (N) (CitationSokolovski et al., 2002), organic and inorganic phosphorus (CitationMyers and Leake, 1996), and iron (CitationShaw et al., 1990). A direct link between ERM colonization and plant uptake of organic N is indicated by depletion of 15N in shoot tissue that occurs as N is accessed by ERM fungi and transferred to the host plant (CitationMichelsen et al., 1996; CitationYang et al., 2002; CitationHobbie and Hobbie, 2006; CitationStackpoole et al., 2008). In addition to nutrient scavenging, ERM increase plant tolerance to various edaphic stresses, including phytotoxic accumulation of aluminum (CitationYang and Goulart, 2000), manganese (CitationHashem, 1995), and organic acids (CitationLeake and Read, 1991), which may occur in acidic soils and under waterlogged conditions (CitationKorcak, 1989).
ERM colonization levels have been assessed in cultivated blueberries and cranberries in Pennsylvania, Michigan, New Jersey (CitationGoulart et al., 1993), Florida (CitationJacobs et al., 1982), Oregon (CitationScagel and Yang, 2005; CitationScagel, 2003), and Wisconsin (CitationKosola and Workmaster, 2007; CitationStackpoole et al., 2008) and are highly variable among and within production regions. The causes that underlie variation in ERM colonization in commercial fields are not entirely known.
Experiments conducted under controlled conditions suggest that high levels of available N reduce ERM colonization. For example, CitationStribley and Read (1976) demonstrated that seedlings of cranberry, V. macrocarpon Ait., inoculated with ERM fungi and grown in sterile sand had only sparse ERM colonization at high rates of ammonium-N, especially at the highest rate supplied, 56 ppm. Suppression of mycorrhizae by N fertilization does not appear to be unique to ericaceous plants. In a meta-analysis of the effect of N amendments on arbuscular or ectomycorrhizal mycorrhizal colonization of non-ericaceous plants, mycorrhizal colonization was reduced by an average of 6% (CitationTreseder, 2004). A study of ectomycorrhizae suggested that plants possess a mechanism to detect the nutritional status of potential fungal symbionts and regulate mycorrhizal colonization according to the differential between the nutritional status of the fungus and plant (CitationKemppainen et al., 2009). In field studies of blueberries, negative correlations between ERM colonization and soil ammonium (CitationScagel and Yang, 2005) and leaf tissue N (CitationStevens et al., 1997) imply that mycorrhizal colonization is reduced as N supply increases. However, studies have failed to consistently demonstrate that increasing rates of N reduce ERM colonization in fields of highbush blueberry (CitationGoulart et al., 1993, 1995, Citation1997; CitationYang et al., 2002), lowbush blueberry (CitationJeliazkova and Percival, 2003 CitationPercival and Burnham, 2006), and native Ericaceae (CitationCaporn et al., 1995; CitationJohansson, 2000; CitationHofland-Zijlstra and Berendse, 2009; CitationIshida and Nordin, 2010). CitationGoulart et al. (1993) stated that the relationship between fertilization practices and ERM colonization “may not be as straightforward as simple suppression of the fungal symbiont by nitrogen.” Under field conditions, ERM colonization may also be influenced by mechanical soil disturbance (CitationHutton et al., 1997), organic vs. inorganic fertilizer (CitationScagel, 2005a), sulfur applied to lower soil pH (CitationDiaz et al., 2008), synthetic fungicides (CitationPercival and Burnham, 2006), seasonal colonization dynamics (CitationStevens et al., 1997; CitationJohansson, 2000; CitationKemp et al., 2003; CitationScagel and Yang, 2005), soil depth (CitationScagel and Yang, 2005), soil moisture (CitationHutton et al., 1994; CitationJohansson, 2000), field age (CitationScagel, 2003; CitationKosola and Workmaster, 2007), and the quantity and quality of soil organic matter (CitationGoulart et al., 1995, Citation1997; CitationYang et al., 2002; CitationKosola and Workmaster, 2007).
Dark septate endophytes (DSE) are fungi that frequently colonize ericaceous roots and can be recognized by inter- and intracellular brown to hyaline hyphae and microsclerotia (CitationHambleton and Currah, 1997; CitationJumpponen and Trappe, 1998). DSE colonization does not necessarily coincide with root development or include a specialized interface for nutrient and carbohydrate exchange, which are two defining characteristics of mycorrhizae (CitationBrundrett, 2004; CitationJones and Smith, 2004; CitationPeterson et al., 2008). DSE associations are not considered mutualisms in the same class as mycorrhizae (CitationSmith and Read, 2008). Despite their widespread occurrence, the functions of DSE in the Ericaceae and other plants have yet to be established (CitationMandyam and Jumpponen, 2005; CitationPeterson et al., 2008). In a native stand of huckleberry (V. membranaceum Douglas) in Oregon, DSE colonization was positively correlated with extractable soil phosphorus (CitationStocking, 2006). Inoculation of lodgepole pine (Pinus contorta Douglas), a non-ericaceous plant, with the DSE Phialocephala fortinii Wang & Wilcox increased plant growth as well as absorption of P and N under non-N-limiting conditions (CitationJumpponen and Trappe, 1998). DSE fungi accumulate polyphosphates in their hyphae (CitationSaito et al., 2006). These observations suggest that DSE play a role in phosphorus mobilization. However, inoculation with P. fortinii was also shown to increase seedling mortality of the ericaceous plant Menziezia ferruginea Smith (CitationStoyke and Currah, 1993) and reduce growth of inoculated pine (P. resinosa Ait.) and spruce (Picea rubens Sarg.) trees (CitationWilcox and Wang, 1987). DSE may form intracellular hyphal coils in hair roots that resemble ERM (CitationUsuki and Narisawa, 2005; CitationVohník et al., 2007; CitationOhtaka and Narisawa, 2008), but also abundant microsclerotia in xylem and meristem tissues in roots, indicating a potential for parasitism of plants (CitationPeterson et al., 2008). Even if they play a lesser role in nutrient uptake than ERM, DSE may benefit their host plants in other ways, possibly via suppression of root pathogens or release of plant-growth-stimulating hormones (CitationAddy et al., 2005; CitationMandyam and Jumpponen, 2005; CitationPeterson et al., 2008).
