Abstract
Inoculation of detached strawberry leaves with Colletotrichum species may provide an accurate, rapid, non-destructive method of identifying anthracnose resistant germplasm. Two assessments of anthracnose disease severity were compared on detached strawberry leaves inoculated with Colletotrichum fragariae and C. gloeosporioides: a quantitative assessment made via computer-based image analysis and a visual assessment made by two independent raters. The image analysis provided a precise measurement of percent lesion area of infected leaves. There was a strong positive correlation between percent lesion area and the visual disease scores of the raters.
Keywords:
INTRODUCTION
Anthracnose is a destructive disease of commercial strawberries that spreads rapidly in production fields during warm, rainy conditions. Conidia are dispersed by rain and may also be moved throughout a field by people, animals, or equipment. Three species of Colletotrichum cause anthracnose diseases of strawberry (CitationSmith, 1998b). Colletotrichum fragariae is the primary causal fungus of anthracnose crown rot in the southeastern United States and has been reported in Central and South America, India, Japan, and South Africa. It infects all above-ground parts of the strawberry plant and has a restricted host range of strawberry and a few non-crop plants. Colletotrichum gloeosporioides causes symptoms indistinguishable from those caused by C. fragariae but has a broader host and geographic range. Colletotrichum acutatum generally causes anthracnose fruit rot that can cause severe losses in production fields. It has a broad host range and occurs in most areas of the world where strawberries are grown.
Two anthracnose diseases occur on strawberry leaves: anthracnose leaf spot or black leaf spot, caused by C. fragariae and C. gloeosporioides and irregular leaf spot caused by C. acutatum. Anthracnose leaf spot lesions are usually light gray to black in color, round, and resemble ink spots on the leaves. These lesions generally do not become necrotic and the fungus does not produce acervuli or spores. Irregular leaf spots are dark brown to black in color and usually occur on the margins and tips of the leaflets. In addition, the fungus produces acervuli and conidia, which may serve to infect young leaves. Both anthracnose leaf spot and irregular leaf spot can serve as an early warning sign that abundant inoculum is present in production fields and fungicides should be applied (CitationSmith, 1998a).
Strawberry breeding programs generally require many years to produce disease-resistant cultivars. Accurate and reproducible disease assessment techniques are required to determine the disease response in breeding material. The degree of anthracnose resistance or susceptibility of strawberry germplasm is often determined by inoculating whole plants with conidia of the causal Colletotrichum species. These inoculation trials are time consuming and require a large number of plants to be assessed for disease development 30 or more days after inoculation. Detached leaf inoculation techniques allow researchers to test strawberry germplasm for disease susceptibility without destroying whole plants, thus decreasing the space needed for inoculated plants. These techniques also reduce the time between inoculation and disease assessment and may protect the environment since the pathogen is confined to the laboratory.
Visual rating scales are often used to quickly assess disease severity on inoculated plant tissues. These scales may be percentage or numerical with specific grades or levels. Percentage rating scales are often in 1% or 5% increments and adapted from the Horsfall-Barratt (HB) scale (CitationHorsfall and Barratt, 1945). The accuracy of these scales may be influenced by the visual bias that is related to our ability to distinguish moderate levels of disease severity. Diseased tissue is the visual focus when lesions are few or small surrounded by healthy green tissue, and it is easier to determine disease severity less than 5% than disease severity between 20% and 40%. On the other end of the spectrum, green tissue is the visual focus when most of the tissue is diseased, and it is easier to determine disease severity greater than 95% than between 65% and 85%. The HB scale was designed with 12 grades, each grade covering a range of percent affected area. The middle grades span wider ranges of infection than the low and high grades since small differences are easier to differentiate visually at the extreme ends of the scale. More categories in a rating scale may make the scale more accurate but they also increase the time required for disease assessment. CitationSlopek (1989) tested five variations of a 1–5 scale for estimating the percent leaf area disease of barley plants and found that two of the five variations tested worked well for estimating the percent leaf area disease and were as precise as the HB scale with fewer categories, thus reducing the overall time for disease assessment.
