Abstract
Different timings and methods of N application modify N dynamics in the plant and could have differential effects on fruit color. To evaluate this possibility, an experiment was conducted during three seasons in a ‘Royal Gala’/EM9 apple orchard. The treatments were: (a) soil N after harvest; (b) foliar N after harvest; and (c) soil N in spring. No nitrogen was applied to the control. HUE angle, lightness, percentage of coverage color, and anthocyanin concentration were determined in the fruit skin. Among the treatments that received fertilization, the postharvest treatments resulted in fruits with darker colorations, higher percentages of coverage color, and higher concentration of anthocyanins. These could be related, at least partly, to lower values of light interception by the canopy, in comparison to the spring treatment. Our results suggest that the negative effects of N on fruit coloration can be mitigated by differing N application to the postharvest period.
INTRODUCTION
In apples, fruit coloration, together with size, is one of the most important quality factors determining the final destination of the fruit production and consumer acceptance. Many factors, including genotype, radiation, temperature, mineral nutrition, pruning thinning, and the application of growth regulators, regulate the red color development in apples (CitationSaure, 1990; CitationLancaster, 1992; CitationCrisosto et al., 1997; CitationRitenour and Khemira, 2007). Nitrogen is the most important mineral nutrient affecting red coloration in apples. It has been reported that high levels of this nutrient in the tree are associated to a reduction in the production of anthocyanins and, consequently, in the development of the red coloration of fruits (CitationAwad and de Jager, 2002; CitationWargo et al., 2003; CitationRacskó et al., 2005; CitationNeilsen et al., 2006; CitationNava et al., 2008). Several authors suggested that the effect of N can be either direct through contribution for precursors for the synthesis of certain proteins, or indirect through the shading caused by the increase in the foliage in response to fertilization (CitationFaust, 1965; CitationUbi, 2004; CitationRacskó et al., 2005; CitationRitenour and Khemira, 2007).
In commercial orchards, annual nitrogen application is needed to ensure high levels of production year after year. These fertilizations can be performed in different times of the year by different methods (foliar or soil applications). It has been reported that the time and method of nitrogen fertilizer application can affect both plant growth and production of the following year and it can also affect the different forms of storage and nitrogen mobilization in the tree (CitationTagliavini et al., 1997; CitationQuartieri et al., 2002; CitationCheng et al., 2004; CitationNeilsen et al., 2006). In late-summer and early-autumn N applications, nitrogen is diverted mainly to the storage organs and increases the nitrogen reserves available for the initial growth of leaves, flowers, and fruits during early spring (CitationSánchez et al., 1990a, Citation1990b, Citation1991; CitationTagliavini et al., 1999; CitationToselli et al., 2000; CitationFallahi et al., 2002; CitationDong et al., 2005). Generally speaking, late N applications do not promote excess of vigor and high N status in the fruits because the partitioning of the N reserve is similar to the spur leaves, shoots, and fruits (CitationSánchez et al., 1990b). A foliar N application, such as urea, also increases reserve N, but the allocation of the N is mainly in the aerial part of the tree (CitationForshey, 1963; CitationSánchez et al., 1990a; CitationFallahi et al., 2002). On the other hand, soil N application at that time is partitioned preferably to the roots. Conversely, in early-spring N applications, N is partitioned to the vegetative organs and fruits (Sánchez, et al., 1990; CitationFallahi et al., 2002; CitationCheng et al., 2004; CitationDong et al., 2005). This differentiated allocation of N promotes vegetative growth that may shade the inner part of the canopy (CitationBarritt et al., 1991) and increase N concentration in fruits that can negatively affect the quality, such as less surface color, more susceptibility to physiological disorders, and lower conservation capacity during storage (CitationFaust, 1989; CitationWargo et al., 2003; CitationRacskó et al., 2005).
Because different timings and N application methods differentially affect the dynamics of this nutrient in the plant, it is possible that the negative effects of the N on the coverage color of the fruit can also vary with the timing and method of the fertilizer application. Although these possible effects are very important for some of the main quality attributes of the fruits, no data has been reported yet. The aim of this work was to assess the effect of the timing and method of nitrogen fertilization in apple cv ‘Royal Gala’ over the concentration of anthocyanins and the skin cover coloration of the fruits.