Study Rationale and Objectives
Despite the long history of blueberry production in Michigan, available data on ERM colonization in Michigan blueberry fields are limited. Blueberry bushes in Michigan may remain productive for more than 50 years. They are cultivated predominantly on soils where highbush blueberries are native. A prior history of native ericaceous plants in field sites may contribute to high levels of mycorrhizal colonization (CitationHutton et al., 1997; CitationScagel, 2003). A survey of ERM colonization that included four sites in Michigan found higher-than-expected levels of root colonization given that intensive management practices are thought to suppress ERM colonization (CitationGoulart et al., 1993). ERM colonization may contribute to the longevity of production fields, but an extensive survey of ERM in Michigan blueberries has, to our knowledge, never been carried out.
In Michigan, organic blueberry acreage is increasing. However, ERM colonization in organic blueberry fields has not been evaluated (CitationDemchak et al., 2010). Some studies suggest that ERM may play an important role in plant nutrition in organic blueberry production. In a study of growth and nutrition of mycorrhizal and non-mycorrhizal nursery-grown blueberries provided with organic or synthetic fertilizers, CitationScagel (2005a) concluded that ericoid mycorrhizal colonization was more prevalent in and beneficial to plants grown in media amended with organic fertilizer. CitationXiao and Berch (1999) observed that ERM colonization of Gaultheria shallon Pursh grown in axenic culture increased as the complexity of N source increased from ammonium to protein. This relationship may be analogous to fertilization practices in conventional and organic blueberry fields in Michigan, where N is often supplied as inorganic salts or protein-based fertilizers, respectively (personal communication, Michigan blueberry growers).
In previous surveys of root symbioses of cultivated Vaccinium spp., DSE were classified as “other fungi” (CitationGoulart et al., 1993) or not mentioned. Despite ambiguity in the nature of the symbiosis between DSE and ericaceous plants, investigation into DSE is necessary because of the potential of these fungi to influence plant growth. One study of native Ericaceae in Canada noted that DSE were nearly absent in acidic bog soils (CitationHambleton and Currah, 1997), but a follow-up study did not report a similar trend (CitationAddy et al., 2000). In Michigan, only a small proportion of blueberries are planted on peat and muck soils, but these soils are an interesting contrast to the coarse-textured soils on which the vast majority of blueberries are grown.
The objectives of this study were to: (1) quantify colonization of blueberry roots by ERM and DSE in organic and conventional fields on different soil types, and (2) describe the relationship between ERM and DSE colonization and soil properties, field age, and management practices.
MATERIALS AND METHODS
We matched eight pairs of organic and conventionally managed fields by soil series (websoilsurvey.nrcs.usda.gov/app/WebSoilSurvey.aspx), and plant age and cultivar where possible. The fields ranged from 10 to 65 years old and received annual inputs of organic or inorganic N fertilizers. All organic fields were managed with conventional practices prior to receiving organic certification. Roots and soil were collected at 0- to 30-cm depth below the canopy of eight bushes per site on July 6–7, August 21–22, and October 9–10, 2009. Hair roots were excised from root pieces of 5 to 20 cm in length, placed in histology capsules (Sakura Tissue-Tek 4090, Sakura Finetec, Torrance, CA, USA), cleared for 2 days in 10% KOH at room temperature, acidified in 2% HCl, and stained in 0.05% methyl blue (adapted from CitationKormanik et al., 1980; CitationGrace and Stribley, 1991; CitationBrundrett et al., 1996). Root colonization was assessed at 600–960× magnification on an Olympus IX71 microscope with differential interference contrast (Olympus America Inc., Center Valley, PA, USA) as the number of ERM hyphal coils or DSE microsclerotia in epidermal cells of 50- to 150-μm-diameter hair roots (CitationMcGonigle et al., 1990; CitationUsuki and Narisawa, 2005; CitationOlsrud et al., 2007). Occurrence of intra- or extraradical DSE hyphae was not recorded due to the nature of our measurement scheme (percentage of epidermal hair root cells colonized) and difficulty in distinguishing between surface hyphae of DSE and other rhizoplane-colonizing fungi (CitationMandyam and Jumpponen, 2005). Fifty contiguous epidermal cells of 25 hair root segments per bush from four randomly selected bushes (eight bushes in July) were examined per field site. Hair root diameter was measured on the midpoint of root segments and was only assessed on samples collected in October 2009.
Soil was passed through a 2-mm sieve and dried at 70°C within 3 days of collection. Ammonium and nitrate were extracted from dried soil in a 5:1 ratio (wt/wt) of 1 M KCl to soil and measured according to CitationDoane and Horwath (2003) and CitationNelson (1983). Soil pH was measured with an electrode in a 1:1 mixture of soil and deionized water (CitationWatson and Brown, 1998). Soil carbon (C) and N were determined with an elemental analyzer (Costech ECS 4010, Costech Analytical Technologies, Inc., Valencia, CA, USA). Information on the field history and cultural practices such as fertilizer type and rate and fungicide applications was provided by the owners of the blueberry fields.
A repeated measures ANOVA of the effects of management practices, soil type (sand and muck), and sampling date was conducted using the LSMEANS option in SAS PROC GLIMMIX SAS 9.2 (SAS Institute, Cary, NC, USA) with field pairs specified as a random factor. The relationships between ERM and DSE and field variables across field sites was determined in SAS PROC CORR using the mean value of three sampling dates at each site. Correlations between measured variables were analyzed for all sampled sites (n = 16), and for sandy soils only (n = 12). Mucks were excluded in the latter case due to extreme values in soil C, soil N, and inorganic N.
