Abstract
The efficacy of biological controls and application of mulch for control of the blight stage of mummy berry disease was examined in different lowbush blueberry fields in Maine over 5 years. Biological controls tested included compost teas and commercial products containing Trichoderma harzianum, Bacillus pumilus, B. subtilis, Streptomyces lydicus, and plant extracts of neem, garlic, citrus, and giant knotweed. Treatment with compost teas did not affect numbers of fungal and bacterial colony-forming units on leaves compared to controls. Early application of peat mulch before leaf bud break was partially effective at controlling disease in the field. In 2010, extracts of citrus and giant knotweed and a B. subtilis commercial product were effective at controlling disease in at least one of two fields. The other biological controls did not significantly decrease disease incidence compared to untreated controls.
INTRODUCTION
Mummy berry disease, caused by the fungus Monilinia vaccinii-corymbosi (Reade) Honey, is one of the major diseases affecting lowbush blueberry (CitationHildebrand and Braun, 1991). M. vaccinii-corymbosi overwinters as pseudosclerotia (mummy berries) that produce apothecia in the spring (CitationCaruso and Ramsdell, 1995). Lowbush blueberries typically are managed on a 2-year crop cycle by pruning. The inoculum for the primary infection stage of this disease is ascospores that are carried by wind to developing leaf and flower buds. New stems produced in the first year emerge too late in the spring to be infected by the ascospores. It is the leaf and flower buds of the second year's stems that develop and are susceptible at the time of apothecia production. The ascospores can germinate and penetrate into susceptible tissue if a sufficient leaf wetness period occurs; longer periods of leaf wetness are required for infection at cooler temperatures (CitationHildebrand and Braun, 1991). Leaf and flower blight symptoms appear approximately 8 to 9 days after infection, and gray masses of conidia form on the dead tissue (CitationBatra, 1983). Conidia are thought to be carried by wind and insects to healthy flowers and cause the secondary stage of infection by growing down the style and then into the ovaries of the flowers (CitationShinners and Olson, 1996). As the fruit develops, the fungus replaces the plant tissue, producing a pseudosclerotia that is noticeably as smaller and gray in color compared to the healthy fruit at harvest (CitationCaruso and Ramsdell, 1995). These “mummified” fruit fall to the ground and overwinter before germinating in subsequent years.
Mummy berry disease can seriously damage blueberry production when environmental conditions are favorable. Disease incidence of greater than 30% blighted leaves reduces berry size (CitationPenman and Annis, 2005), and in most years, this level of disease is a regular occurrence in fields that have mummy berry disease inoculum and do not use control measures. Currently, the only organically acceptable options recommended for decreasing mummy berry disease incidence in lowbush blueberries are burn pruning of fields and applications of mulch (CitationDrummond et al., 2008; CitationLambert, 1990). Burn pruning decreases the level of mummy berry blight in the subsequent year, presumably by decreasing the number of pseudosclerotia surviving over the winter (CitationLambert, 1990). In rabbiteye blueberry plantings, apothecia do not emerge from mummified fruit that are buried more than 2.6 cm below the ground (CitationNgugi et al., 2002). In lowbush blueberries, mulch applications of at least 5 cm are recommended as a possible control method (CitationYarborough, 2004), but have not been tested for efficacy. Biological control materials can suppress leaf diseases in other crops and are suggested to have both protective and curative effects by promoting plant defenses or by their competition, antagonism, or parasitism to pathogens (CitationMcSpadden-Gardener and Fravel, 2002). From 2005 to 2010, we conducted field trials to determine the efficacy of biological control materials consisting of either living organisms or extracts from plants for control of mummy berry disease in lowbush blueberry. Materials containing living organisms were compost teas and commercially available products containing Trichoderma harzianum, Bacillus pumilus, B.subtilis, or Streptomyces lydicus. Plant-based materials that were tested included extracts from neem (Azadirachta indica), garlic (Allium sativum), citrus, and giant knotweed (Reynoutria sachalinensis).
Compost teas are water extracts prepared by soaking compost for varying lengths of time with or without aeration. The organisms and their metabolites either introduced with or encouraged by the application of compost teas are thought to provide disease suppression, but there is no consensus on the most effective fermentation process or the type of compost necessary for disease suppression (CitationWeltzien, 1991; CitationLitterick et al., 2004).
All of the living organisms tested as biological controls are commonly found in soil and may cause disease suppression by competing with pathogens for space and nutrients on the plant and producing antagonistic materials, as well as parasitizing pathogens and inducing plant defense mechanisms. Both B. subtilis and B. pumilus are spore-forming, gram-positive bacteria that excrete antibiotics and possibly trigger a host's natural defense mechanisms (CitationWulff et al., 2002). B. subtilis produces antifungal polypeptides that decrease both spore germination and hyphal growth (CitationMcKeen et al., 1986). Serenade, a commercial formulation of B. subtilis, limits the radial growth of M. vaccinii-corymbosi in vitro using antibiosis and is also suggested to compete with M. vaccinii-corymbosi conidia for infection sites on flower stigmas and induce resistance in rabbiteye blueberry (CitationScherm et al., 2004). Trichoderma species parasitize other fungi, compete with pathogens for nutrients and space, produce antibiotics and chitinases, and degrade enzymes that are essential for pathogenic fungi to penetrate leaf surfaces (CitationBrunner et al., 2005; CitationElad, 2000; CitationHarman et al., 2004). T. harzianum is able to induce local and systemic resistance in many plants, lasting up to several months (CitationHarman et al., 2004). Streptomyces lydicus suppresses the growth of pathogenic fungi in vitro and Botrytis cinerea (CitationLu et al., 2008), Alternaria solani, and Colletotrichum cocciodes on tomatoes in the greenhouse (CitationHigham et al., 2006). S. lydicus produces the antifungal material natamycin (CitationLu et al., 2008) and colonizes hyphae of pathogenic fungi (CitationHigham et al., 2006).
