Abstract
During the past few years, white grubs have become recognized as a pest of southern highbush blueberries in California. White grubs feed on plant roots causing the plant to be stunted. In some cases, plant death has occurred when large grub populations attack newly planted fields. The predominant white grub species in California blueberries was identified as Cyclocephala longula. Research on flight characteristics determined that grubs are primarily in the third instar in April, pupate in May, and fly from mid-June through mid-July. Egg hatch begins in mid-July. Adult beetles begin flying about 30 min after dark and can be collected for a period of about 2 hours with black-light traps. Evaluation of control methods found that the entomopathogenic nematode Heterorhabditis bacteriophora and the insecticide imidacloprid can both provide control of the grub. Applications of Heterorhabditis bacteriophora on April 1 initially only provided 8.3% control, but resulted in secondary spread that led to an epizootic within the grub population. Applications of Heterorhabditis bacteriophora and imidacloprid in August resulted in 81.6 and 71.1% control, respectively, the following June.
INTRODUCTION
The white grub larval stages of Cyclocephala spp. are recognized throughout much of the world as pests of turfgrass (CitationPotter, 1998). Within turf and other grassy areas, the grubs thrive in an environment containing shallow roots, high organic matter, and high moisture. Until recently in California, white grubs were rarely considered pests outside of turf. However, with the introduction of southern highbush blueberry varieties, Vaccinium corymbosum L. x V. darrowi Camp, blueberry production in California has been made possible (CitationJimenez et al., 2005; CitationStrik and Yarborough, 2005). Since the late 1990s and early 2000s, expansion of blueberry production into California has allowed white grubs to expand their host range and status as a pest.
Southern highbush blueberries in California provide an excellent host environment for white grubs. Blueberries are similar to turf in that they are shallow-rooted, and planted into soil that is maintained at a high level of moisture and amended with high amounts of compost and other organic matter. Most fields are heavily amended prior to planting blueberries to reduce soil pH to a level conducive to blueberry production. During and after planting, drip irrigation is typically placed along the top of the amended soil, after which several centimeters of mulch are maintained from year to year. This system of drip irrigation within a mulch layer on top of amended soil provides an environment analogous to the thatch layer found in turf where the grubs typically reside.
Most plantings of southern highbush blueberries in California have been planted since the late 1990s (CitationStrik and Yarborough, 2005). Through the first several years of production, there was little to no recognition of grubs as a blueberry pest. However, this has changed since about 2007. The first to recognize the problem were growers who planted new blueberry fields adjacent to mature fields. What appears to have happened is that grub populations slowly built up over several years in the initial blueberry plantings. However, increased plant size as populations grew, coupled with the lack of below-ground inspections by growers, caused the problem to go undetected.
When new plantings of blueberries were being planned in adjacent fields, high amounts of compost and other organic matter were incorporated into fields that were pre-irrigated during the period of time that we now know to be the adult flight. Volatiles coming from freshly-hydrated compost in the new fields were highly attractive to the adult beetles that laid their eggs in the field such that small grubs were present in high numbers prior to planting. Then, once plants were in the ground, grubs migrated to the roots of the new plants and fed on the limited root systems, often times causing plant death. Because of this experience by multiple blueberry growers, grubs were initially recognized as a pest of young plants. However, now that an increasing number of blueberry fields are becoming mature, grubs have been documented to also cause significant stunting of mature plants. In some commercial blueberry fields, the density of grubs has been found to exceed several dozen to over one hundred grubs per plant.
In 2007, we began our efforts to identify the grub pest attacking California blueberries, particularly in the lower San Joaquin Valley. Efforts to rear out grubs into adult beetles, coupled with black-light trapping efforts, led us to identify Cyclocephala longula LeConte as the predominant white grub species. This was based on the dissection of the aedeagi from 100 adult males from June 15 and June 29, 2009 using the key written by CitationSaylor (1945). Cyclocephala longula was reported by Saylor as being a widely distributed species, known from Oregon, Arizona, Lower California, Utah, and extremely common throughout California.
Once the white grubs were identified as a pest of blueberries in California, we began our efforts to determine how they could be controlled. Initial efforts focused on grub biology, particularly to determine seasonal differences in developmental stages present in the field. We also used black-light traps to better understand characteristics of the June beetle flight. Lastly, we did two trials, one of which was observational and one of which was replicated to evaluate the potential role of entomopathogenic nematodes and insecticides as part of a control program.