Numbered grade scales, sometimes called arbitrary scales, are often employed to evaluate disease symptoms on whole plants or leaves (CitationCouture, 1980) and are adequate when used by experienced observers for ranking test plants or plots in order of increasing symptom severity (CitationRussell, 1978). CitationCouture (1980) noted that percentage scales are difficult to use when relating to plants or plant organs that exhibit noticeably different amounts of infections, such as rusts, powdery mildews, and leaf spots. Another approach relies on a scale of 0–5 (0 = no symptoms and 5 = severe symptoms) that allows the observer to divide the plant or leaf visually into fifths and determine the portion that is diseased. CitationAbril et al. (2009) used photographs of strawberry detached leaves for visual assessment of disease severity in a study testing the efficacy of natural product-based fungicides. They used an arbitrary scale of 0–3 (0 = no disease and 3 = most severe disease) to assess the percent diseased leaf area.
Software has been developed that can be used to perform very precise quantitative analysis on images of lesions on plant tissues. Although precise, the analysis software depends on a value judgment by the computer operator to determine at what level the color change on the image is considered a lesion. Digital imaging and analytical software was used by CitationWang et al. (2008) to determine percent disease and percent phytotoxicity on strawberry leaves in a miniaturized antifungal bioassay. CitationKwack et al. (2005) used digital image analysis to assess the severity of cucumber anthracnose caused by Colletotrichum orbiculare. However, the time it took to photograph inoculated tissues, utilize software to identify necrotic tissue (lesions), and determine the ratio of lesion to healthy tissue was considerable.
The objective of our study was to compare computer-based image analysis of anthracnose disease on inoculated detached strawberry leaves with visual assessments made by two trained independent raters. The goal was to determine how well the visual assessments correlated to the computer assessment. A secondary goal was to determine if a detached leaf inoculation technique could reliably distinguish anthracnose-resistant germplasm from susceptible germplasm.
MATERIALS AND METHODS
Growth of fungal isolates and preparation of inoculum: Isolates of C. fragariae (CF63 and CF75, and C. gloeosporioides (CG162) (CitationSmith and Black, 1990) were initiated from silica gel cultures maintained at USDA/ARS in Poplarville, MS. Cultures were grown on a 1:1 oatmeal agar (O-3506 Sigma-Aldrich, Inc., St. Louis, MO, USA):potato dextrose agar (PX2549J A. Daigger & Co., Inc., Vernon Hills, IL, USA) under fluorescent light with a 12-hr photoperiod and at a room temperature of 20–28°C. Conidial suspensions used for inoculations were prepared from 7- to 14-day-old cultures. Inoculum was prepared by flooding each culture plate with sterile deionized water containing 0.5 ml Tween® 20 (P7949 Sigma-Aldrich, Inc.) per liter as a surfactant and gently scraping the agar surface with a glass rod to remove conidia. The resulting conidial suspension was filtered through cheesecloth and adjusted to a concentration of 1.5 × 106 conidia/ml by diluting with sterile distilled water containing Tween® 20.
Detached Leaf Inoculations
Detached leaves were collected from two groups of strawberry clones. The cultivar group consisted of eight named cultivars known or assumed to be susceptible to anthracnose. Plants of these cultivars were obtained from a commercial strawberry nursery as bare root plants. The MS group consisted of 13 clones selected as anthracnose resistant in the anthracnose screening project at USDA/ARS in Poplarville, MS, and included one named cultivar (Pelican), two germplasm releases (US159 and US438), and ten MSUS breeding lines (CitationSmith and Black, 1990). Plants of these clones were increased by rooting daughter plants from stock plants grown at Poplarville. Twenty or more plants of each clone were grown in 10-cm-diameter containers in a 1:1 mixture of Jiffy-Mix (Harris Seed Co., Rochester, NY, USA):sand in a greenhouse at 28°C day/18°C night ±6°C with a 16-hr photoperiod for at least 6 weeks before collecting leaves for inoculation.