MATERIALS AND METHODS
Experimental Site and Orchard Management
The study was conducted in an apple orchard of cv ‘Royal Gala’ (central leader, 4 × 1.25 m, 6 years old) located in the Agricultural Experiment Station INTA Alto Valle de Rio Negro, Argentina (latitude 39° 01′ S; longitude 67° 40′ O), during 3 seasons: 2006/2007; 2007/2008, and 2008/2009.
The cultural practices were the standard for commercial orchards in the region. ‘Royal Gala’ trees were irrigated by flooding with irrigation at 15–20 day intervals during the spring and summer and every month from harvest through late April. A chemical thinning was performed in October (fruits of 5 mm diameter). In early November, the trees were hand-thinned leaving one fruit per spur. Harvest took place from January 20 to January 31 in all seasons.
Fertilization Treatments
The treatments were: (1) SNAH: soil ammonium nitrate (100 kg N ha−1) application after harvest in a single dose; (2) FNAH: foliar urea application 4% w/v after harvest; (3) SNS: soil ammonium nitrate (100 kg N ha−1) application in spring in two equal doses. In the 2007/2008 and 2008/2009 seasons, foliar urea application was divided into two doses of 2% W/V each because in the first season a slight necrosis was observed on the edges of the leaves. Treatments were always applied to the same trees throughout the study. Postharvest treatments were applied in mid-February. Spring applications were performed at full bloom and 30 days after full bloom.
Skin Coverage Color
At harvest time, the skin coverage color was determined in 60 fruits per treatment using a portable tristimulus colorimeter (Minolta CR-400 Co, Osaka, Japan). The fruits were randomly sampled from the middle portion of the canopy in the four quadrants. Chromaticity was recorded in Commission Internationale de l'Eclairage (CIELAB) L*, a*, and b* color space coordinates. The HUE angle was calculated as tan−1 b*/a* (in degrees). L* represents the relative lightness of colors, showing low values for dark colors and high values for light colors (CitationDussi et al., 1995; CitationDussi et al., 1997). The measurements were performed on the red surface of the fruit. In the 2007/2008 and 2008/2009 seasons, a visual estimation was conducted to determine the percentage of the red surface over the fruit on the sun-exposed and shaded surfaces. The final percentage corresponds to the average of both calculations.
Anthocyanin Concentration Determination
Immediately after harvest, 30 fruits per treatment were selected at random. The samples were washed with distilled water and then they were completely peeled. The skin of each sample was immediately frozen using liquid nitrogen and then kept at −80°C (CitationAwad and de Jager, 2000) before being analyzed according to the method described by CitationWhale and Singh (2007) with modifications. Briefly, 1 g of peel per fruit was shattered and blended with liquid nitrogen. The powdered sample was mixed with 10 ml of aqueous methanol (95%)–1% HCL. The mixture was allowed to rest in the darkness at 4°C during the night. After decantation, the solution was centrifuged at 3000 rpm (centrifuge Rolco, model 2070, rotor C440 Rolco SRL, Buenos Aires, Argentina) for 30 min. The absorbance of the supernatant was measured in a Milton Roy (Spectronic 1201) spectrophotometer (Milton Roy, Ivyland, PA, USA) set at a wavelength of 530 nm, and the anthocyanin concentration was calculated using the formula proposed by CitationWrolstad (1993) and CitationWrolstad et al. (2005):
-
A = Absorbance at 530 nm;
-
MW = Molecular weight of 3-cyanidin galactoside (= 445.2; it does not include the chloride ion or crystallization water);
-
DF = Dilution factor;
-
ϵ = Molar extinction coefficient, L × mol−1 × cm−1 = 30,200 (for the solvent 1% HCL MeOH);
-
l = Pathlength.
Light Interception
In the first season (2006/2007), photosynthetically active radiation (PAR) was recorded using a PAR Ceptometer (Cavadevices, Buenos Aires, Argentina) every 15 days, from December 18 to January 30 (after harvest) between 12:30 and 13:30 under completely clear skies (CitationWünsche and Lakso, 2000). PAR interception was determined under each tree (0–25 m above floor level), performed from the tree trunk (0 cm) every 0.25 m along 1.50 m at both sides of the trees (North-South). Each reading was compared to the above canopy reading in order to express the result as a percentage of the available sunlight. Four replicates per treatment were carried out. In the second season only one measurement was performed with six replicates per treatment before harvest (up to 0.75 m). Readings were recorded on January 28, 2008.