RESULTS
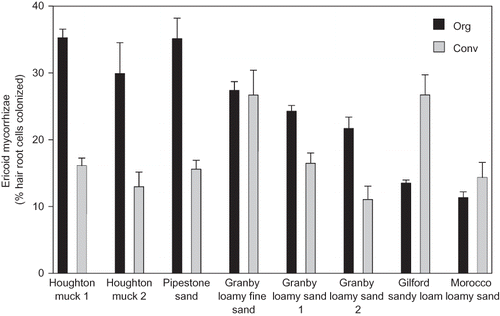
ERM colonization of blueberry roots was visible as loosely arranged hyphal loops or more densely coiled hyphae with limited hyphal extension from the root surface (, , and ). We also occasionally observed hyphae that formed a partial mantle, similar to that described by CitationDighton and Coleman (1992) CitationSmith et al. (1995), and CitationRains et al. (2003), and were closely associated with ERM coils (, , and ). ERM colonization ranged from 11 to 35% of root epidermal cells () and was significantly affected by management (organic or conventional) (P = 0.045) but did not vary by soil type (muck or sand) (P = 0.48) or sampling date (P = 0.22). ERM colonization of blueberry roots was higher under organic production in 6 out of 8 field pairs, particularly on high organic matter muck soils ().
FIGURE 1 Morphology of ericoid mycorrhizae and dark septate endophytes (DSE) in hair roots of northern highbush blueberry (Vaccinium corymbosum L. ‘Bluecrop’) stained with methyl blue. (A) Intracellular hyphal coils (black arrows), (B) partial mantle on surface of epidermal cells (white arrows), (C) intracellular hyphal coils (black arrows) and DSE microsclerotia (white arrows), and (D) extensive partial mantle of hyphae on surface of hair root (white arrows). Scale bar = 12.5 μm (color figure available online).
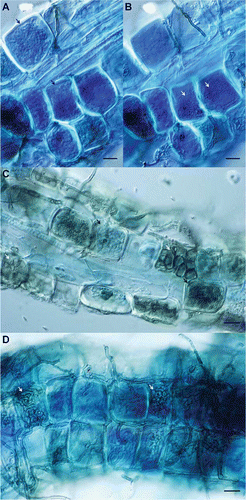
FIGURE 2 The percentage of hair root cells colonized by ericoid mycorrhizae in organic (Org) and conventional (Conv) blueberry fields paired by soil series in Michigan. Error bars represent one standard error of the mean of samples collected on July 6–7, August 21–22, and October 9–10, 2009.
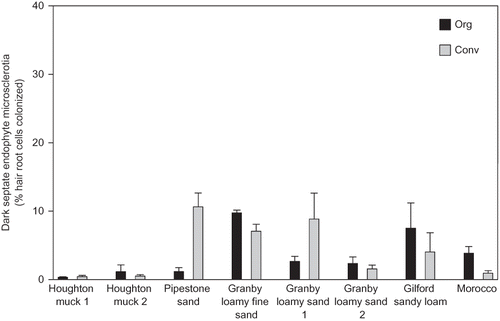
The morphology of DSE colonization was consistent with that described by CitationStoyke and Currah (1991) (). DSE microsclerotia occurred most often in epidermal cells but were occasionally observed in xylem and meristem tissues. Across field sites, DSE microsclerotia were present in 0.3 to 11% of root epidermal cells (). DSE colonization was higher in July and August than in October (ANOVA P = 0.003) and in sandy compared to muck soils (P = 0.04), but was not significantly affected by management (P = 0.54).
FIGURE 3 The percentage of hair root cells colonized by dark septate endophyte microsclerotia in organic (Org) and conventional (Conv) blueberry fields paired by NRCS soil series in Michigan. Error bars represent one standard error of the mean of samples collected on July 6–7, August 21–22, and October 9–10, 2009.
With both sandy and muck soils included in the analysis of the relationship between ERM colonization and field variables, only field age was significantly negatively correlated with ERM colonization (P = 0.001; ), although the correlation between ERM colonization and root diameter was marginally significant and positive (P = 0.09). On sandy soils, there were significant negative correlations of ERM colonization with field age (P = 0.0001) and mean soil ammonium levels (P = 0.05), a marginally significant negative correlation of ERM colonization with total soil N (P = 0.06; ), and significant positive correlations of ERM colonization with soil pH (P = 0.02; ) and root diameter (P = 0.02; ). ERM colonization was not significantly correlated with mean soil nitrate concentration, inorganic N (nitrate + ammonium), total soil carbon, soil C:N ratios, the number of fungicide applications, or time under organic management. When sands and mucks were analyzed together, DSE colonization was negatively correlated with total soil N (P = 0.04), ammonium-N (P = 0.03), and total soil C (P = 0.04), largely due to low DSE colonization levels and high values of the above-mentioned soil parameters in muck soils. On sandy soils, DSE colonization was not significantly correlated with any of the measured variables. Hair-root diameter was negatively correlated with field age on sandy soils (P = 0.04), and this relationship was marginally significant (P = 0.09) when muck soils were included in the analysis. There was no significant correlation between ERM and DSE colonization.
DISCUSSION
Levels of ERM colonization of blueberries in Michigan roughly agreed with colonization levels reported in other northern highbush blueberry production regions in North America (CitationGoulart et al., 1993; CitationScagel and Yang, 2005). The upper limit of ERM colonization in the fields we sampled was 35%, less than half of that reported by CitationGoulart et al. (1993) but in line with that reported for highbush blueberry fields in Oregon (44%). However, the unit of measure reported by CitationGoulart et al. (1993) was the percentage of hair-root segments containing mycorrhizae, which is expected to be much higher than the degree of colonization of individual roots. For example, across sites in the present study, the percentage of root segments with ERM colonization ranged from 68% to 98% and had an overall mean of 89%. CitationScagel and Yang (2005) reported the percentage of hair-root length infected, which is similar to the unit of measurement, percentage epidermal cells colonized, that we used.
Mycorrhizal colonization was generally higher in organic compared to conventional fields, which suggests ERM colonization is enhanced by organic management. CitationStribley and Read (1976) demonstrated that high levels of ammonium-N decreased ERM colonization, while CitationXiao and Berch (1999) observed increased ERM colonization with increasing molecular complexity of supplied N. Our results suggest that the relationship between fertilizer-N type and ERM colonization may play out in blueberry production fields in Michigan as well, since N is added mainly as ammonium or urea on conventional farms and as protein-based fertilizer and compost on organic farms. Several studies have suggested that the primary role of ERM is in allowing ericaceous plants to exploit organic pools of N (CitationYang et al., 2002; CitationWalker et al., 2010). ERM fungi produce abundant proteases (CitationBajwa et al., 1985) and may aid in uptake of N from protein meal-based fertilizers. It would be valuable to quantify the contribution of ERM to organic-N uptake in cultivated Vaccinium spp. and determine how different fertilizer types influence organic N pools in blueberry soils.