Extracts from various plant species have antifungal activity. An Allium sativum (garlic) water extract significantly inhibits spore germination and infections of Phytophthora infestans on detached potato leaves (CitationCao and van Bruggen, 2001; CitationCao et al., 2004). Neem, an extract from Azadirachta indica, is mostly tested as an insecticide, but also decreases wheat leaf rust by 60% in 2 years of field trials (CitationAyoub and Niazi, 2001) and has some effectiveness on decreasing B. cinerea infection of inoculated apples (CitationMoline and Locke, 1993). An extract from giant knotweed (Reynoutria sachalinensis) elicits rapid accumulation of phenolic compounds, methyl esters, and other antifungal compounds in leaves (CitationDaayf et al., 1995, 2000). A commercial formulation of this extract is used as a preventative to control powdery mildew on tomato leaves, and directly affects the germination of conidia (CitationKonstantinidou-Doltsinis et al., 2006). The rate and timing of applications of this extract is critical for its protection of plants (CitationKonstantinidou-Doltsinis et al., 2006), since there needs to be enough time before infection to indirectly induce the plants' responses (CitationDaayf et al., 2000). Procidic (Greenspire Global Inc., Waukee, IA, USA), formerly known as Citrex, is a combination of ascorbic acid with citric and lactic acids. Procidic can directly damage fungal and bacterial cell walls, but is also suggested to work indirectly by stimulating the plants' own defenses. Procidic decreases scab severity on apples, effectively reduces rust and powdery mildew on apple shoots, and is effective in the short term at controlling powdery mildew on grapes (CitationTravis et al., 2006). In one rabbiteye blueberry cultivar, Procidic reduces relative mummy berry blight (CitationScherm and Krewer, 2008). A major factor in the efficacy of plant extracts for disease control is the timing and frequency of rainfall (Cao et al., 2004) because this affects their retention and concentration on plant surfaces.
Our objectives were to find organically acceptable methods to control mummy berry blight of lowbush blueberry. We tried two approaches to (a) control the primary infection stage of mummy berry disease by preventing apothecial germination, or (b) suppress infection by M. vaccinii-corymbosi using biological control materials.
MATERIALS AND METHODS
Fields and Treatments: Selection and Application
Each year, selected materials were tested at two lowbush blueberry fields with a history of mummy berry disease in different regions of Maine. All fields were conventionally managed with varying levels of pesticide input; the organically managed Jonesboro field used in 2006 was the exception. Treatments were replicated in a randomized complete block design with four blocks with plots of 2 m × 20 m in 2005 and 2006, and eight blocks with 2 m × 9 m plots in 2007, 2009, and 2010. Biological control materials () were applied with a CO2 backpack sprayer equipped with 80002VS Tjet nozzles with 50 mesh screens (Bellspray, Inc. R&D Sprayers, Opelousas, LA, USA) at 276 kPa and 187 L of solution per ha. In 2005, control plots were sprayed with water and in 2006 the effect of the water treatment was compared to that of a control with nothing applied. There was no significant difference between water treated and untreated controls, so in all subsequent years, controls were untreated. In 2006, peat mulch (Worcester Peat Co., Deblois, ME, USA), chosen for its availability close to experimental fields, was applied to a depth of 3 to 4 cm at or before bud break to plots in both fields. The mulch treatment plots were each 4 m × 10 m in an attempt to decrease leaf infections from apothecia outside the plots. In 2009 and 2010, the recommended rate of propiconazole (Bumper, rate 0.44L/ha, Makhteshim Agan of North America Inc., Raleigh, NC, USA) was included as a treatment for comparison. Applications were made on a twice weekly schedule in 2005 and 2006; in subsequent years, they were applied every 7 to 10 days beginning as soon as the plants had susceptible buds and continuing as long as mature apothecia were present in the fields.
TABLE 1 Materials Tested for Their Efficacy to Control Mummy Berry Blight and Their Respective Source, Preparation, Application Rates
Compost teas were freshly made for each application in a fan-ventilated greenhouse that ranged in temperature from 11 to 32°C. In 2005, compost teas were made with two sources of compost, Kinney Fish and Farm compost (Knox, ME, USA) and Coast of Maine Lobster compost (Portland, ME, USA). Non-aerated compost teas () were prepared by adding 272 g of dry compost to 7.57 L distilled water in a 5-gallon plastic bucket and then the mixture was stirred, covered loosely, and allowed to ferment for 6 to 8 days before use. The tea was strained through cheesecloth and diluted with distilled water. The aerated compost teas () were brewed by placing dry compost (272 g) into a mesh bag and submersing it in 7.57 L of distilled water with aeration using an Alaska Bountea Compost Tea brewer (Palmer, AK, USA) for 2 to 3 days. Aerated compost teas were diluted as described above. In 2006, a single undiluted, aerated compost tea () was prepared by the above method with aeration for 24 to 48 hr between 10 and 21°C.