Vegetative Growth
In July 2009, the length of the current-year shoots was measured (representing the growth during the 2008/2009 season) in each sampled plant.
Climatic Conditions
For the three seasons, during the month prior to harvest, temperature and radiation were recorded. Data were obtained from a meteorological station located 300 m from the experimental site. Mean daily maximum temperature, mean daily minimum temperature, and mean daily maximum global radiation for the month prior to harvest in the three seasons are presented in . All values were among the typical range for the region (CitationCordon et al., 2000).
TABLE 1 Mean Daily Maximum Temperature, Mean Daily Minimum Temperature, and Mean Daily Maximum Global Radiation during a 30-Day Period Prior to Harvest for the 2006/2007, 2007/2008, and 2008/2009 Seasons
Experimental Design and Data Analysis
The experimental design was completely randomized with six replicates. In all of the cases, three contiguous plants were treated, taking as the experimental unit the central one and the two adjacent as borders. The treatments were randomly distributed among the selected plants. A one-way variance analysis with a significance level of 5% (α = 0.05) was used to analyze data on coloration and anthocyanin concentration. The Tukey test was used to compare means in the cases where differences were detected.
RESULTS
Lightness and HUE Angle
In the 2006/2007 season, no significant differences among treatments were found for fruit color (). However, fruits from the spring treatment tended to show the highest HUE angle values (lighter red colorations), while the lowest values corresponded to the control (redder colorations).
Table 2 Lightness and HUE Angle Mean Values ± SE of Fruits Apple Trees of cv ‘Royal Gala’ under Different Fertilization Treatments in 2006/2007, 2007/2008, and 2008/2009 Seasons
In the 2007/2008 season, significant differences were observed in the lightness of fruits among FNAH and SNS treatments (), with the lowest values for FNAH treatment and consequently the darkest fruits. Significant differences were also found in HUE angle values among the fruits from SNAH and FNAH treatments () compared to the fruits from the control. Postharvest treatments showed lower values, indicating redder colorations. The fruits from the spring treatment showed a high variability in the values of this parameter.
In the 2008/2009 season, significant differences were observed in the lightness between the spring treatment and the control (); the lowest value for C treatment indicates the darkest coloration. The postharvest treatments showed an intermediate condition, while the highest values and, consequently, the clearest coloration were observed in the spring treatment. Differences were also found in HUE angle values among treatments (). Fruits from the spring treatment showed the highest HUE values and consequently the least red coloration, while the reddest coloration (lowest HUE value) was found in the control. Once again, it can be observed that the postharvest treatments showed an intermediate condition between the spring treatment and the control.
Percentage of Coverage Color
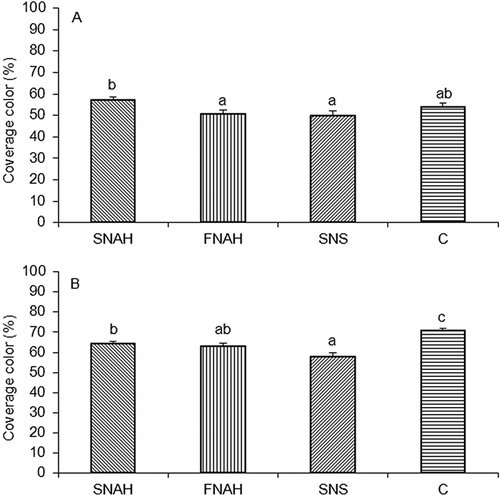
Significant differences in the percentage of coverage color were observed in the two analyzed seasons for this parameter (A and B). In the 2007/2008 season, fruits from SNAH treatment showed the highest percentage, while the lowest value was found in the spring treatment (A). In the 2008/2009 season, the control treatment showed differences compared to the other treatments (B), displaying the highest percentages, also fruits from SNS treatment showed differences in relation to SNAH and the covering percentage was lower.
FIGURE 1 Mean values ± SE of percentage of coverage color of the fruits of cv ‘Royal Gala’ apple trees under different fertilization treatments. (A) 2007/2008 season; (B) 2008/2009 season. SNAH: soil N application after harvest; FNAH: foliar N application after harvest; SNS: soil N application in spring; C: control (no N applied). Different letters indicate significant differences (p < 0.05).