ERM colonization was strongly negatively correlated with field age. In previous studies, the relationship between the age of Vaccinium spp. production fields and levels of ERM colonization were inconsistent among crop species and production regions. In cranberry beds, ERM colonization increased with field age in Oregon (CitationScagel, 2003), but decreased with field age in Wisconsin (CitationKosola and Workmaster, 2007). Rabbiteye blueberry (V. ashei Reade) fields in Florida had higher ERM colonization levels in the oldest fields surveyed (CitationJacobs et al., 1982), and a similar trend was reported in Oregon (CitationScagel and Yang, 2005), which contrasts with the negative association between ERM and field age that we observed. CitationKosola and Workmaster (2007) attributed lower levels of ERM colonization in older cranberry fields to accumulation of organic matter with a low C:N ratio in deeper soil horizons. In the present study, ERM colonization was not related to the soil C:N ratio but tended to decrease with increasing total soil N on sandy soils (discussed below). We also observed a trend of higher total soil N with increasing field age on sandy soils (r = 0.48), but the relationship was not statistically significant (P = 0.12). Therefore, a decrease in ERM colonization levels in older Michigan blueberry fields on sandy soils may be related to enrichment of soil N over time, in addition to other factors not accounted for here. It is clear that ERM colonization of blueberries and cranberries is affected by various factors related to the age of production fields, but the relationship appears to be specific to both the production region and crop species.
We observed a negative relationship between ERM and total soil N. In contrast, CitationJohansson (2000) reported that total soil N and ERM colonization of Calluna vulgaris (L.) Hull (Ericaceae) in a native heathland were unrelated (P = 0.83) when ammonium nitrate was added at up to 70 kg N ha-1 yr-1 for 3 years. In our study, the fields were fertilized annually with variable rates of organic or inorganic N for 10 to 65 years. Perhaps a longer duration of N fertilization is required to saturate N in field soils and result in an inverse relationship between soil N and ERM colonization that is not typically observed in shorter studies. A negative correlation between ERM colonization and ammonium-N was anticipated based on a similar finding in a survey of ERM colonization of blueberries in Oregon (CitationScagel and Yang, 2005). However, in the present study, ERM colonization was not related to concentrations of inorganic extractable N or nitrate, suggesting ammonium and nitrate may differ in their effects on ERM colonization. CitationKosola et al. (2007) reported that ERM colonization improved uptake of nitrate-N by cranberry (V. macrocarpon). Our results indicate that ERM are ubiquitous and may play a role in nitrate uptake in Michigan blueberries. However, it is not known whether high levels of ERM colonization enhance nitrate uptake and assimilation in cultivated blueberry fields.
We observed a positive relationship between soil pH and mycorrhizal colonization. In contrast, CitationStevens et al. (1997) reported a negative relationship between ERM colonization and soil pH in blueberries in Pennsylvania, and CitationScagel and Yang (2005) found a general trend of decreased ERM colonization at a soil pH above 5.2. In the study by CitationStevens et al. (1997), roots were collected from native as well as commercial field sites, and native sites had the highest levels of ERM colonization but lowest soil pH. We only sampled roots from commercial fields and the positive linear relationship between ERM colonization and soil pH was consistent among both organic and conventionally managed fields. In our study, one site had a soil pH of 5.6, while the remainder had a soil pH in the range of 4.0 to 5.2. Therefore, it appears that at the typical pH range of Michigan blueberry soils, ERM colonization levels increase linearly with soil pH. Soil pH may directly or indirectly affect the growth and survival of ERM fungi. In general, the growth of both fungi and bacteria is reduced as the soil pH decreases to 4.0 (CitationRousk et al., 2010; Sadowsky et al., unpublished data). ERM colonization appears to be an important means for ericaceous plants to compete with saprotrophic microbes for available soil N (CitationWalker et al., 2010), and it is possible that in blueberry soils, plant-microbe competition for N intensifies with increasing soil pH. Further investigation into interactions between soil pH, soil nitrogen, and ERM colonization may provide additional insights into the correlative data presented here.
DSE colonization levels did not appear to be related to soil C or N content in sandy soils. DSE were observed in all fields sampled, but at very low levels on muck soils. The latter finding is in accordance with the near-absence of DSE in bog soils previously reported by CitationHambleton and Currah (1997). Ultrastructural observation has shown that epidermal hair root cells colonized by DSE appear to be senescent (CitationCurrah et al., 1993). Increased abundance of DSE microsclerotia in some soils may be related to some edaphic stress factors not accounted for in the variables we measured, which may have resulted in increased root senescence and turnover. The occasional observation of microsclerotia in vascular tissues or root apices is a concern and may indicate pathogenic behavior. This type of DSE colonization should be monitored in future studies, especially if observed in relation to above-ground symptoms of root disease in the bushes.