Young, fully developed, blemish-free leaves with petioles were removed from randomly chosen plants no more than 4 hr before inoculation, rinsed in distilled water, and placed in 10 mm × 150 mm test tubes filled with distilled water. A minimum of eight detached leaves of each of the 21 clones were inoculated with each of the three isolates of Colletotrichum species. A hand pump sprayer was used to mist the adaxial surface of each leaf until the point of runoff with the conidial suspension of each test isolate. Detached leaves of each clone sprayed with distilled water served as ‘not inoculated’ controls. Inoculated leaves were immediately placed in a dew chamber at 100% RH, 30°C, and incubated in the dark for 48 hr after inoculation. Leaves were then transferred to sealed plastic containers at 100% RH and 28°C with continuous fluorescent light for an additional 3 to 5 days before assessing disease symptoms. Disease severity on each leaf was assessed visually by two trained independent raters. A visual scoring scale of 0 (no disease) to 5 (lesion covering the entire leaf surface) was used to determine the amount of diseased area. The observations were made in a laboratory setting with overhead fluorescent lighting and no magnification. Average visual scores for each leaf were calculated by averaging the scores of each of the two raters.
Image Analysis
Each leaf was separated into individual leaflets for photographing, placed on a light box with a 247 × 197 mm viewing area (P-Frame, Model A-5A, Logan Electric Specialty Mfg. Co., Chicago, IL, USA), and covered with a 203 mm × 254 mm glass plate to position the leaflets flat against the light box, removing folds. Visualization of lesions was enhanced by the backlighting provided by the light box. Photographs were taken either with a DXCB151A color video camera (Hitachi Instruments, Inc., Houston, TX, USA) and captured with the Bioquant® 98 image analysis software package (R&M Biometrics, Inc., Nashville, TN, USA) or with a Nikon COOLPIX 5000 digital camera (Nikon Corp., Tokyo, Japan). Photographs were enlarged electronically 200% and lesions were individually marked and colorized for image analysis as healthy leaf tissue green and lesions black, using Corel® Photo-Paint X4 or X5 (Corel Corp., Ottawa, ON, Canada). The total leaf area was calculated as the green area (healthy) plus the black (lesion) area using Image Pro Plus 7.0 (Media Cybernetics, Bethesda, MD, USA). The percent lesion area was calculated as lesion area divided by total leaf area multiplied by 100. The number of discrete lesions was counted (electronically) for each leaf. Hereafter the electronic disease ratings will be termed percent lesion area and lesion count, and the scores for the visual disease ratings will be considered the disease score.
Data Analysis
Leaves were collected and inoculated periodically over 2 years. The availability of leaves at each collection date determined the number of leaves inoculated. The experimental design was totally random. Eight to 17 leaves of each clone were inoculated with each of the three isolates. Additional leaves collected from the same plants as those being inoculated served as “not inoculated” controls. The average disease score of the “not inoculated” controls was less than 0.1 at each inoculation date, and therefore removed from the data set before analysis. Data were subjected to analysis of variance (ANOVA) and means were separated by Fisher's protected least significant difference (LSD) method at p < 0.05. Pearson correlation coefficient was used to compare the percent lesion area, lesion count, and disease scores of each leaf. All statistical analyses were conducted using SAS software (SAS Institute Inc., Cary, NC, USA).
RESULTS AND DISCUSSION
Disease symptoms were assessed on 8–17 detached leaves of each of 21 strawberry clones following inoculation with C. fragariae isolates CF63 and CF75 and C. gloeosporioides isolate CG162. There were significant differences between the two groups of strawberry clones in the average percent lesion area, lesion count, and disease scores of raters 1 and 2 and their average score (). The cultivar group had an average lesion area of 14%, an average lesion count of 38, and an average disease score of 1.7, which were significantly higher than those of the MS group, i.e., average lesion area of 6%, average lesion count of 17, and average disease score of 1.0. The higher values of the cultivar group confirmed that as a group these clones were more susceptible to anthracnose than the MS group.
FIGURE 1 Main effect due to Group (A) and Isolate (B) of detached strawberry leaves inoculated with two Colletotrichum fragariae (CF63 and CF75) isolates and one C. gloeosporioides (CG162) isolate. Within each variable, bars with different letters are significantly different at p < 0.05 according to Fisher's protected least significant difference test.