MATERIALS AND METHODS
Grub Biology
Seasonal biology of the grubs was evaluated using black-light traps coupled with periodic sampling in the soil. A black-light bucket trap (Universal Black Light Trap, Bioquip Products, Rancho Dominguez, CA, USA) was placed in a commercial blueberry field in Mettler, Kern Co., California from May 13 to July 12, 2007 and was evaluated weekly for the number of adult beetles caught. In 2009, black-light traps were placed in two different commercial blueberry fields on June 2 approximately 8 km apart near Righgrove, Tulare Co., California and were evaluated weekly for the number of adult beetles caught through August 11. Data from all three trials were evaluated by plotting the data on a chart to visualize flight characteristics.
On the evening of June 15, 2009, additional data were collected to determine the period of time each day that flights took place. Four black-light traps were placed within four different adjacent 20 ha blueberry fields. Traps were turned on at 20:30 hr and were evaluated for the number of adult beetles caught at 30-min intervals until activity stopped. Data were analyzed by plotting the mean ± SEM of the number of beetles caught during each 30-min period.
Collections of grubs and other life stages in the soil took place on May 6, June 2, and July 13, 2009 at one of the Richgrove sites to help validate the stages of grubs present in the soil prior to and after the adult beetle flight. On each of these dates, hand trowels were used to dig around the blueberry plants until at least 50 individuals were found. These observational data were converted into percentage of Cyclocephala by developmental stage to assist in understanding pest biology as well as estimate the most effective treatment timings after the flight was over.
Grub Management
During 2009, we conducted two trials to evaluate management options for blueberry growers. In the first experiment, we made observations of the effectiveness of a commercial application of the entomopathogenic nematode, Heterorhabditis bacteriophora Poinar, in a 40 ha commercial blueberry field. Prior to the application, a total of 36 healthy third-instar grubs were collected from the field and placed with soil into six 7.6 cm × 7.6 cm × 20.3 cm long cylinders made out of 3.3-mm wire mesh. Three of these cages were placed at one site towards the head end of a random irrigation drip line and the other three were placed at a second site near the tail end of the line. At each site, one cage of sentinel grubs was placed about 15 cm deep at locations that were about 5, 25, and 50 cm perpendicular to the drip line.
TerranemTM (Koppert Biological Systems, Inc., Romulus, MI, USA) was applied to the entire field through the commercial injection system on April 1, 2009 at a rate of 1 billion infective juveniles per treated ha, which was calculated as the 1-m wide top of the berm on a field with a 3.3-m row spacing. This timing coincided with soil temperatures of approximately 15°C, which is the approximate minimum temperature threshold for when the product label states that the infective juveniles can maintain activity. Approximately 1 month after application, on May 6, the sentinel grubs were excavated from the field and evaluated for the presence or absence of Heterorhabditis bacteriophora. Additional sampling was conducted on June 2, July 13, and August 6. On each evaluation date, grubs and other life stages were collected from random locations within the treated field. Individuals were recorded by life stage, evaluated to determine if they were alive or dead, and were dissected to determine the presence of entomopathogenic nematodes. Data were summarized to determine if nematode treatments had successfully started a self-perpetuating epizootic in the field that warranted further evaluation in a replicated field trial.
The second trial to evaluate management options for growers was conducted to determine the effects of imidacloprid (Admire® Pro, Bayer CropScience LP, Research Triangle Park, NC, USA) and TerranemTM on grub populations compared to an untreated check. Twelve rows (= 0.87 ha) of a commercial blueberry field in Mettler, Kern Co., California were organized into a randomized complete block design of four blocks of two treatments and an untreated check. Each plot was 200 m long by one row wide and contained approximately 220 blueberry plants. The first treatment was Admire® Pro at a rate of 167 ml of product per treated ha. Applications were made by the grower cooperator on August 13, 2009 by injection through the drip system. The drip system was composed of two drip lines along the top of the berm, with one line on each side of the blueberry bushes. Emitters on each line were spaced at 0.41 m apart with a flow rate of 0.95 l per hr per emitter. Applications included a 24-hour pre-irrigation, followed by 84 ml of product, followed by 20 more hours of irrigation, followed by the other 84 ml of product. The second treatment was TerranemTM at a rate of 1 billion Heterorhabditis bacteriophora infective juveniles per treated ha within the 1-m band down the top of the berm. Applications were made on August 13, 2009 by splicing into the drip lines of each row to be treated and injecting the nematodes using an Air Driven Diaphragm Pump (Model G575205, Flojet-ITT, Foothill Ranch, CA, USA) hooked up to a pressurized CO2 tank. To do this, the TerranemTM product for the treated area of one row (= 245 sq m) was mixed with 15 l of water and injected over a period of approximately 20 min following a 12-hr preirrigation and followed by a 12-hr postirrigation.