Anthocyanin Concentration
In the 2006/2007 season, no significant differences in the anthocyanin concentration were observed in the fruit skin among treatments (A). However, fruits from SNS treatment showed the lowest concentration, while those from treatment SNAH showed the highest.
FIGURE 2 Anthocyanin concentration ± SE (μg g−1 fresh weight) of the fruit skin of cv ‘Royal Gala’ apple trees under different fertilization treatments. (A) 2006/2007 season; (B) 2007/2008 season; (C) 2008/2009 season. SNAH: soil N application after harvest; FNAH: foliar N application after harvest; SNS: soil N application in spring; C: control (no N applied). Different letters indicate significant differences (p < 0.05).
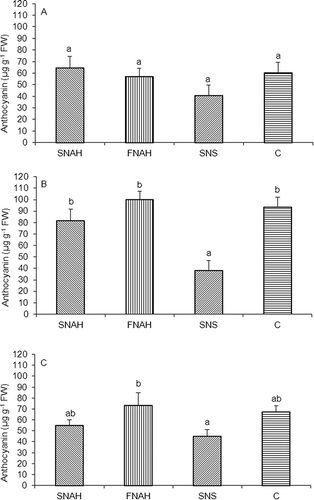
Significant differences were found in the following two seasons (B and C). In the 2007/2008 season, fruits from the spring treatment showed the lowest anthocyanin concentration and differed from the other three treatments. However, in the 2008/2009 season, despite the fact that there were no differences between SNAH, SNS, and C treatments, fruits from the SNS treatment tended to show the lowest concentrations of anthocyanins.
PAR Interception and Vegetative Growth
SNS treatment showed the highest values of PAR interception (57.3 ± 6.3 and 65.5 ± 5.2% for the first and second season, respectively) while C treatment showed the lowest values (48.8 ± 5.6 and 58.3 ± 5.1% for the first and second season, respectively). With regard to the vegetative growth, SNS treatment showed the highest value in the 2008/2009 season (26.8 ± 3.6 m), while the lowest values were found in the control (23.9 ± 5.9 m). SNAH and FNAH treatments showed intermediate values for both analyzed parameters (data not shown).
DISCUSSION
N fertilization is a common practice in commercial apple orchards in order to sustain high yields. Considering that the cost of the fertilizer is a minimal portion of the total production, growers usually apply more N than recommended. Depending on the timing and rate of the applications, N can have detrimental effects on some of the main quality attributes, such as fruit coloration. After three periods of successive application of nitrogen, our results suggest that timing and method of nitrogen fertilization modify the response of fruit coloration to the applied nitrogen.
Among the treatments that received fertilization (SNAH, FNAH y SNS), postharvest treatments tended to display fruits with darker coloration (lower lightness and Hue angles together with higher percentages of coverage color) (, A and B). In accordance to these results, postharvest treatments tended to show the highest contents of anthocyanins (A–C), in agreement with CitationFallahi et al. (2001). Fruits from SNS treatment showed the lowest colorations and the lowest anthocyanin content. In contrast, fruits from C treatments showed high values of anthocyanins and good coloration.
Observations on PAR interception in the different treatments suggest that the observed effects on fruit color could be, at least in part, explained by differences in the amount of light that reaches the fruits in the canopies of the different treatments. Thus, the lowest colorations observed in SNS treatment with respect to the postharvest fertilizations, would be related to the shading generated by the higher foliar growth in SNS treatment, in response to a higher availability of nitrogen during the active vegetative growth period (CitationWilliams and Billingsley, 1974; CitationFaust, 1989; CitationRacskó et al., 2005; CitationNava et al., 2008). Also, the higher coloration in fruits from C treatment would be related to the higher exposure to light due to the lower vigor of the plants in response to the lack of fertilization.
Additionally, it should be taken into account that in the spring fertilization a higher amount of partitioned nitrogen is assigned to the fruit (CitationFaust, 1989; CitationKhemira et al., 1998; CitationWargo et al., 2003; CitationRacskó et al., 2005), and it has been reported that a higher nitrogen content is negatively correlated with the anthocyanin synthesis and the development of fruit coloration (CitationAwad and de Jager, 2002; CitationWargo et al., 2003; CitationRacskó et al., 2005; CitationNeilsen et al., 2006, CitationNava et al., 2008).