In 2005, Plant Shield (Bioworks, Fairport, NY, USA) containing T. harzianum was tested at 10 times its recommended rate for greenhouse soil drenches, due to the high volume of liquid and the damaging number of passes that would be required to apply it at the soil drench recommended rate (). We applied PlantShield at this rate in the hope it would inhibit the germination of apothecia and possibly serve as a protectant to developing plant surfaces. Actinovate (Natural Industries, Inc., Houston, TX, USA), containing Streptomyces lydicus, was applied at the highest recommended rate of product per ha, but in the typical volume of liquid per ha for lowbush blueberries, which was 40% of the recommended volume. The commercial formulations of Sonata (AgraQuest, Davis, CA, USA), containing B. pumilus, and Serenade (AgraQuest), containing B. subtilis, were tested as liquid formulations in 2005 and 2006 at their recommended rates. In 2006, Serenade, Sonata, and the compost tea solutions were applied with an adjuvant, Biolink (garlic-yucca based, Westbridge Ag Products, Vista, CA, USA), to aid in their spread and retention on plant surfaces. Biolink was also tested alone for its possible disease suppression. SerenadeMax (AgraQuest; wettable powder formulation) was tested at its recommended rate in 2007 and 2009 and then at 2.7 times its recommended rate in 2010 following promising results from studies in Canada (CitationLangdon et al., 2009). The commercial formulations of Procidic (Greenspire Global Inc., Waukee, IA, USA), containing a water-based citrus extract, and Regalia and MBI-106020 (Marrone Bio Innovations, Inc., Davis, CA, USA) containing an extract from Reynoutria sachalinensis were tested at their recommended rates ().
Leaf Collection and Determination of Organisms on Leaves
In 2005, leaves were collected before treatments and immediately after treatment materials had dried on the leaves. In 2006, an additional leaf collection at 2 to 3 days after application was also taken. One leaf bud from ten randomly selected stems along a transect down the center of each plot was collected. Leaves were weighed, 10 mL of 0.1% Tween 20 (SigmaUltra Co., St. Louis, MO, USA) was added, and then the leaves were vortexed for 30 sec. For each sample, dilutions were replicate-plated onto malt yeast extract agar with 0.01% penicillin G and streptomycin sulfate for counting fungi and trypticase soy agar (Becton, Dickinson and Company, Sparks, MD, USA) for bacterial counts. Plates were incubated at 25°C for 7 to 10 days and then the average colony forming units (CFU) of fungi and bacteria per gram of leaf material was determined and then compared among treatments, weeks, and collection times for each field. In the second and third week of applications in 2005, samples of the treatment solutions were collected for determining fungal and bacterial CFU. Dilutions of the treatment solutions were plated and incubated as described above. Bacterial and fungal CFU per 0.5 mL of treatment solution were calculated. All comparisons were made using Kruskal-Wallis nonparametric tests (KW) and Bonferroni multiple pairwise comparisons of means (BMCM) (SYSTAT 11, 2004, Systat Software, Inc., San Jose, CA, USA).
Effect of Treated Leaves on In Vitro Monilinia Growth
In 2005, fully opened leaves were collected from plots before and after the treatment applications made in the third week. A mycelium plug from one of three M. vaccinii-corymbosi isolates was placed in the middle of a Petri dish of malt yeast extract agar medium, and four leaves from a plot were placed around the plug. Controls were mycelium without leaves grown on the above medium. The plates were incubated for 7 days at 25°C and mycelial growth was measured beyond the initial plug. Growth was averaged across isolates and across fields since there was no significant difference among them. Differences among treatments within collection time were compared using KW and BPCM at P < 0.05.
Mummy Berry Blight Disease Assessment
Plots were assessed in late May or early June when mummy berry blight symptoms were visible. In 2005, 2006, and 2007, four subsamples per plot were assessed by randomly tossing a 0.07-m2 rectangle into a plot and counting the number of diseased stems and the total number of stems in the rectangle. The percent incidence of disease was calculated for each subsample and then averaged for each plot. Starting in 2009, a different method of rating disease was used in which 70 stems in 2009 and 40 in 2010 from a transect through the middle of each plot were rated for leaf or flower blight on a scale of 0 to 3, where 0 = no blight, 1 = one leaf or flower cluster with blight, 2 = 2 to 3 leaves or flower clusters blighted, and 3 = 4 or more leaves or flower clusters blighted. Fields were analyzed separately because the field sites had different management methods, timing of leaf bud development, and level of disease found in the controls. Incidence data were transformed using the arcsin of the square root of percent disease if data were not normally distributed, but is presented as percent disease for reader interpretation. Treatments were compared within each field using analysis of variance (proc GLM) and Tukey's multiple comparisons test at P < 0.05 (SAS, SAS Institute, Cary, NC, USA). For the 2009 and 2010 data, average disease rating per plot and percent blighted stems per plot were consistent for each treatment so only disease incidence is presented.