The positive relationship between ERM colonization and root diameter in sandy soils is intriguing. In arbuscular mycorrhizal plants, mycorrhizal colonization often increases with increasing root diameter (CitationBerta et al., 1995) or reduced specific root length (SRL) (CitationEissenstat et al., 2000), a measure of root length per mass of root tissue that is inversely related to root diameter (CitationHodge, 2004) Auxins produced by ERM fungi may alter the morphology of colonized roots (CitationBerta et al., 1988). CitationScagel (2005b) reported that ERM colonization levels in rooted cuttings of Leucothoe fontanestana (Steud) Sleumer grown in a bark-peat substrate were higher as SRL decreased, which agrees with our findings. However, ERM colonization increased with increasing SRL in blueberry fields in the northwest U.S. (CitationScagel and Yang, 2005), and a study of highbush blueberry in Pennsylvania showed that ERM colonization decreased with increasing root diameter (CitationValenzuela-Estrada et al., 2008). It is important to point out that in the latter study (CitationValenzuela-Estrada et al., 2008), ERM colonization and diameter of roots was assessed on only 3 to 5 bushes in one field, and higher-order roots with secondary xylem thickening were included in the assessment. In the present study, roots were collected from several fields over a range of soil types, and only hair roots, lacking secondary xylem, were observed for ERM colonization. Furthermore, the positive relationship between ERM and hair root diameter that we observed reflects site-to-site variation in edaphic properties and management that are likely to influence hair-root diameter and ERM colonization. ERM are an adaptation that allows ericaceous plants to mobilize nutrients from organic matter (CitationRead et al., 2004). It is possible that the positive relationship between ERM and hair root diameter is related to soil nutrient availability among field sites, with lower average root diameter associated with higher SRL and root surface area for absorption of nutrients in mineral form, and higher average root diameter associated with higher ERM colonization for mobilization of nutrients from organic matter. An inverse correlation between hair-root diameter and field age supports this hypothesis, since fertilization may enrich inorganic nutrient pools in soil over time. However, root diameter was not correlated with ammonium, nitrate, or inorganic N levels in October and was not assessed at earlier sampling dates. Closer examination of the relationship between hair-root diameter, ERM colonization, and nutrient availability may help to explain the relationship observed in our study. A slightly inflated estimate of root diameter in our study may have resulted from flattening roots onto microscope slides, which was necessary for microscopic observation of fungal colonization. This may explain why the average hair root diameter in some fields was above 100 μm, which is considered the upper limit of hair-root diameter in the Ericaceae (CitationRead, 1996).
Although we did not attempt to identify ERM and DSE fungi, we observed a range of fungal morphology in colonized roots, including mantle-like hyphae closely associated with intracellular ERM hyphal coils. In the future, methodologies such as single cell dissection and DNA sequencing (CitationBalestrini et al., 2009) will be helpful to identify species and assess genetic diversity, especially since many ERM fungi are difficult if not impossible to culture on laboratory media (CitationAllen et al., 2003b; CitationKosola et al., 2007; CitationIshida and Nordin, 2010).
In conclusion, organic fields generally had higher levels of ERM colonization than conventional fields situated on the same soil type. ERM colonization levels varied in relation to field age, soil pH, total soil N, soil ammonium, and root diameter. Michigan blueberries are grown mostly on soils where highbush blueberries are native, and cultivated varieties are only a few generations removed from their wild relatives. It is likely that ERM play a beneficial role in the health and productivity of bushes. An alternative view suggests that ERM may be a relic of wild populations and of marginal importance in intensively managed crop production (CitationSmagula and Litten, 1989; CitationKorcak, 1988) or even mild plant parasites where intensive fertilization practices select for “less mutualistic” mycorrhizae (CitationJohnson, 1993). Perhaps the relative benefit provisioned to plants by ERM depends on management intensity (e.g., conventional vs. organic) or other factors unrelated to cultural practices, such as soil type or field age. Nonetheless, highbush blueberry hair roots comprise about 40% of total root biomass in highbush blueberry fields (CitationValenzuela-Estrada et al., 2008), and when colonized by ERM, are nearly devoid of plant organelles and occupied almost entirely by fungal hyphae (CitationDuddridge and Read, 1982). CitationAllen et al. (2003a) and CitationHobbie and Hobbie (2006) estimated that mycorrhizae are a sink for 10 to 20% of the carbon fixed by plants. Therefore, it is likely that blueberry bushes in Michigan allocate significant photosynthetic energy to ERM and DSE symbioses. Further studies to determine the functions of ERM and DSE and ways to attain maximum benefit from these symbioses in blueberry production fields are warranted.
ACKNOWLEDGMENTS
We would like to thank the North Central Region Sustainable Agriculture Research and Education program for providing funding for the study, K. Haynes from the MSU Department of Forestry for help with soil C and N analysis, W. Q. Yang and K. Demchak for advice on mycorrhizal assessment methods, and the blueberry growers who participated in this study for kindly allowing access to their fields and providing production information.
LITERATURE CITED
- Addy , H.D. , Piercey , M.M. and Currah , R.S. 2005 . Microfungal endophytes in roots. Can . J. Bot. , 83 : 1 – 13 .
- Addy , H.D. , Hambleton , S. and Currah , R.S. 2000 . Distribution and molecular characterization of the root endophyte Phialocephala fortinii along an environmental gradient in the boreal forest of Alberta . Mycol. Res. , 104 : 1213 – 1221 .
- Allen , M.F. , Swenson , W. , Querejeta , J.I. , Egerton-Warburton , L.M. and Treseder , K.K. 2003a . Ecology of mycorrhizae: A conceptual framework for complex interactions among plants and fungi . Annu. Rev. Phytopathol. , 41 : 271 – 303 .
- Allen , T.R. , Millar , T. , Berch , S.M. and Berbee , M.L. 2003b . Culturing and direct DNA extraction find different fungi from the same ericoid mycorrhizal roots . New Phytol. , 160 : 255 – 272 .
- Balestrini , R. , Gomez-Ariza , J. , Klink , V.P. and Bonfante , P. 2009 . Application of laser microdissection to plant pathogenic and symbiotic interactions . J. Plant Interac. , 4 : 81 – 92 .
- Bajwa , R. , Abuarghub , S. and Read , D.J. 1985 . The biology of mycorrhiza in the Ericaceae. X. The utilization of proteins and the production of proteolytic enzymes by the mycorrhizal endophyte and by mycorrhizal plants . New Phytol. , 101 : 469 – 486 .
- Berta , G. , Trotta , A. , Fusconi , A. , Hooker , J.E. , Munro , M. , Atkinson , D. , Giovannetti , M. , Morini , S. , Fortuna , P. , Tisserant , B. , Gianinazzi-Pearson , V. and Gianinazzi , S. 1995 . Arbuscular mycorrhizal induced changes to plant growth and root system morphology in Prunus cerasifera . Tree Physiol. , 15 : 281 – 293 .
- Berta , G. , Gianinazzi-Pearson , V. , Gay , G. and Torri , G. 1988 . Morphogenetic effects of endomycorrhizae formation on the root system of Calluna vulgaris (L.) Hull . Symbiosis , 5 : 33 – 44 .
- Brundrett , M. 2004 . Diversity and classification of mycorrhizal associations . Biol. Rev. , 79 : 473 – 495 .