The effects of treatments were evaluated the following spring on June 10, 2010 by determining grub density around blueberry roots. In each plot, shovels and trowels were used to excavate soil from areas approximately 0.6 sq m in size. This was done by excavating soil from a rectangular sampling area bordered on the ends by the trunks of two plants and on the sides by the center line of the row and another that ran approximately 60 cm perpendicular to the center of the berm. Twenty excavations of this type were done per each plot and the total number of grubs found was recorded. Data were organized as total grubs per 20 excavations and are reported as average number of grubs per 10 plants. Calculations were also made of the percentage of excavations positive for grubs. All data were analyzed by ANOVA with means separated by Fisher's Protected LSD (α = 0.05) (SAS CitationInstitute, 1999).
RESULTS AND DISCUSSION
Grub Biology
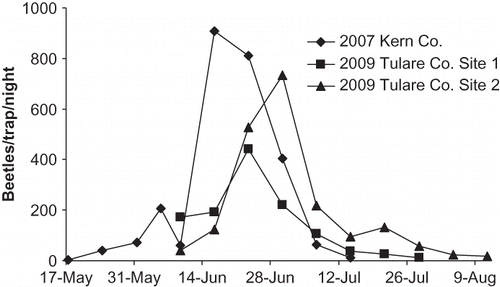
Black-light traps at the Kern Co., Tulare Co. site 1, and Tulare Co. site 2 locations caught 16,450, 7,504, and 13,324 beetles, respectively (). In all three cases, beetle flight began approximately 1 week into June, peaked during the last 2 weeks of June, dropped off by mid-July and was over by the first of August. The greatest numbers of catches, constituting 84.6, 85.2, and 72.4% of all adults, respectively, were collected during a 3-week period from June 9 to June 30. Subsamples of 50 adult male beetles per trap were collected on June 15 and July 21 2009 from each of the two Tulare Co. sites and were found to be greater than 97% Cyclocephala longula.
FIGURE 1 Per night beetle catches in black-light traps at three sites in the lower San Joaquin Valley.
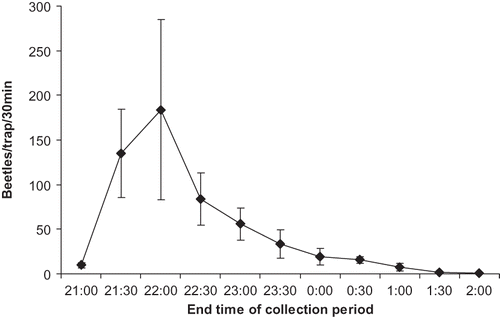
Trapping data from June 15, 2009 documented that beetles begin flying about one half hour after sunset, which occurred at 20:31 hr on that date (). Very few beetles were collected prior to the 20:30 to 21:00 hr collection period, after which high numbers of beetles comprising 24.7% and 33.6% of the total beetles collected were captured from 21:00 to 21:30 hr and 21:30 to 22:00 hr, respectively. Only 8.3% of the beetles were collected after 21:30 hr.
FIGURE 2 Beetles collected during 30-min periods from 20:00 to 01:30 hr on June 15, 2009 in Richgrove, CA.
Field collections of life stages in the soil prior to and just after the flight were consistent with what would be expected based on the flight period. On May 6, a total of 82 Cyclocephala were collected with 70.7% still third-instar grubs and 29.3% pupae. By June 2, approximately 1 week before the flight began, a total of 75 Cyclocephala were collected, of which 85.3% were teneral and other young adults that had not yet left their pupal chamber, 4.0% were pupae, and 10.7% were still grubs. Observations noted that many of the grubs were becoming a lighter color indicating that they were about to pupate. By July 13 as the flight was concluding, a total of 88 Cyclocephala were collected, comprised of 14.7% adults, 22.7% eggs, 55.7% first-instar grubs, and 6.8% second-instar grubs.
Grub Management
Evaluations of the April 1 commercial application of TerranemTM documented that the entomopathogenic nematodes were successfully applied through the irrigation system and that they were able to attack grubs in the soil. On May 6, approximately 1 month after application, the 36 sentinel grubs located within cages in the soil were excavated. Of these, 33% were found to be sick or dying. Dissection of these 12 grubs found that three contained thousands of Heterorhabditis bacteriophora, whereas the other 9 were dying of causes that were not due to entomopathogenic nematodes or could not be confirmed as such. Despite the relatively low mortality rate confirmed to be caused by nematodes (8.3% of all sentinel grubs), these data indicated that the entomopathogenic nematodes had become established in the soil.