In conclusion, our results showed that the detrimental effects of N on fruit coloration, either direct through the synthesis of anthocyanins or indirect through the foliar development and change of light environment, can be avoided or at least reduced if application of the nutrient is performed in the postharvest period.
ACKNOWLEDGMENTS
The study was supported by funds from INTA (PNFRU2181), UNMdP (AGR 301), and Comisión de Investigaciones Científicas de la Provincia de Buenos Aires (CIC). This work is part of a lecture presented at the National University of Mar del Plata by Verónica De Angelis. The authors wish to thank Trans. María Cecilia Moreno (CIC) and Cecilia Gittins for the English version of the paper.
LITERATURE CITED
- Awad , M.A. and de Jager , A. 2000 . Flavonoid and chlorogenic acid concentrations in skin of ‘Jonagold’ and ‘Elstar’ apples during and after regular and ultra low oxygen storage . Postharvest Biol. Technol. , 20 : 15 – 24 .
- Awad , M.A. and de Jager , A. 2002 . Relationships between fruit nutrients and concentrations of flavonoids and chlorogenic acid in ‘Elstar’ apple skin . Sci. Hort. , 92 : 265 – 276 .
- Barritt , B.H. , Rom , C.R. , Konishi , B.J. and Dilley , M.A. 1991 . Light level influences spur quality and canopy development and light interception influence fruit production in apple . HortScience , 26 : 993 – 999 .
- Cheng , L. , Ma , F. and Ranwala , D. 2004 . Nitrogen storage and its interaction with carbohydrates of young apple trees in response to nitrogen supply . Tree Physiol. , 24 : 91 – 98 .
- Cordon , V.H. , Forquera , J.C. , Gastiazoro , J. , Lassig , J. , Bastanski , M. and Nordenstrom , G. 2000 . Climatic Characterization of Rio Negro, Neuquén, and Lower Limay Upper Valley. Comahue University, Agricultural Sciences Faculty , Argentina : Cinco Saltos, Río Negro .
- Crisosto , C.H. , Johnson , R.S. , DeJong , T. and Day , K.R. 1997 . Orchard factors affecting postharvest stone fruit quality . HortScience , 32 : 820 – 823 .
- Dong , S. , Cheng , L. , Scagel , C.F. and Fuchigami , L. 2005 . Timing of urea application affects leaf and root N uptake in young Fuji/M.9 apple trees . J. Hort. Sci. Biotechnol. , 80 : 116 – 120 .
- Dussi , M.C. , Sugar , D. and Wrolstad , R.E. 1995 . Characterizing and quantifying anthocyanins in red pears and the effect of light quality on fruit color . J. Amer. Soc. Hort. Sci. , 120 : 785 – 789 .
- Dussi , M.C. , Sugar , D. , Azarenko , A.N. and Righetti , T.L. 1997 . Colorimetric characterization of red pear cultivars . Fruit Var. J. , 51 : 39 – 43 .
- Fallahi , E. , Colt , W.M. , Fallahi , B. and Chun , I.J. 2001 . Influence of different rates of nitrogen on fruit quality, yield and photosynthesis of ‘Fiju’ apple . Acta Hort. , 594 : 261 – 268 .
- Fallahi , E. , Khemira , H. , Righetti , T.L. and Azarenko , A.N. 2002 . Influence of foliar application of urea on tree growth, fruit quality, leaf minerals, and distribution of urea-derived nitrogen in apples . Acta Hort. , 594 : 603 – 610 .
- Faust , M. 1965 . Physiology of anthocyanin development in McIntosh apple II: Relationship between protein synthesis and anthocyanin development . Proc. Amer. Soc. Hort. Sci. , 87 : 10 – 20 .
- Faust , M. 1989 . Physiology of temperate zone fruit trees , Hoboken, NJ, USA : Wiley Interscience .
- Forshey , C.G. 1963 . A comparison of soil nitrogen fertilization and urea sprays as sources of nitrogen for apple trees in sand culture . Proc. Amer. Soc. Hort. Sci. , 83 : 32 – 45 .
- Khemira , H. , Righetti , T.L. and Azarenko , A.N. 1998 . Nitrogen partitioning in apple as affected by timing and tree growth habit . J. Hort. Sci. Biotechnol. , 73 : 217 – 223 .
- Lancaster , J. E. 1992 . Regulation of skin color in apples . Crit. Rev. Plant Sci. , 10 : 487 – 502 .