- Brundrett , M. , Bougher , N. , Dell , B. , Grove , T. and Malajczuk , N. 1996 . “ Working with mycorrhizae in forestry and agriculture ” . In ACIAR Monograph 32 , Canberra , , Australia : Australian Centre for International Agricultural Research .
- Caporn , S.J.M. , Song , W. , Read , D.J. and Lee , J.A. 1995 . The effect of repeated nitrogen fertilization on mycorrhizal infection in heather [Calluna vulgaris (L.) Hull] . New Phytol. , 129 : 605 – 609 .
- Currah , R.S. , Tsuneda , A. and Murakami , S. 1993 . Morphology and ecology of Phialocephala fortinii in roots of Rhododendron brachycarpum . Can. J. Bot. , 71 : 1071 – 1078 .
- Demchak, K., et al. 2010. Blueberries, p. 109–160. In: A. Kirsten (ed.). The mid-Atlantic berry guide. Retrieved from http://pubs.cas.psu.edu/freepubs/pdfs/AGRS097h.pdf (http://pubs.cas.psu.edu/freepubs/pdfs/AGRS097h.pdf)
- Diaz , A. , Green , I. and Tibbett , M. 2008 . Re-creation of heathland on improved pasture using top soil removal and sulphur amendments: Edaphic drivers and impacts on ericoid mycorrhizas . Biol. Conserv. , 141 : 1628 – 1635 .
- Dighton , J. and Coleman , D. C. 1992 . Phosphorus relations of roots and mycorrhizas of Rhododendron maximum in the Southern Appalachians, North Carolina . Mycorrhiza , 1 : 175 – 184 .
- Doane , T.A. and Horwath , W.R. 2003 . Spectrophotometric determination of nitrate with a single reagent . Anal. Lett. , 36 : 2713 – 2722 .
- Duddridge , J. and Read , D.J. 1982 . An ultrastructural analysis of the development of mycorrhizas in Rhododendron ponticum . Can. J. Bot. , 60 : 2345 – 2356 .
- Eissenstat , D.M. , Wells , C.E. , Yanai , R.D. and Whitbeck , J.L. 2000 . Building roots in a changing environment: Implications for root longevity . New Phytol. , 147 : 33 – 42 .
- Goulart , B.L. , Demchak , K. and Yang , W.Q. 1995 . Organic matter and nitrogen level effects on mycorrhizal infection in ‘Bluecrop’ highbush blueberry plants . J. Small Fruit & Viticulture , 3 : 151 – 164 .
- Goulart , B.L. , Demchak , K. and Yang , W.Q. 1997 . Effect of cultural practices on field grown ‘Bluecrop’ highbush blueberries, with emphasis on mycorrhizal infection levels . Acta Hort. , 446 : 271 – 280 .
- Goulart , B.L. , Schroeder , M.L. , Demchak , K. , Lynch , J.P. , Clark , J.R. and Darnell , R.L. 1993 . Blueberry mycorrhizae: Current knowledge and future directions . Acta Hort. , 346 : 230 – 239 .
- Grace , C. and Stribley , D.P. 1991 . A safer procedure for routine staining of vesicular-arbuscular fungi . Mycol. Res. , 95 : 1160 – 1162 .
- Hambleton , S. and Currah , R.S. 1997 . Fungal endophytes from the roots of alpine and boreal Ericaceae . Can. J. Bot. , 75 : 157 – 1581 .
- Hashem , A.R. 1995 . The role of mycorrhizal infection in the resistance of Vaccinium macrocarpon to manganese . Mycorrhiza , 5 : 289 – 291 .
- Hobbie , J.E. and Hobbie , E.A. 2006 . 15N in symbiotic fungi and plants estimates nitrogen and carbon flux rates in arctic tundra . Ecology , 87 : 816 – 822 .
- Hodge , A. 2004 . The plastic plant: root responses to heterogeneous supplies of nutrients . New Phytol. , 162 : 9 – 24 .
- Hofland-Zijlstra , J.D. and Berendse , F. 2009 . The effect of nutrient supply and light intensity on tannins and mycorrhizal colonization in Dutch heathland ecosystems . Plant Ecol. , 201 : 661 – 675 .
- Hutton , B.J. , Dixon , K.W. and Sivasithamparam , K. 1994 . Ericoid endophytes of western Australian heaths (Epacridaceae) . New Phytol. , 127 : 557 – 566 .
- Hutton , B.J. , Dixon , K.W. , Sivasithamparam , K. and Pate , J.S. 1997 . Effect of habitat disturbance on inoculum potential of ericoid endophytes of Western Australian heaths (Epacridaceae) . New Phytol. , 135 : 739 – 744 .
- Ishida , T.A. and Nordin , A. 2010 . No evidence that nitrogen enrichment affect fungal communities of Vaccinium roots in two contrasting boreal forest types . Soil Biol. Biochem. , 42 : 234 – 243 .
- Jacobs , L.A. , Davies , F.S. and Kimbrough , J.M. 1982 . Mycorrhizal distribution in Florida rabbiteye blueberries . HortScience , 17 : 951 – 953 .
- Jeliazkova , E.A. and Percival , D.C. 2003 . N and P fertilizers, some growth variables, and mycorrhizae in wild blueberry (Vaccinium angustifolium) . Acta Hort. , 626 : 297 – 304 .
- Johansson , M. 2000 . The influence of ammonium nitrate on the root growth and ericoid mycorrhizal colonization of Calluna vulgaris (L.) Hull from a Danish Heathland . Oecologia , 123 : 418 – 424 .
- Johnson , N.C. 1993 . Can fertilization of soil select less mutualistic mycorrhizae? Ecol . Appl. , 3 : 749 – 757 .
- Jones , M.D. and Smith , S.E. 2004 . Exploring functional definitions of mycorrhizas: Are mycorrhizas always mutualisms? . Can. J. Bot. , 82 : 1089 – 1109 .
- Jumpponen , A. and Trappe , J.M. 1998 . Dark septate Endophytes: A review of facultative ebiotrophic root-colonizing fungi . New Phytol. , 140 : 295 – 310 .