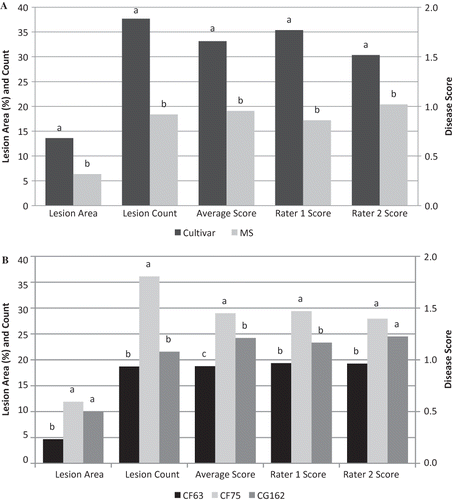
Significant differences were also found in percent lesion area, lesion count, and disease scores among the three fungal isolates (). Colletotrichum fragariae isolate CF75 had the highest values with an average lesion area of 12%, average lesion count of 36, and an average disease score of 1.5 indicating that it is the most aggressive of the three isolates. Colletotrichum gloeosporioides isolate CG162 had the next highest values with an average lesion area of 10%, average lesion count of 22, and an average disease score of 1.2, and C. fragariae isolate CF63 had the lowest values of the three fungal isolates with an average lesion area of 5%, average lesion count of 19, and an average disease score of 1 indicating that it was the least aggressive of the three isolates. There was no significant interaction between isolate and group.
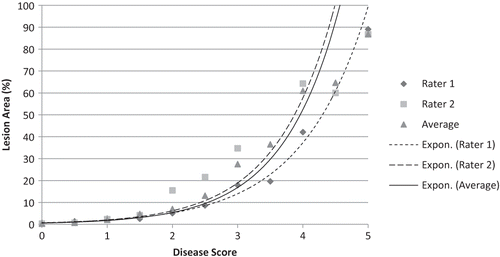
The Pearson correlation coefficient showed a strong positive correlation between the disease scores of the two raters (r = 0.82; p < 0.0001) and between the percent lesion area of each rater: (r = 0.77; p < 0.0001) for Rater 1, (r = 0.87; p < 0.0001) for Rater 2, and (r = 0.85; p < 0.0001) for the average score of the two raters. Lesion count had a poor correlation with the percent lesion area (r = 0.1; p = 0.0056) and the average disease scores (r = 0.32; p < 0.0001).
The data were also analyzed using disease scores as independent variables. The average percent lesion area that corresponded to each value on the 0–5 visual scale was calculated for each of the raters and for their average scores (). The disease scores of Raters 1 and 2 were not significantly different based on Fisher's protected least significant difference (p = 0.05); however, Rater 1 scored more than twice as many leaves “0” (no disease symptoms) compared to Rater 2, while Rater 2 scored more than twice as many leaves “1” compared to Rater 1. Even so, there was still good agreement in the percent lesion area of each rater at each score; i.e., the average percent lesion area of the 335 leaves scored “0” by Rater 1 was 0.6% while the average of the 124 leaves scored “0” by Rater 2 was 0.3%. The average lesion area scored “1” by Raters 1 and 2 was 2.1% and 2.2%, respectively. The differences between the two raters in the percent average lesion area became greater as the values of the visual scale increased. The average percentage lesion area for Rater 1 for a disease score of “3” was nearer to Rater 2's average for a disease score of “2”, and Rater 1's “4” score corresponded to a lesion area of 35%, which was closer to that of Rater 2's score for a “3”. The increase in percent lesion area as each value of the visual scale increased was exponential as indicated in
TABLE 1 Average Percent Lesion Area Corresponding to Each Value of the 0–5 Visual Scale for Each of Two Independent Raters and Their Average Disease Scores Following Inoculation of Detached Strawberry Leaves with Three Colletotrichum Species
FIGURE 2 Average percent lesion area vs. disease score of 21 strawbery clones following inoculation with three isolates of Colletotrichum spp. Percent lesion area was calculated from computer image analysis of photographed leaves. Disease scores are based on visual ratings by two independent raters and the average of their ratings. Exponential trend lines were calculated from the disease scores of each of two raters and their average score.