On June 2, approximately 2 months after the application, the second evaluation was conducted. Based on an estimated 2-week period required for the new generation of infective juvenile Heterorhabditis bacteriophora to move from host to host, the presence of the nematodes within any hosts would confirm that the nematodes could reproduce and cycle through the white grubs. During this evaluation, a total of 135 Cyclocephala were excavated from the soil. Of these, 68 were grubs, 3 were pupae, and 64 were adults. Out of the 68 grubs found, only 8 were alive while the remaining 60 (88.2%) expressed typical signs of death by Heterorhabditis bacteriophora. Dissection of these 60 grubs resulted in positive recognition of entomopathogenic nematodes in 49 of them, while the remaining 11 had been dead for an extended period of time such that they expressed signs of death by Heterorhabditis bacteriophora, but the nematodes had emerged from the hosts. Of the pupae evaluated, all appeared healthy. Of the 64 adults found, 37 were alive and 27 were dead. Upon dissection, 16 of the dead adults (59.2%) were found to be infested with entomopathogenic nematodes. On July 13, after the flight was over, additional observations were made to determine if Heterorhabditis bacteriophora was able to continue to the next generation of grubs. A total of 49 first-instar and 6 second-instar grubs were collected from the soil. Of these, 12.3 and 16.7%, respectively, contained entomopathogenic nematodes. On August 6, no grubs were found after 3 hr of searching.
These data, though unreplicated and only observational in nature, suggested that applications of Heterorhabditis bacteriophora caused an epizootic within grub populations below the soil. This information, coupled with dozens of observations from untreated fields that entomopathogenic nematode populations are negligible in fields where they have not been introduced, confirmed the potential for nematode treatments as a viable control strategy for farmers.
In mid-August 2009, the Kern Co. replicated field trial was established to further evaluate nematode treatments, as well as the use of the commercial insecticide, Admire® Pro. At the time of treatment, grubs were primarily in the first, and sometimes second, instar of development. Data from these plots on June 10 the following year confirmed the value of nematode, as well as insecticide, treatments (). Plots treated with Admire® Pro and TerranemTM had significant reductions of grub populations at 5.5 ± 1.9 and 3.5 ± 1.3 grubs per ten plants, respectively, compared to 19.0 ± 4.3 for the untreated check (F = 27.6; df = 2,6; P = 0.0008). Similar results were seen in the average percentage of excavations with grubs (F = 17.69, df = 2,6; P = 0.0030).
TABLE 1 The Effects of Fall 2009 Treatments on Grubs Density in June 2010
CONCLUSIONS
The series of research projects described within this article generated the basic information necessary to help growers develop in-field management programs for white grubs in California blueberry fields. Surveys of beetle stages present in the ground coupled with black-light trap catches documented that white grubs are in the third-instar grub stage in April, transition to pupae through the month of May, are adults from mid-June through mid-July, and produce eggs that hatch by early August. Data also showed that adults begin flying about 30 min after sunset and can be collected for a period of about 2–2½ hr.
Based on this information, management options that target adult beetles, such as foliar applications of broad-spectrum insecticides, are unlikely to be effective. Applications would need to be used during the second half of June and would be best applied during the period from 30 min to 2–2½ hr after sunset. However, observations showed that adult beetles do not feed and are primarily in search of mates. Once a mate is found, paired-off adult beetles can be seen digging themselves back into the soil where they would no longer be exposed to foliar applications of insecticides. This leaves a very short window of opportunity for control. This is a very different situation than what occurs on the East Coast where the adult stage of the predominant grub species, the oriental beetle, Anomala orientalis Waterhouse, remains above ground to feed on foliage and fruit where they can be exposed directly to insecticide applications and have the potential to eat pesticide residues on treated plant surfaces (CitationPolavarapu, 1996).
Treatments of the entomopathogenic nematode Heterorhabditis bacteriophora were documented in this project to be an effective tool in management programs for Cyclocephala longula. This was true when applications were made against third-instar larvae in April once soil temperatures reached 15°C, as well as in August when new larvae hatched. This is in contrast to the results of CitationPolavarapu et al. (2007) that showed a lack of effectiveness of this entomopathogenic species of nematode against third-instar larvae of the oriental beetle, Anomala orientalis. Data also showed that, over time, nematode applications in April that initially only controlled a small percentage of the white grubs led to an epizootic within the grub population as secondary, tertiary, and additional spread occurred within the blueberry field. This information is particularly useful for organic blueberry growers that have limited options regarding control with chemical insecticides.
