Abstract
Beginning at least as early as 2005 and continuing through 2010, the California strawberry industry has suffered production losses caused by soilborne fungi not previously recognized as strawberry pathogens in California. The vast majority of these problems took place in fields that did not receive the traditional pre-plant fumigation treatment of methyl bromide + chloropicrin. These new disease developments have been consistently associated with two pathogens: Macrophomina phaseolina and Fusarium oxysporum f. sp. fragariae. Pathogenicity tests confirmed that these fungi caused symptoms similar to those observed in the field. Other experiments indicated that some strawberry cultivars are apparently less susceptible than others. Field trials using alternative fumigants provided some control of both diseases. In California, both Fusarium and Macrophomina are appearing in previously uninfested areas, indicating that these pathogens will be long-term concerns for this industry.
INTRODUCTION
Strawberry is an important high value crop for the state of California. In 2009, approximately 39,800 acres of strawberries were grown in California, which accounted for 67% of the US crop. The value of the 2009 crop was approximately $1.73 billion, representing 81% of the US total value for strawberry. The majority of the California strawberry crop is produced in the central to south central coastal counties stretching from Santa Cruz County in the north through Monterey, San Luis Obispo, and Santa Barbara counties, ending in Ventura County in the south. Most California strawberries are grown as annual crops, with second year berries making up a small percentage of the total acreage. Prior to recent changes in fumigation practices, virtually all fields that were planted with conventional strawberries were pre-plant fumigated with some combination of methyl bromide and chloropicrin injected with shanks to flat ground and covered with tarp.
First Development
Beginning in 2005 or before, strawberry growers reported an increasing problem with collapsing strawberry plants. Symptoms consisted of wilting of foliage, plant stunting, and drying and death of older leaves, with the central youngest leaves often remaining green and alive. Plants eventually collapsed and died, especially if such plants were subject to environmental stresses or were bearing a heavy load of fruit. When plant crowns were cut open, internal vascular and cortex tissues were dark brown to orange brown. The fungus Macrophomina phaseolina was consistently associated with these problems (CitationKoike, 2008a; 2008b). Macrophomina phaseolina has been reported as the cause of charcoal rot in Illinois and Macrophomina root rot in Egypt (CitationMaas, 1998), Florida (Merteley et al., 2005), Isreal (CitationZveibil & Freeman, 2005) and elsewhere. In 2005, the Macrophomina problem was restricted to the southern part of the state (Ventura and Orange counties). However, by 2010, cases of charcoal rot were also documented in Santa Cruz, Santa Clara, Alameda, and Sacramento counties.
Second Development
A second concern was first confirmed in 2006. Strawberry growers again reported collapsing strawberry plants that exhibited the same foliar wilting, leaf death, and vascular and crown discoloration. In this case, the fungus Fusarium oxysporum was consistently associated with the affected plants. Fusarium oxysporum f. sp. fragariae has been reported to cause Fusarium wilt or Fusarium yellows in Australia and Japan (CitationMaas, 1998). In 2006, this distinct second problem was found only in Ventura County, the county having the largest strawberry acreage. However, in 2010 the problem was confirmed in the second largest strawberry-producing county, Monterey.
Other pathogens, such as Verticillium, Phytophthora, and Colletotrichum species, were never isolated from affected plants. There are indications that the fields most seriously affected by these novel plant collapse problems are fields that no longer are treated with the traditional pre-plant fumigation but have been bed-fumigated with alternatives to the methyl bromide + chloropicrin standard.
MATERIALS AND METHODS
Cultivar Susceptibility in the Field
Strawberry cultivars were evaluated at sites with histories of Macrophomina phaseolina (Ventura) or Fusarium oxysporum (Oxnard). The Ventura site was not fumigated and the Oxnard site was treated pre-plant with InLine soil fumigant (61% 1,3-dichloropropene and 33% chloropicrin, Dow Agrosciences LLC., Indianapolis, IN, USA) at 224 kg/hectare (200 lbs/acre). At both sites, the cultivars Albion, Camarosa, Monterey, Palomar, San Andreas, and Ventana were planted in a randomized complete block design with four replications. Transplants were handled, planted, and grown according to commercial practices. We periodically evaluated the plants for symptoms of plant collapse and recorded disease incidence. Representative samples were collected and analyzed in the UC Cooperative Extension diagnostic lab (Salinas, CA) to determine the presence of Macrophomina, Fusarium, or other pathogens.