There were significant differences among clones in the percent lesion area and in the disease scores of the two raters and their average disease score (). These differences can be used to identify resistant and susceptible clones. When data from all three isolates are averaged across clones, those with average percent lesion area ≤7% are considered resistant, those with an average percent lesion area >12% are considered susceptible, and those with average percent lesion areas >7% to 12% are intermediate. Similarly average disease scores can also be used for determining resistance or susceptibility. Clones with average disease scores ≤1.2 are considered resistant, those with scores >1.5 are susceptible, and those whose scores are >1.2 to 1.5 are intermediate. Based on the percent lesion area criteria, eight of the 13 clones in the MS group are resistant and five are intermediate, and one of the eight cultivars in the cultivar group is resistant, two are intermediate, and five are susceptible (). Based on the average disease score criteria, 12 of 13 clones in the MS group are resistant and one is intermediate. One of eight clones in the cultivar group is resistant, two are intermediate, and five are susceptible ().
TABLE 2 Average Percent Lesion Area and Average Disease Score for Two Raters for Each of 21 Strawberry Clones Following Inoculation of Detached Strawberry Leaves with Three Colletotrichum Species
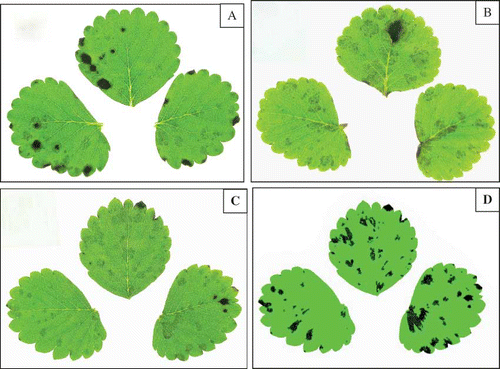
Photographs of inoculated detached leaves clearly show lesions ( and ), and even without digital analysis, these photographs can be used to visually score the leaves at a time convenient for each rater. Computer analysis of the photographs was capable of identifying more lesions than the observers who rated the leaves in the laboratory. and show the difference in an original leaf photograph and the same photograph after computer enhancement. Backlighting the leaflets in the photograph () enhanced the lesion areas by increasing the contrast between healthy and diseased tissue. This contrast would have increased the ability of raters to visualize the disease incidence more clearly than was possible when viewing the leaves under laboratory lights. The computer enhanced photograph () shows the increased contrast achieved by artificially coloring the healthy tissue pure green and diseased tissue pure black, thus enhancing the computer assessment of percent lesion area.
FIGURE 3 Anthracnose leaf spot on strawberry leaves following inoculation with Colletotrichum gloeosporioides (A) and C. fragariae (B). Original photograph of a leaf inoculated with C. gloeosporioides (C) and the same photograph computer enhanced (D). Visual scores for this leaf were 3 (Rater 1) and 2 (Rater 2), and 2.5 (average). Computer analysis determined the percent lesion area to be 10.2% (color figure available online).
CONCLUSIONS
The choice of a disease rating method must take into consideration the purpose of the research as well as the time, practicality, cost, and accuracy of the ratings. CitationKwack et al. (2005) compared visual to image assessment of cucumber leaf anthracnose lesions and found the visual assessment had significantly higher scores than the image analysis on almost all tests. The over estimation of disease severity by visual assessment at the lower disease incidence was confirmed by CitationSherwood et al. (1983) with the greatest overestimation occurring in leaves with the least infected areas. Overestimating disease severity could lead to discarding possible breeding stock that may have better rate-limiting resistance than other plants in the trial. Image analysis may decrease the possibility of making such mistakes.
CitationKwack et al. (2005) used scanned images of cucumber leaves and digitally manipulated the photographs for disease assessment. They determined that the photographic manipulation required about 3 min per cucumber leaf sample and surmised their method would work well for a small number of samples. Three minutes is not a large amount of time, but if a study requires hundreds or thousands of photographs to be manipulated for assessment, time becomes critical in completing the task. Strawberry leaves require an additional step of removing leaflets from the petiole before photographing, which adds more time to the overall process.