Insecticide treatments with imidacloprid were also documented to be effective against grubs when applied in August to California blueberries. This is consistent with recommendations in turf that imidacloprid applications are most effective if made when grubs are still in early-instar stages (CitationFlint et al., 2009). This timing would also not be detrimental to any Tiphia sp. parasitoids that are found in California (CitationFlint et al., 2009) and that may or may not begin to provide biological control of grubs in commercial blueberries as they do in other hosts (CitationRogers and Potter, 2003). Growers choosing to use imidacloprid as a control method should use a monitoring program of black-light traps and soil excavations to determine when eggs are being laid and are starting to hatch. Then, as the eggs hatch, treatments should be made, likely some time from late July through mid-August. Growers might also consider trying combination treatments of imidacloprid as a synergist that may or may not make entomopathogenic nematodes more effective (CitationKoppenhöfer et al., 2002, Citation2003).
Combining all of this information, California blueberry growers should have the basic tools to successfully monitor for white grubs within their fields and should be able to successfully reduce pest populations to levels below those that will cause economic damage.
ACKNOWLEDGEMENTS
We wish to thank grower-cooperators John Ojalla, Misty Rex, and Bruce Frost, for their assistance in providing field locations and in-kind donations that made this project possible. We also wish to thank Dr. Harry Kaya and Dr. Edwin Lewis for providing advice that focused this project in a positive direction that could quickly provide critical information that would lead to a management program for blueberry farmers.
LITERATURE CITED
- Flint , M.L. , Harivandi , M.A. and Kaya , H.K. 2009 . “ Insects and mites ” . In UC IPM pest management guidelines: Turfgrass , Univ. of Calif. Agric. and Natural Resources Publ. 3464-T .
- Jimenez , M. , Carpenter , F. , Molinar , R.H. , Wright , K. and Day , K.R. 2005 . Blueberry research launches exciting new California specialty crop . Calif. Agric. , 59 ( 2 ) : 65 – 69 .
- Koppenhöfer , A.M. , Cowles , R.S. , Cowles , E.A. , Fuzy , E.M. and Baumgartner , L. 2002 . Comparison of neonicotinoids insecticides as synergists for entomopathogenic nematodes . Biol. Control , 24 : 90 – 97 .
- Koppenhöfer , A.M. , Cowles , R.S. , Cowles , E.A. , Fuzy , E.M. and Kaya , H.K. 2003 . Effect of neonicotinoids synergists on entomopathogenic nematode fitness . Entomol. Exp. Appl. , 106 ( 1 ) : 7 – 18 .
- Polavarapu , S. 1996 . Species composition of scarab grubs and seasonal life-history of oriental beetle in blueberries . Hort. News , 76 : 8 – 11 .
- Polavarapu , S. , Koppenhöfer , A.M. , Barry , J.D. , Holdcraftr , R.J. and Fuzy , E.M. 2007 . Entomopathogenic nematodes and neonicotinoids for remedial control of oriental beetle, Anomala orientalis (Coleoptera: Scarabaeidae), in highbush blueberry . Crop Protec. , 26 : 1266 – 1271 .
- Potter , D.A. 1998 . Destructive turfgrass insects. Biology, diagnosis and control , Chelsea , MI : Ann Arbor Press .
- Rogers , M.E. and Potter , D.A. 2003 . Effects of spring imidacloprid application for white grub control on parasitism of Japanese beetle (Coleoptera: Scarabaeidae) by Tiphia vernalis (Hymenoptera: Tiphiidae) . J. Econ. Entomol. , 96 ( 5 ) : 1412 – 1419 .
- Institute , SAS . 1999 . User's manual, Version 8.0 , Cary , NC : SAS Institute .
- Saylor , L.W. 1945 . Synoptic revision of the United States scarab beetles of the subfamily Dynastinae, No. 1: Tribe Cyclocephalini . J. Washington Acad. Sci. , 35 ( 12 ) : 378 – 386 .
- Strik , B.C. and Yarborough , D. 2005 . Blueberry production trends in North America, 1992–2003, and predictions for growth . HortTechnology , 15 ( 2 ) : 391 – 398 .