Cultivar Susceptibility under Shadehouse Conditions
Three M. phaseolina isolates (UC 3, UC 4, and UC 14) were used for this experiment. In previous research, isolates UC 3 and UC 4 were very aggressive and caused rapid dieback symptoms when inoculated into strawberry. UC 14 was significantly less aggressive but still caused dieback symptoms. To prepare inoculum, a 3:1 mixture of fine washed sand and cornmeal was placed into 500-ml Erlenmeyer flasks; a total of 200 ml of the mixture was added to each flask. All flasks then received 120 ml of distilled water, were sealed with foil, and autoclaved two times (24 hr between autoclaving). Agar plugs colonized with active M. phaseolina growth were added to each flask. Inoculated flasks were maintained at room temperature for 3 to 4 months. When flask cultures were ready, as evidenced by the black color due to microsclerotia formation, flask contents were emptied out onto trays and allowed to dry at room temperature.
For this project the plant materials tested consisted of 11 public (University of California) and 24 proprietary cultivars. In November, strawberry transplants were planted into shallow nursery flats to establish initial root and leaf growth. The following April, plants were uprooted and their roots were rinsed free of the peat moss potting mix. Roots were trimmed to approximately 15 to 20 cm (6 to 8 inch) lengths, placed on top of fresh peat moss in nursery flats, inoculated by sprinkling the sand + cornmeal inoculum onto the roots (20 grams inoculum per flat), and covered with fresh peat moss. A total of 15 plants were placed in each flat, which was maintained in a shadehouse. Two flats were prepared for each cultivar and for each of the three isolates.
Evaluation of Fumigants
At a site with a history of M. phaseolina, drip applied fumigants were evaluated for managing charcoal rot. The treatment plots were arranged in a randomized complete block design with four replications. Each plot was one bed wide and 100 m (300 ft) long. After application of treatments to pre-formed beds, the field was later planted with the cultivar Camarosa. Transplants were handled, planted, and grown according to commercial practices. We periodically evaluated the plants for symptoms of plant collapse and recorded both disease incidence and strawberry fruit yields.
Detecting Macrophomina in Field Soils
Soils were collected from three locations in Ventura County and assayed for Macrophomina. Five grams of soil from each sample were mixed with 250 ml of 10% bleach in a blender three times for 30 sec each time (CitationMihail & Alcorn, 1982) The mixture was washed with distilled water through a 70-μm mesh US standard sieve. Using sterilized water, the residue was then back washed into a 125-ml autoclaved flask. One hundred milliliters of cooled PDA amended with rifampicin (Sigma-Aldrich, St. Louis, MO, USA) (0.05 g/l) and Tergitol (Sigma-Aldrich) (1 ml/l) was added to each flask separately and thoroughly mixed. The mixture was poured into six Petri plates and incubated at 32°C in the dark for 4 days. The number of Macrophomina colonies (colony forming units or CFUs) typically forming fluffy white aerial mycelium surrounding a central area with black sclerotia was enumerated under a stereomicroscope.
A second sample consisted of soils taken from the Ventura cultivar experiment site. Soil was collected from two depths (6 and 12 in.) from each of three locations on the plant beds: near one of the two drip-lines, from the shoulder area of bed, and from the center of bed.
RESULTS
Cultivar Susceptibility in the Field
Even without soil fumigation (Ventura site), M. phaseolina had little impact on plant mortality until March (). As the season progressed and temperatures increased, plant collapse started to occur in the cultivar Albion and later in cultivars Camarosa and San Andreas. By early June, 48.5% of ‘Albion’, 22.4% of ‘Camarosa’, and 30.6% of ‘San Andreas’ plants were dead. ‘Ventana’, ‘Monterey’, and ‘Palomar’ strawberry had higher survival percentages. However, randomly selected plants from all cultivars were tested in the lab and were individually positive for either Macrophomina or Fusarium. Therefore, interpretation of these results is complicated by the recovery of both pathogens from plants collected at this location. No other pathogens were recovered from tested plants.
TABLE 1 Number of Live Plants for Each of Six Strawberry Cultivars at a Non-Fumigated Ventura Site Infested with Both Macrophomina and Fusarium
Fumigation with InLine at the Oxnard site provided good protection from F. oxysporum until June (). Similar to our observations in previous seasons, cultivar Camarosa had the greatest dieback in the presence of F. oxysporum, confirming its previously established high susceptibility to this pathogen. Randomly selected plants were tested in the lab and were positive for F. oxysporum. No other pathogens were recovered from tested plants.
TABLE 2 Number of Live Plants of Six Strawberry Cultivars at an Oxnard Site That Was Infested with Fusarium and Treated with 200 lbs/acre (224 kg/hectare) InLine Soil Fumigant
Cultivar Susceptibility under Shadehouse Conditions
Overall results (grand means) for the three isolates were consistent with previous studies. Isolates UC 3 and UC 4 (11.14 and 8.76% grand mean survival rates, respectively) were again significantly more aggressive than isolate UC 14 (18.86% survival rate). For many cultivars, the results were consistent when separately inoculated with the three isolates. For example, from previous experiments the cultivar Ventana was rated as very susceptible to M. phaseolina. In this experiment, the UC 3/UC 4/UC 14 survival percentages for cultivar Ventana were 3.3, 0, and 0, respectively. However, results were not consistent across the three isolates for other cultivars. For example, the UC 3/UC 4/UC 14 survival percentages for cultivar Albion were 0, 30, and 6.7, respectively. Under these very challenging inoculation conditions, however, none of the tested UC or proprietary cultivars showed resistance to M. phaseolina.