Blueberry Yield
In early August, the center of each plot was harvested with a 45-cm-wide hand rake or a 3-ft-wide mechanical harvester. Yield was weighed with a standard pound scale, converted to g for analysis, and extrapolated to kg per hectare for presentation. Non-normal data was log-transformed for analysis, and data was compared among treatments using analysis of variance (proc GLM) and Tukey's multiple comparisons test with P < 0.05 (SAS).
RESULTS AND DISCUSSION
Mummy Berry Disease Incidence
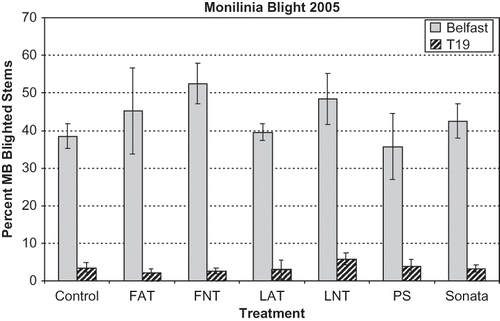
The mummy berry disease incidence in the control treatments varied greatly among fields and years, ranging from 5 to 87%, and is presumed to be affected by the level of inoculum in a field and the local weather conditions. In 2005, the control treatments had 5% mummy berry disease incidence in the Township 19 field and 38% in the Belfast field (). None of the treatments, compost teas, Sonata, or Plant Shield, had a significant effect on disease incidence compared to the controls in both fields in 2005 (). All of the compost tea solutions had approximately 105 bacterial CFU and from 102 to 103 fungal CFU. None of the compost tea treatments increased the number of bacteria or fungi on blueberry leaves compared to the water-treated controls. The Sonata solution contained 106 bacterial CFU and no fungi, and was the only treatment that significantly increased the number of bacterial CFU on leaves after treatment. The Plant Shield solution contained 108 fungal CFU, but did not consistently affect levels of fungal CFU on leaves. Monilinia vaccinii-corymbosi had significantly less growth compared to the controls only in the presence of leaves from Plant Shield-treated plots. Growth of Trichoderma from the treated leaves indicated there were viable spores present, but they were not effective at controlling M. vaccinii-corymbosi in the field. CitationHowell (2003) suggested that the production and activity of enzymes produced by T. harzianum are temperature-sensitive, and air temperatures were above 15°C only two times in the first 24 days of May 2005 (Old Town, Maine, www.noaa.gov), which is typical in Maine springs. There may not have been high enough levels of fungal or bacterial CFU from the treatments for effective competition or inhibition of Monilinia on the plants. Unfortunately, there is no consensus on what levels of bacterial or fungal CFU are required for disease suppression (CitationWeltzien, 1991; CitationLitterick et al., 2004).
FIGURE 1 Incidence of mummy berry (MB) blight at Belfast and Township 19 (T19) fields in 2005. Disease incidence was the percent of diseased stems with blight symptoms. Treatments included Control = water-treatment, FAT = aerated compost tea of Fish and Farm compost, FNT = non-aerated compost tea of Fish and Farm, LAT = aerated compost tea of Lobster compost, LNT = non-aerated compost tea of Lobster compost, PS = Plant Shield (Trichoderma harzianum formulation), Sonata = Bacillus pumilus formulation. See for application rates. Bars represent standard error of the means; none of the treatments were significantly different from the control or each other at P < 0.05.
In 2006, an undiluted aerated compost tea was tested to try to increase the levels of bacterial and fungal CFU applied, since all of the compost tea treatments in 2005 were diluted 1:3 compost tea:water. In 2006, there were no significant differences in mummy berry disease incidence (which ranged from 27 to 34%) between water treated and untreated controls for both fields (Deblois and Jonesboro) (). There were also no significant differences in disease incidence among the treatments, compost tea, Serenade, Sonata, Trilogy, and Biolink, or compared to the controls (). We anticipated that the use of an adjuvant (BioLink: garlic and yucca-based) added to the Serenade, Sonata, and compost tea treatments would increase bacterial retention on the leaf surfaces and increase the efficacy of these treatments, but these effects were not seen. Only the Serenade and Sonata treatments increased bacterial CFU on leaves after treatment, but bacterial levels had decreased to control leaves by 2 days after treatment. None of the treatments increased fungal CFU on the leaves. A 2% garlic extract exhibited 100% inhibition of P. infestans spore germination in vitro (CitationCao and van Bruggen, 2001), but the BioLink product recommended rate resulted in 0.2% garlic solution, which may not have been a high enough concentration for antifungal activity. Most neem studies performed in vitro have had some success in suppressing disease, but in field studies, the efficacy of neem was inconclusive (CitationBowers and Locke, 2000), as we also found.
FIGURE 2 Incidence of mummy berry blight in Deblois and Jonesboro fields in 2006. Disease incidence was the percent of diseased stems with blight symptoms. Treatments included Water control = water-treatment, Untreated control, Biolink = garlic-yucca-based adjuvant, Compost tea = aerated compost tea of Lobster compost, Serenade = Bacillus subtilis formulation, Sonata = Bacillus pumilus formulation, Trilogy = neem extract. See for application rates. Bars represent standard error of the means; none of the treatments were significantly different from the control or each other at P < 0.05.