Image analysis is more precise than visual ratings, so we compared percent lesion area obtained using image analysis to visual disease scores and found a strong positive correlation. Image analysis is appealing as a means of assessing percent lesion areas, but it has the major disadvantage of requiring considerable time to prepare and photograph the leaves, enhance the photographs, and mark each lesion. The strong positive correlation of the percent lesion area obtained by image analysis and the visual disease scores of the two independent raters and their average disease score suggests that visual disease scores are sufficiently reliable and reproducible to determine disease response in inoculated plant material. The use of an average score calculated from the scores of two or more independent, trained raters should increase the reliability of visual scores.
Results from the comparison of the disease scores and percent lesion area from our inoculations of detached strawberry leaves indicate that anthracnose resistant germplasm can be distinguished from susceptible germplasm. Compared to whole plant inoculations, inoculation of detached leaves eliminates the need to sacrifice whole plants, decreases the space needed for the plants, and can be accomplished in a laboratory setting without the possibility of the pathogen being introduced into the environment. Results are obtained more rapidly, thus benefiting breeding programs whose goals are to develop resistant cultivars. However, detached leaf inoculation techniques may not entirely capture all nuances of strawberry leaf disease caused by Colletotrichum species. Detached leaf inoculations can be used as a rapid preliminary screen to eliminate susceptible germplasm from large populations in breeding programs.
ACKNOWLEDGMENT
We wish to acknowledge the North American Strawberry Growers Association, Inc. for funding part of this research.
LITERATURE CITED
- Abril , M. , Curry , K.J. , Smith , B.J. , Delucca , A.J. , Boue , S. and Wedge , D.E. 2009 . Greenhouse and field evaluation of the natural saponin CAY-1 for control of several strawberry diseases . Intl. J. Fruit Sci. , 9 : 211 – 220 .
- Couture , L. 1980 . Assessment of severity of foliage diseases of cereals in cooperative evaluation tests . Can. Plant Dis. Survey , 60 : 8 – 10 .
- Horsfall , J.G. and Barratt , R.W. 1945 . An improved grading system for measuring plant disease . Phytopathology , 35 : 655
- Kwack , M.S. , Kim , E.N. , Lee , H. , Kim , J. , Chun , S. and Kim , K.D. 2005 . Digital image analysis to measure lesion area of cucumber anthracnose by Colletotrichum orbiculare . J. Gen. Plant Pathol. , 71 : 418 – 421 .
- Russell , G.E. 1978 . Plant breeding for pest and disease resistance , 485 London : Butterworths .
- Sherwood , R.T. , Berg , C.C. , Hoover , M.R. and Zeiders , K.E. 1983 . Illusions in visual assessment of Stagonospora leaf spot of orchard grass . Phytopathology , 73 : 173 – 177 .
- Slopek , S.W. 1989 . An improved method of estimating percent leaf area diseased using a 1 to 5 disease assessment scale . Can. J. Plant Pathol. , 11 : 381 – 387 .
- Smith , B.J. 1998a . “ Anthracnose leaf spot and irregular leaf spot ” . In Compendium of strawberry diseases , 2nd , Edited by: Maas , J.L. 24 – 25 . St. Paul , MN : American Phytopathological Society .
- Smith , B.J. 1998b . “ Anthracnose fruit rot ” . In Compendium of strawberry diseases , 2nd , Edited by: Maas , J.L. 31 – 33 . St. Paul , MN : American Phytopathological Society .
- Smith , B.J. and Black , L.L. 1990 . Morphological, cultural and pathogenic variation among Colletotrichum species isolated from strawberry . Plant Dis. , 74 : 69 – 76 .
- Wang , X. , Wedge , D.E. , Nurhayat , T. , Johnson , R.D. , Cutler , S.J. , Pace , P.F. , Smith , B.J. and Zhou , L. 2008 . Development of a miniaturized 24-well strawberry leaf disk bioassay for evaluating natural fungicides . Natural Prod. Commun. , 3 : 1079 – 1084 .