- Kemp , E. , Adam , P. and Ashford , A.E. 2003 . Seasonal changes in hair roots and mycorrhizal colonization in Woollsia pungens (Cav.) F. Muell. (Epacridaceae) . Plant Soil , 250 : 241 – 248 .
- Kemppainen , M. , Duplessis , S. , Martin , F. and Pardo , A.G. 2009 . RNA silencing in the model mycorrhizal fungus Laccaria bicolor: Gene knock-down of nitrate reductase results in inhibition of symbiosis with Populus. Environ . Microbiol. , 11 : 1878 – 1896 .
- Korcak , R.F. 1988 . Nutrition of blueberry and other calcifuges . Hort. Rev. , 10 : 183 – 227 .
- Korcak , R.F. 1989 . Variation in nutrient requirements of blueberries and other calcifuges . HortScience , 24 : 573 – 578 .
- Kormanik , P.P. , Bryan , W.C. and Schultz , R.C. 1980 . Procedures and equipment for staining large numbers of plant root samples for endomycorrhizal assay . Can. J. Microbiol. , 26 : 536 – 538 .
- Kosola , K.R. and Workmaster , B.A. 2007 . Mycorrhizal colonization of cranberry: Effects of cultivar, soil type, and leaf litter composition . J. Am. Soc. Hort. Sci. , 132 : 134 – 141 .
- Kosola , K.R. , Workmaster , B.A. and Spada , P.A. 2007 . Inoculation of cranberry (Vaccinium macrocarpon) with the ericoid mycorrhizal fungus Rhizoscyphus ericae increases nitrate influx . New Phytol. , 176 : 184 – 196 .
- Leake , J.R. and Read , D.J. 1991 . “ Experiments with ericoid mycorrhiza, p ” . In Methods in microbiology , Edited by: Norris , J.R. , Read , D.J. and Varma , A. K. Vol. 23 , 435 – 459 . London, UK : Academic Press .
- Mandyam , K. and Jumpponen , A. 2005 . Seeking the elusive function of the root-colonising dark septate endophytic fungi . Stud. Mycol. , 53 : 173 – 189 .
- McGonigle , T.P. , Millers , M.H. , Evans , D.G. , Fairchild , G.L. and Swan , J.A. 1990 . A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi . New Phytol. , 115 : 495 – 501 .
- Michelsen , I.K. , Schmidt , S.J. , Quarmby , C. and Sleep , D. 1996 . Leaf 15N abundance of subarctic plants provides field evidence that ericoid, ectomycorrhizal and non- and arbuscular mycorrhizal species access different sources of soil nitrogen . Oecologia , 105 : 53 – 63 .
- Myers , M.D. and Leake , J.R. 1996 . Phosphodiesters as mycorrhizal P sources. II. Ericoid mycorrhiza and the utilization of nuclei as a phosphorus and nitrogen source byVaccinium macrocarpon . New Phytol. , 132 : 445 – 451 .
- Nelson , D.W. 1983 . Determination of ammonium in KCl extracts of soils by the salicylate method . Commun. Soil Sci. Plant , 14 : 1051 – 1062 .
- Ohtaka , N. and Narisawa , K. 2008 . Molecular characterization and endophytic nature of the root-associated fungus Meliniomyces variabilis (LtVB3) . J. Gen. Plant Pathol. , 74 : 24 – 31 .
- Olsrud , M. , Michelsen , A. and Wallander , H. 2007 . Ergosterol content in ericaceous hair roots correlates with dark septate endophytes but not with ericoid mycorrhizal colonization . Soil Biol. Biochem. , 39 : 1218 – 1221 .
- Percival , D. and Burnham , J. 2006 . Impact of mycorrhizal associations and soil-applied nitrogen and phosphorus on lowbush blueberry (Vaccinium angustifolium Ait.) . Acta Hort. , 715 : 381 – 387 .
- Peterson , R.L. , Wagg , C. and Paulter , M. 2008 . Associations between microfungal endophytes and roots . do structural features indicate function? Botany , 86 : 445 – 456 .
- Rains , K.C. , Nadkarni , N.M. and Bledsoe , C.S. 2003 . Epiphytic and terrestrial mycorrhizas in a lower montane Costa Rican cloud forest . Mycorrhiza , 13 : 257 – 264 .
- Read , D.J. 1984 . “ The structure and function of the vegetative mycelium of mycorrhizal roots ” . In The ecology and physiology of the fungal mycelium. British Mycological Society Symposium 8 , Edited by: Jennings , D.H. and Rayner , A.D.M. 215 – 240 . Cambridge , UK : Cambridge University Press .
- Read , D.J. 1996 . The structure and function of the ericoid mycorrhizal root . Ann. Bot. , 77 : 365 – 374 .
- Read , D.J. , Leake , J.L. and Perez-Moreno , J. 2004 . Mycorrhizal fungi as drivers of ecosystem processes in heathland and boreal forest biomes . Can. J. Bot. , 82 : 1243 – 1263 .
- Rousk , J. , Brookes , P.C. and Baath , E. 2010 . Investigating the mechanisms for the opposing pH relationships of fungal and bacterial growth in soil . Soil Biol. Biochem. , 42 : 926 – 934 .
- Saito , K. , Ueteke , Y.K. , Saito , M. and Peterson , R.L. 2006 . Vacuolar localization of phosphorus in hyphae of Phialocephala fortinii, a dark septate fungal root endophyte . Can. J. Microbiol. , 52 : 643 – 650 .
- Scagel , C.F. 2003 . Mycorrhizal status of sand-based cranberry (Vaccinium macrocarpon) bogs in southern Oregon . Small Fruits Rev. , 2 : 31 – 41 .
- Scagel , C.F. 2005a . Inoculation with ericoid mycorrhizal fungi alters fertilizer use of highbush blueberry cultivars . HortScience , 40 : 786 – 794 .
- Scagel , C.F. 2005b . Isolate-specific rooting responses of Leucothoe fontanesiana cuttings to inoculation with ericoid mycorrhizal fungi . J. Hort. Sci. Biotechnol. , 80 : 254 – 262 .