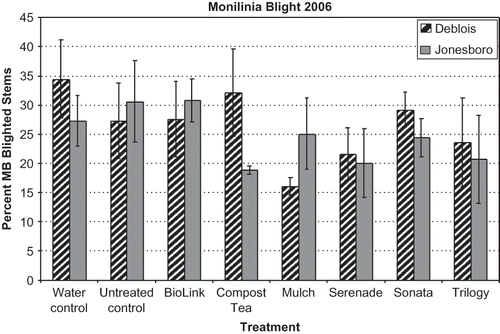
In 2006, the mulch treatment had less mummy berry disease incidence (P = 0.082) than the water-treated control plots in Deblois, but not in Jonesboro () probably due to the timing of the mulch application. The development of blueberry leaf and flower buds is correlated to germination of M. vaccinii-corymbosi apothecia in highbush blueberries (CitationLehman and Oudemans, 2000) and to mummy berry disease incidence in lowbush blueberry (CitationPenman and Annis, 2005). At the Jonesboro field, the blueberry leaf bud development was ahead of the Deblois field by approximately 1 week, but mulch was applied at Jonesboro 1 day before the Deblois field. It is possible that we missed the appropriate time for mulch application at the Jonesboro site and, therefore, some pseudosclerotia germinated and produced spores that infected the plants before the mulch was applied.
In 2007, the only organically acceptable material tested was SerenadeMax, a wettable powder formulation of B. subtilis. Blight incidence was 36% in the control plots at the Deblois field and 22% at the T19 field (data not shown). SerenadeMax-treated plots had 24% disease incidence at the Deblois field and 8% at the T19 field (data not shown), but these differences were not significant at P < 0.05.
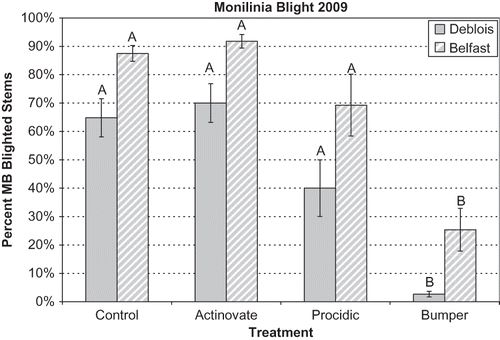
In 2009, mummy berry disease incidence was higher in controls at the Belfast field (87%) than in the Deblois field (64%; ). Of the treatments tested (propiconazole, Actinovate, and Procidic), only the propiconazole (Bumper) treatment had significantly less disease incidence than the controls for both fields (P < 0.05) (). The 2009 spring was very wet, and this produced both high mummy berry disease pressure (9 to 10 infection periods for the fungus) and conditions that could affect the retention of biological materials on plant surfaces. Actinovate had no effect on disease incidence at either field, even though it was applied at the maximum recommended rate and in a more concentrated solution then recommended. The Procidic treatment had lower, but not significant, disease incidence compared to the controls in both fields. We conjectured that with less disease pressure, Procidic might perform better, so it was chosen for further testing in 2010.
FIGURE 3 Incidence of mummy berry blight in Deblois and Belfast fields in 2009. Disease incidence was the percent of diseased stems with blight symptoms. Treatments included Control = untreated, Actinovate = Streptomyces lydicus formulation, Procidic = an extract from citrus, and Bumper = propiconazole. See for application rates. Bars represent standard error of the means; treatments labeled with different letters are significantly different at P < 0.05.
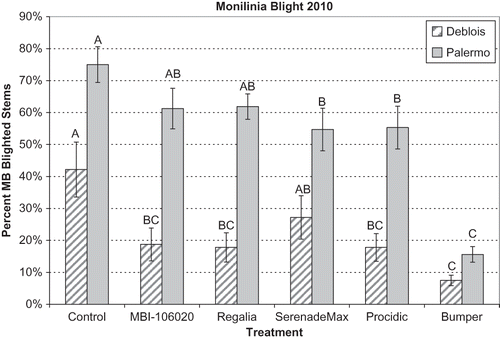
In 2010, the control plots at the Deblois field had 42% disease incidence as compared to 75% at the Palermo field (). Of the treatments tested (propiconazole, Procidic, SerenadeMax, Regalia, and MBI-106020), both propiconazole (Bumper) and Procidic treatments had significantly less disease incidence than the controls in both fields, but the Bumper treatment also had significantly less disease (15%) than the Procidic treatment (55%) in the Palermo field. The other treatments had inconsistent results between the fields. MBI-106020 and Regalia had significantly less disease than the controls in the Deblois field, but not in the Palermo field. Plots treated with SerenadeMax at 2.7× the recommended rate had significantly less disease than controls in the Palermo field, but not in the Deblois field. In the Deblois field with lower disease pressure, MBI-106020, Regalia, and Procidic had similar levels of disease control as propiconazole. The first application of these materials in the Deblois field was at the very beginning of apothecial production and before the first infection period for the fungus, which may have provided better protection than at the Palermo site where apothecia emerged earlier.