- Nava , G. , Dechen , A.R. and Nachtigall , G.R. 2008 . Nitrogen and potassium fertilization affect apple fruit quality in southern Brazil . Commun. Soil Sci. Plant Anal. , 39 : 96 – 107 .
- Neilsen , D. , Neilsen , G.H. , Herbert , L.C. , Millard , P. and Guak , S. 2006 . Allocation of dry matter and N to fruit and shoots in dwarf apple in response to sink size and N availability . Acta Hort. , 721 : 33 – 40 .
- Quartieri , M. , Millard , P. and Tagliavini , M. 2002 . Storage and remobilisation of nitrogen by pear (Pyrus communis L.) trees as affected by timing of N supply . Eur. J. Agron. , 17 : 105 – 110 .
- Racskó , J. , Szabó , Z. and Nyéki , J. 2005 . Effect of nutrient supply on fruit quality of apple (Malus domestica Borkh.) . J. Cent. Eur. Agr. , 6 : 35 – 42 .
- Ritenour, M. and H. Khemira. 2007. Red color development of apple: A literature review. Tree Fruit Research and Extension Center—Washington State University. Retrieved April 25, 2011, from < http://postharvest.tfrec.wsu.edu/REP2007A.pdf (http://postharvest.tfrec.wsu.edu/REP2007A.pdf)
- Sánchez , E.E. , Righetti , T.L. , Sugar , D. and Lombard , P.B. 1990a . Response of ‘Comice’ pear trees to a postharvest urea spray . J. Hort. Sci. , 65 : 541 – 546 .
- Sánchez , E.E. , Righetti , T.L. , Sugar , D. and Lombard , P.B. 1990b . Seasonal differences, soil texture and uptake of newly absorbed nitrogen in field-grown pear trees . J. Hort. Sci. , 65 : 395 – 400 .
- Sánchez , E.E. , Righetti , T.L. , Sugar , D. and Lombard , P.B. 1991 . Recycling of nitrogen in field-grown ‘Comice’ pears . J. Hort. Sci. , 66 : 479 – 486 .
- Saure , M.C. 1990 . External control of anthocyanin formation in apple . Sci. Hort. , 42 : 181 – 218 .
- Tagliavini , M. , Quartieri , M. and Millard , P. 1997 . Remobilised nitrogen and root uptake of nitrate for spring leaf growth, flowers and developing fruits of pear (Pyrus communis L.) . trees. Plant Soil , 195 : 137 – 142 .
- Tagliavini , M. , Millard , P. , Quartieri , M. and Marangoni , B. 1999 . Timing of nitrogen uptake affects winter storage and spring remobilisation of nitrogen in nectarine (Prunus persica var. nectarine) trees . Plant Soil , 211 : 149 – 153 .
- Toselli , M. , Flore , J.A. , Zavalloni , C. and Marangoni , B. 2000 . Nitrogen partitioning in apple trees as affected by application time . HortTechnology , 10 : 136 – 141 .
- Ubi , B.J. 2004 . External stimulation of anthocyanin biosynthesis in apple fruit . J. Food Agr. Environ. , 2 : 65 – 70 .
- Wargo , J.M. , Merwin , I.A. and Watkins , C.B. 2003 . Fruit size, yield, and market value of ‘GoldRush’ apple are affected by amount, timing and method of nitrogen fertilization . HortTechnology , 13 : 153 – 161 .
- Whale , S.K. and Singh , Z. 2007 . Endogenous ethylene and color development in the skin of ‘Pink Lady’ apple . J. Amer. Soc. Hort. Sci. , 132 : 20 – 28 .
- Williams , M.W. and Billingsley , H.D. 1974 . Effect of nitrogen fertilizer on yield, size and color of ‘Golden Delicious’ apple . J. Amer. Soc. Hort. Sci. , 99 : 144 – 145 .
- Wrolstad , R.E. 1993 . Color and pigment analyses in fruit products . Oregon Agr. Expt. Sta. Bul. 624. ,
- Wrolstad , R.E. , Durst , R.W. and Lee , J. 2005 . Tracking color and pigment changes in anthocyanin products . Trends Food Sci. Technol. , 16 : 423 – 428 .
- Wünsche , J. and Lakso , A.N. 2000 . The relationship between leaf area and light interception by spur and extension shoot leaves to apple orchard productivity . HortScience , 35 : 1202 – 1206 .