- Scagel , C.F. and Yang , W.Q. 2005 . Cultural variation and mycorrhizal status of blueberry plants in NW Oregon commercial production fields . Int. J. Fruit Sci. , 5 : 85 – 111 .
- Shaw , G. , Leake , J.R. , Baker , A.J.M. and Read , D.J. 1990 . The biology of mycorrhiza in the Ericaceae. XVII. The role of mycorrhizal infection in the regulation of iron uptake by ericaceous plants . New Phytol. , 115 : 251 – 258 .
- Smagula , J.M. and Litten , W. 1989 . Effect of ericoid mycorrhizae isolates on growth and development of lowbush blueberry tissue culture plantlets . Acta Hort. , 241 : 110 – 114 .
- Smith , J.E. , Molina , R. and Perry , D.A. 1995 . Occurrence of ectomycorrhizas on ericaceous and coniferous seedlings grown in soils from the Oregon Coast Range . New Phytol. , 129 : 73 – 81 .
- Smith , S.E. and Read , D.J. 2008 . Mycorrhizal Symbiosis , New York : Academic Press .
- Sokolovski , S.G. , Meharg , A.A. and Haathuis , F.J.M. 2002 . Calluna vulgaris root cells show increased capacity for amino acid uptake when colonized with the mycorrhizal fungus Hymenoscyphus ericae . New Phytol. , 155 : 525 – 530 .
- Stackpoole , S.M. , Workmaster , B.A.A. , Jackson , R.D. and Kosola , K.R. 2008 . Nitrogen conservation strategies of cranberry plants and ericoid mycorrhizal fungi in an agroecosystem . Soil Biol. Biochem. , 40 : 2736 – 2742 .
- Stevens , C.M. , Goulart , B.L. , Demchak , K. , Hancock , J.F. , Dalpé , Y. and Yang , W.Q. 1997 . The presence, isolation and characterization of ericoid mycorrhizal symbionts in two native and two commercial Vaccinium populations in central Pennsylvania . Acta Hort. , 46 : 411 – 420 .
- Stocking , P. 2006 . “ In search of ecological advantages: Mycorrhizal facilitation of big huckleberry ” . In Vaccinium membranaceum Doug. B.S. Thesis , Aurora , OR : Oregon State University .
- Stoyke , G. and Currah , R.S. 1991 . Endophytic fungi from the mycorrhizae of alpine ericoid plants . Can. J. Bot. , 69 : 347 – 352 .
- Stoyke , G. and Currah , R.S. 1993 . Resynthesis in pure culture of a common subalpine fungus-root association using Phialocephala fortinii and Menziesia ferruginea (Ericaceae) . Arctic Alpine Res. , 25 : 189 – 193 .
- Stribley , D.P. and Read , D.J. 1976 . The biology of mycorrhiza in the Ericaceae VI. The effects of mycorrhizal infection and concentration of ammonium nitrogen on growth of cranberry (Vaccinium macrocarpon Ait.) in sand culture . New Phytol. , 77 : 63 – 72 .
- Treseder , K. 2004 . A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies . New Phytol. , 164 : 347 – 355 .
- Usuki , F. and Narisawa , K. 2005 . Formation of structures resembling ericoid mycorrhizas by the root endophytic fungus Heteroconium chaetospora within roots of Rhododendron obtusum var . kaempferi. Mycorrhiza , 15 : 61 – 64 .
- Valenzuela-Estrada , L.R. , Vera-Caracalla , V. , Ruch , L.E. and Eissenstat , D.M. 2008 . Root anatomy, morphology, and longevity among root orders in Vaccinium corymbosum (Ericaceae) . Am. J. Botany , 95 : 1506 – 1514 .
- Vohník , M. , Fendrych , M. , Albrechtová , J. and Vosátka , M. 2007 . Intracellular colonization of Rhododendron and Vaccinium roots by Cenococcum geophilum, Geomyces pannorum, and Meliniomyces variabilis . Folia Microbiol. , 52 : 407 – 414 .
- Walker , J.F. , Johnson , N.B. , Simpson , L.C. , Bill , M. and Jumpponen , A. 2010 . Application of fungistatics in soil reduces N uptake by an arctic ericoid shrub (Vaccinium vitis-idaea) . Mycologia , 102 : 822 – 834 .
- Watson , M.E. and Brown , A. 1998 . “ pH and lime requirement ” . In Recommended Chemical Soil Test Procedures for the North Central Region , Edited by: Brown , A. 13 – 20 . Columbia , MI : Missouri Agricultural Experiment Station . North Central Regional Research Publication No. 221 (Revised)
- Wilcox , H.E. and Wang , C.J.K. 1987 . Mycorrhizal and pathological associations of dematiaceous fungi in roots of 7-month-old tree seedlings . Can. J. Forest Res. , 17 : 884 – 889 .
- Xiao , G. and Berch , S.M. 1999 . Organic nitrogen use by salad ericoid mycorrhizal fungi from northern Vancouver Island and impacts on growth in vitro of . Gaultheria shallon. Mycorrhiza , 9 : 145 – 149 .
- Yang , W.Q. and Goulart , B.L. 2000 . Mycorrhizal infection reduces short-term aluminum uptake and increases root cation exchange capacity of highbush blueberry plants . HortScience , 35 : 1083 – 1086 .
- Yang , W.Q. , Goulart , B.L. , Demchak , K. and Li , Y. 2002 . Interactive effects of mycorrhizal inoculation and organic soil amendments on nitrogen acquisition and growth of highbush blueberry . J. Am. Soc. Hort. Sci. , 127 : 742 – 748 .
- Yang , W.Q. , Tien , M. and Goulart , B.L. 2006 . Characterization of extracellular proteases produced by four ericoid mycorrhizal fungi in pure culture and symbiotic states . Acta Hort. , 715 : 403 – 410 .
![FIGURE 4 Correlation between ericoid mycorrhizal colonization and (A) field age, (B) total soil nitrogen, (C) soil pH, and (D) hair root diameter in organic (Org) and conventionally (Conv) managed blueberry fields on sandy soils, n = 12 [sand and muck soils in (A), n = 16] in Michigan.](/cms/asset/c205354e-1bbd-4454-b8c0-68e1556a30c1/wsfr_a_619346_o_f0004g.gif)