FIGURE 4 Incidence of mummy berry blight in Deblois and Palermo fields in 2010. Disease incidence was the percent of diseased stems with blight symptoms. Treatments included Control = untreated, MBI-106020 and Regalia = extracts from giant knotweed, SerenadeMax = a B. subtilis formulation, Procidic = an extract from citrus, and Bumper = propiconazole. See for application rates. Bars represent standard error of the means; treatments labeled with different letters are significantly different at P < 0.05.
Blueberry Yield
There were no significant differences among the treatments in yield for 2005, 2006, and 2007. In 2009, yields were significantly higher for the Bumper (2,707.5 kg/ha) and Procidic (987.6 kg/ha) treatments compared to controls (581.9 kg/ha) in the Belfast field but not in the Deblois field (data not shown). There were no significant differences in harvest yield among any of the 2010 treatments, but the Palermo field had lower overall yields than the Deblois field (data not shown).
CONCLUSIONS
Organic producers often have limited options for disease control, and, therefore, it has been suggested that they consider trying methods that provide even small improvements in disease control (CitationScheuerell and Mahaffee, 2002). There is a need to look beyond only efficacy of a material in decision-making on pesticide inputs to agricultural systems. Organic lowbush blueberry growers can sell a pound of unprocessed blueberries for $0.65 compared to $0.35 for conventional grown unprocessed berries in 2009 (K. McGovern, personal observation). The demand for organic produce has been growing rapidly (CitationThompson, 1998) and is predicted to increase 20% annually (CitationLohr, 2001). Materials that are organically acceptable and produce a small decrease in disease may be cost effective for organic growers but not for conventional growers.
A large problem with products containing living organisms appears to be retention of high enough numbers of organisms on the plant surfaces to be effective. Precipitation between applications can wash off organisms on plant surfaces, but another problem is that the blueberry leaf and flower buds are rapidly developing to maturity during the time that apothecia are present. Although the biological control materials in 2005 and 2006 were applied twice a week, it is likely there was new plant tissue that was unprotected between treatment applications. The only biological control containing a living organism to be effective in controlling mummy berry blight was SerenadeMax, containing B. subtilis, at a high rate of application, but this was only found in one field, Palermo, with high disease pressure in 2010 (). Our findings are consistent with tests of Serenade on highbush and rabbiteye blueberry, where it was inconsistent at decreasing mummy berry blight in trials with varying levels of disease pressure (CitationScherm and Krewer, 2008). SerenadeMax will be further tested and may show improved efficacy when combined with other materials with different modes of action, such as inducers of plant defenses.
The products, Regalia and MBI-106020, consisting of extracts from R. sachalinensis, were effective in decreasing mummy berry blight in the Deblois field with lower disease pressure in 2010 (). This extract induces plant defenses as seen by the accumulation of phenolics and other compounds that are toxic to fungi in treated leaves and by its control of various leaf diseases (CitationDaayf et al., 1995, 2000). Extracts from this plant were effective at reducing powdery mildew on greenhouse tomato, but effectiveness was directly related to application rate, and was diminished when disease pressure was very high or disease symptoms were advanced (CitationKonstantinidou-Doltsinis et al., 2006). Regalia and MBI-106020 may have improved efficacy at higher rates or in combination with other organically acceptable biofungicides with different modes of action, but it will also need to be evaluated in more fields with varying levels of disease pressure to determine its general effectiveness.
The Procidic fungicide, consisting of extracts from citrus, was effective at controlling mummy berry blight at both locations in 2010 (). CitationScherm and Krewer (2008) found Citrex (old name of Procidic) to be effective at reducing the blight and mummy stage of this disease only with certain highbush blueberry cultivars and only when disease pressure was low. At the Deblois field with less disease pressure in 2010, we found Procidic to be statistically as effective as propiconazole, but that was not the case when disease pressure was higher at the Palermo site. In 2009, Procidic showed indications that it may be effective at lowering the incidence of mummy berry blight, but the prolonged and reoccurring rainfall in the spring of 2009 may have washed it from the plants and diminished its effectiveness. CitationVan Eeden and Korsten (2004) suggested that Citrex (Procidic) inhibits the survival of B. subtilis when used in combination with products containing this bacteria to control avocado diseases, and Procidic is promoted as a control for both fungal and bacterial diseases. Procidic will be tested in laboratory studies for its effects on other biological fungicides and will be retested at organically managed blueberry fields with different levels of disease pressure.
Mulch could be a viable cultural control of M. vaccinii-corymbosi in certain situations. It would not be time- or cost-effective to apply mulch to an entire field by hand (K. McGovern, personal observation), but a mechanical manure spreader could be used and may decrease the cost enough to make application feasible. If there is a moisture-retaining low spot in a field or an area with numerous mummified fruit at harvest time, mulching that area in the fall or very early in the spring may be an option for disease control. Other benefits from mulching include increased rhizome development and growth, and decreases in water loss and erosion, all of which can help maintain plant health (CitationDeGomez and Smagula, 1990). The growers and researchers noticed these benefits at the Jonesboro site in 2006, where denser and greener appearing blueberry plants were found in the mulched plots compared to untreated areas. For optimal mummy berry disease control, mulch would need to be applied when all leaf buds are still within their bud scales and are not susceptible to M. vaccinii-corymbosi (CitationHildebrand and Braun, 1991).