Evaluation of Fumigants
No significant differences in plant mortality from M. phaseolina were observed in the first part of the season (). By mid-May plant collapses occurred in all treatments and by May 25 plant losses ranged from 5.1 to 13.2%. Untreated plots had a 10.3% reduction. The fumigant treatments were not statistically different than untreated plots. Midas Gold had the lowest plant loss figure at 5.1% (). These results suggest that fumigants provided little to no protection in this trial against late season infection by M. phaseolina. Randomly selected plants tested positive for both Macrophomina and Fusarium. No other pathogens were recovered from tested plants.
TABLE 3 Number of Surviving ‘Camarosa’ Strawberry Plants Following Pre-Plant Fumigation at a Site Infested with Both Macrophomin a and Fusarium
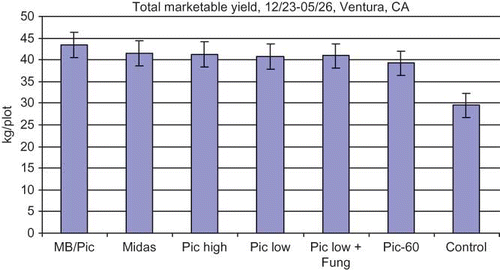
Fruit production was similar in all treatments until the end of March (data not shown), at which time yields in the non-fumigated check started to fall behind yields in the fumigated treatments. This resulted in lower marketable yields () and total yields (data not shown) in non-fumigated check compared to all fumigated treatments. Even though the dieback was not observed until May (as described above), the fruit yield decline in the non-fumigated check since March provides evidence for the potential impact of the pathogens on plant productivity.
Detecting Macrophomina in Field Soils
Macrophomina numbers, counted as colony forming units (CFUs), were low in the four samples tested: 1, 3, 13, and 18 cfu/5 g of soil. A second sample was also assayed for Macrophomina CFUs and consisted of soils taken from two depths (6 and 12 in.) from each of three locations on the beds (). In general, Macrophomina CFUs at the 6-in. depth were significantly higher than at the 12-in. depth regardless of the treatment (P = 0.044). There was no significant interaction between treatments and the depth of sampling (P = 0.546) suggesting that the treatments performed consistently at both depths. Among the treatments, shoulder and center locations on beds were not significantly different at either depth. Soil under drip-lines had the lowest numbers of CFUs at both depths, and both 6 and 12 in. counts were significantly lower than counts for the other two treatments. Delivery of chemicals via drip-lines results in the highest concentrations of the materials in soil directly under and near these lines. Therefore, improved distribution of drip applied fumigants is needed to manage soilborne pathogens resident at locations further away from the drip line.
TABLE 4 Number of Colony Forming Units (CFUs) of Macrophomina phaseolina Recovered from Soil Collected at 15.2 and 30.5 cm (6 and 12 in.) Depths and from Three Locations on Planted Beds
ACKNOWLEDGMENTS
We thank the California Strawberry Commission for their financial support, and thank Joe Coelho, Mike Ferro, Daren Gee, Andy Hooper, Bill Reiman, and Edgar Terry for their assistance and willingness to let us conduct the field trials on their ranches. We thank the following persons for their assistance: Patty Ayala, Surendra Dara, Annika Forester, Emmanuel Gonzalez, Ian Greene, Jonathan Hunzie, Kat Kammeijer, Ian Kile, Dan Legard, Maren Mochizuki, and Paul Penza.
LITERATURE CITED
- Koike , S.T. 2008a . Macrophomina crown rot: Possible new production issue for strawberry in California. University of California Cooperative Extension Crop Notes July–August
- Koike , S.T. 2008b . Crown rot of strawberry, caused by Macrophomina phaseolina, in California . Plant Dis. , 92 : 1253
- Maas , J.L. 1998 . Compendium of strawberry diseases , 2nd , 98 St. Paul , MN : APS Press .
- Mertely , J. , Seijo , T. and Peres , N. 2005 . First report of Macrophomina phaseolina causing a crown rot of strawberry in Florida . Plant Dis. , 89 : 434
- Mihail , J.D. and Alcorn , S.M. 1982 . Quantitative recovery of Macrophomina phaseolina sclerotia from soil . Plant Dis. , 66 : 662 – 663 .
- Zveibil , A. and Freeman , S. 2005 . First report of crown and root rot in strawberry caused by Macrophomina phaseolina in Israel . Plant Dis. , 89 : 1014