Finding the appropriate combinations of biological and cultural controls could potentially decrease the incidence of disease as effectively as chemical fungicides. The cost effectiveness of organically acceptable fungicides in an organic system should also be taken into consideration when determining the effectiveness of a fungicide, since small-scale organic growers often have different economic conditions than conventional growers.
ACKNOWLEDGMENTS
We wish to thank Jennifer D'Appollonio, Tamara Levitsky, Loretta Kreider, Heather Westwood, and Kerry (Lough) Guiseppe for technical support; blueberry land owners and managers; and USDA/Organic Transitions grant, Regional IPM grant, Maine Blueberry Commission, and Marrone Bio Innovations, Inc. for partial research funding.
Notes
Mention of any product or material in this report does not constitute endorsement by the University of Maine, Maine Cooperative Extension, or the Maine Agricultural and Forest Experiment Station.
LITERATURE CITED
- Ayoub , M. and Niazi , A. 2001 . Control of wheat rust by leaves extract of poisonous phanergamic plants . J. Biol. Sci. , 1 : 490 – 491 .
- Batra , L.R. 1983 . Monilinia vaccinii-corymbosi (Sclerotiniaceae): Its biology on blueberry and comparison with related species . Mycologia , 75 : 131 – 152 .
- Bowers , J.H. and Locke , J.C. 2000 . Effect of botanical extracts on the population density of Fusarium oxysporum in soil and control of Fusarium wilt in the greenhouse . Plant Dis. , 84 : 300 – 305 .
- Brunner , K. , Zeilinger , S. , Ciliento , R. , Woo , S.L. , Lorito , M. , Kubicek , C.P. and Mach , R.L. 2005 . Improvement of the fungal biocontrol agent Trichoderma atroviride to enhance both antagonism and the induction of plant systemic disease resistance . Appl. Environ. Microbiol. , 71 : 3959 – 3965 .
- Cao , K. and van Bruggen , A.H.C. 2001 . Inhibitory efficacy of several plant extracts and plant products on Phytophthora infestans . J. Agric. Univ. Hebei , 24 : 90 – 96 .
- Cao, K., S.T. Wang, H.R. Forrer, and P.M. Fried. 2004. Inhibitory effects of Chinese medicinal plants against Phytophthora infestans on potatoes. Regional Workshop on Potato Late Blight for East and Southeast Asia and the Pacific, at Yezin Agricultural University. <http://research.cip.cgiar.org> (<http://research.cip.cgiar.org>) (Accessed: 25 March 2007 ).
- Caruso , F.L. and Ramsdell , D.C. 1995 . Compendium of blueberry and cranberry diseases , St. Paul , MN : (American Pathological Society)APS Press .
- Daayf , F. , Ongena , M. , Boulanger , R. , Hadrami , I.E. and Belanger , R. 2000 . Induction of phenolic compounds in two cultivars of cucumber by treatment of healthy and powdery mildew-infected plants with extracts of Reynoutria sachalinensis . J. Chem. Ecol. , 26 : 1579 – 1593 .
- Daayf , F. , Schmitt , A. and Belanger , R.R. 1995 . The effects of plant extracts of Reynoutria sachalinensis on powdery mildew and leaf physiology of long English cucumber . Plant Dis. , 79 : 577 – 580 .
- DeGomez , T. and Smagula , J.M. 1990 . “ Filling bare spots in blueberry fields ” . In Wild Blueberry Fact Sheet No. 221 , Orono , ME : University of Maine Cooperative Extension . UMCE No. 2057
- Drummond , F. , Smagula , J. , Annis , S. and Yarborough , D. 2008 . “ Organic wild blueberry production ” . In Wild Blueberry Fact Sheet No. 304 , Orono , ME : University of Maine Cooperative Extension . UMCE No. 2029
- Elad , Y. 2000 . Biological control of foliar pathogens by means of Trichoderma harzianum and potential modes of action . Crop Prot. , 19 : 709 – 714 .
- Harman , G.E. , Howell , C.R. , Viterbo , A. , Chet , I. and Lorito , M. 2004 . Trichoderma species—Opportunistic, avirulent plant symbionts . Nature Microbiol. Rev. , 2 : 43 – 56 .
- Higham , J.M. , Cuppels , D.A. and Traquair , J.A. 2006 . Streptomycetous biocontrol agents: Interactions with the tomato plant and tomato fungal pathogens . Phytopathol. , 96 : S48
- Hildebrand , P.D. and Braun , P.G. 1991 . Factors affecting infection of lowbush blueberry by ascospores of Monilinia vaccinii-corymbosi . Can. J. Plant Pathol. , 13 : 232 – 240 .
- Howell , C.R. 2003 . Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts . Plant Dis. , 87 : 4 – 10 .
- Konstantinidou-Doltsinis , S. , Markellou , E. , Kasselaki , A.M. , Fanouraki , M.N. , Koumaki , C.M. , Schmitt , A. , Liopa-Tsakalidis , A. and Malathrakis , N.E. 2006 . Efficacy of Milsana®, a formulated plant extract from Reynoutria sachalinensis, against powdery mildew of tomato (Leveillula taurica). BioControl . 51 : 375 – 392 .
- Lambert , D.H. 1990 . Effects of pruning method on the incidence of mummy berry and other lowbush blueberry diseases . Plant Dis. , 74 : 199 – 201 .
- Langdon , D. , Hildebrand , P.D. , Traquair , J.A. and Boland , G.J. 2009 . Efficacy of the biological control agent SerenadeMax against Monilinia vaccinii-corymbosi in lowbush blueberry . Can. J. Plant Pathol. , 31 : 124
- Lehman , J.S. and Oudemans , P.V. 2000 . Variation and heritability of phenology in the fungus Monilinia vaccinii-corymbosi on blueberry . Phytopathol. , 90 : 390 – 395 .
- Litterick , A.M. , Harrier , L. , Wallace , P. , Watson , C.A. and Wood , M. 2004 . The role of uncomposted materials, composts, manures, and compost extracts in reducing pest and disease incidence and severity in sustainable temperate agricultural and horticultural crop production—A review . Crit. Rev. Plant Sci. , 23 : 453 – 479 .
- Lohr, L. 2001. Factors affecting international demand and trade in organic food products. In: A. Regmi (ed.). Changing structure of global food consumption and trade. Economic Research Service/USDA, 2001 [cited Dec. 2006]. www.ers.usda.govproducts (http://www.ers.usda.govproducts) (Accessed: 2 December 2006 ).
- Lu , C.G. , Liu , W.C. , Qiu , J.Y. , Wang , H.M. , Liu , T. and Liu , D.W. 2008 . Identification of an antifungal metabolite produced by a potential biocontrol Actinomyces strain A01 . Brazilian J. Microbiol. , 39 : 701 – 707 .
- McKeen , C.D. , Reilly , C.C. and Pusey , P.L. 1986 . Production and partial characterization of antifungal substances antagonistic to Monilinia fructicola from Bacillus subtilis. Phytopathol . 76 : 136 – 139 .
- McSpadden Gardener , B.B. and Fravel , D.R. 2002 . Biological control of plant pathogens: Research, commercialization, and application in the USA . Online. Plant Health Progress , doi: 10.1094/PHP-2002-0510-01-RV
- Moline , H.E. and Locke , J.C. 1993 . Comparing neem seed oil with calcium chloride and fungicides for controlling post-harvest apple decay . HortScience , 28 : 719 – 720 .
- Ngugi , H.K. , Scherm , H. and NeSmith , D.S. 2002 . Distribution of pseudosclerotia of Monilinia vaccinii-corymbosi and risk of apothecial emergence following mechanical cultivation . Phytopathol. , 92 : 877 – 883 .
- Penman , L.N. and Annis , S.L. 2005 . Leaf and flower blight caused by Monilinia vaccinii-corymbosi on lowbush blueberry: effects of yield and relationship to bud phenology . Phytopathol. , 95 : 1174 – 1182 .
- Scherm , H. and Krewer , G. 2008 . Disease management in organic rabbiteye blueberries . Int. J. Fruit Sci. , 8 : 69 – 80 .
- Scherm , H. , Ngugi , H.K. , Savelle , A.T. and Edwards , J.R. 2004 . Biological control of infection of blueberry flowers caused by Monilinia vaccinii-corymbosi . Biol. Control , 29 : 199 – 206 .
- Scheuerell , S.J. and Mahaffee , W.F. 2002 . Compost tea: Principles and prospects for plant disease control . Compost Sci. Utiliz. , 10 : 313 – 338 .
- Shinners , T.C. and Olson , A.R. 1996 . The gynoecial infection pathway of Monilinia vaccinii-corymbosi in lowbush blueberry (Vaccinium angustifolium) . Can. J. Plant Sci. , 76 : 493 – 497 .
- Thompson , G.D. 1998 . Consumer demand for organic foods: What we know and what we need to know . Amer. J. Agric. Econ. , 80 : 1113 – 1118 .
- Travis, J.W., M. Saunders, B. Hed, J. Timer, and A. Muza. 2006. Integrated disease and insect management in organic grape production systems. <http://agsci.psu.edu/research/> (<http://agsci.psu.edu/research/>) (Accessed: 25 March 2007 ).
- Van Eeden , M. and Korsten , L. 2004 . Effect of additives and copper fungicide on Bacillus subtilis to control avocado (Persea Americana Mill.) fruit diseases . South African Avocado Growers' Association Yearbook , 27 : 11 – 16 .
- Yarborough , D.E. 2004 . “ Cultural management for insects and diseases in wild blueberries ” . In Wild Blueberry Fact Sheet No.253 , Orono , ME : University of Maine Cooperative Extension .
- Weltzien , H.C. 1991 . “ Biocontrol of foliar fungal diseases with compost extracts ” . In Microbial ecology of leaves , Edited by: Andrews , J.H. and Harino , S.S. 430 – 450 . New York , NY : Springer-Verlag .
- Wulff , E.G. , Mguni , C.M. , Mansfeld-Giese , K. , Fels , J. , Lubeck , M. and Hockenhull , J. 2002 . Biochemical and molecular characterization of Bacillus amyloliquefaciens, B. subtilis, and B. pumilus isolates with distinct antagonistic potential against Xanthomonas campestris . Plant Pathol. , 51 : 574 – 584 .