Abstract
Strawberry production is increasing annually, with the world production exceeding 4 million tons. Virus diseases of strawberry are also increasing as the crop is planted in new regions and exposed to new viruses. A decade ago there were about a dozen viruses known to infect strawberry. There are now seven known aphid transmitted viruses—Strawberry crinkle, Latent C, Mottle, Mild yellow edge, Pseudo mild yellow edge, Vein banding, and Chlorotic fleck. Whitefly transmitted viruses have become more important; four criniviruses and one geminivirus have emerged as new threats to strawberry in areas where vectors are present. The ilarviruses that infect strawberry include Strawberry necrotic shock (previously misdiagnosed as Tobacco streak), Tobacco streak, Fragaria chiloensis latent, and Apple mosaic viruses. Strawberry necrotic shock is the predominant ilarvirus in the United States, whereas Fragaria chiloensis latent has significant presence in Chile. Modern strawberry cultivation has minimized the impact of nematode transmitted viruses but the elimination of methyl bromide may lead to the reemergence of this virus group in the future. With the knowledge we have acquired over the last decade, it is now possible to have robust certification systems, the cornerstone for minimizing the impact and spread of strawberry viruses.
Keywords:
INTRODUCTION
It has been more than 80 years since the initial work on strawberry virus-like diseases in the 1920s by CitationPlakidas (1927). During this period, there have been several diseases of virus or virus-like etiology identified primarily because of the work of Norman Frazier in the second part of the 20th century, the period when the majority of strawberry virus diseases were identified. Since 1990, with the application of molecular biology tools, many of the strawberry graft-transmissible agents were identified and characterized giving new breath to Strawberry Virology. In the last decade, a coordinated effort to characterize agents associated with major disease epidemics, primarily on the west coast of North America, has shed additional light on the complex interactions between strawberry and strawberry viruses. As strawberry production expands—China now has the largest acreage planted in the world—it is almost certain that some of the strawberry viruses and diseases will reemerge, whereas strawberry cultivation in areas without any previous history of the crop will most certainly result in new pathogens and diseases on strawberry. This communication provides a brief review of the current knowledge on strawberry viruses, their biology, and impact in production in North America and beyond. The presentation of the different set of viruses will be based on their mode of transmission, as this is the primary focus of control (see for details). Control strategies developed for any virus transmitted by a given type of vector is usually efficient for control of all viruses transmitted by that vector group, i.e., aphids, whiteflies, and nematodes.
TABLE 1 Strawberry Viruses, Names, Acronyms, Natural Modes of Transmission, Genera, and Means of Laboratory DetectionFootnote z
APHID-TRANSMITTED VIRUSES
Aphid-borne strawberry viruses are the single most important and best studied virus group, both in virus characterization as well as epidemiology. There are now seven strawberry viruses known to be transmitted by aphids, primarily the strawberry aphid, Chaetosiphon fragaefolii. Two of them are very similar as they belong to the same virus family, whereas the transmission properties of the rest vary.
The two similar viruses are Strawberry crinkle virus (SCV) and Strawberry latent C virus (SLCV). Although the diseases associated with the two viruses were described in the 1930s and 1950s, respectively (CitationDemaree and Marcus, 1951; CitationZeller and Vaughan, 1932), it was not until the 1970s and 1980s that the viruses were identified (CitationRichardson et al., 1972; CitationYoshikawa et al., 1986). Both are negative strand RNA viruses belonging to the family Rhabdoviridae and are transmitted in a persistent-replicative manner by Chaetosiphon species (CitationKrczal, 1982). The mode of transmission indicates that the viruses not only infect plants but also their vectors. This property has an important role in virus epidemiology, as those viruses typically have long acquisition and transmission periods, which are heavily influenced by environmental conditions. After acquisition the virus moves across membranes and infects vector cells. Transmission does not actually occur until there is systemic infection and active virus replication in the vector. Once a vector is infected, it can then transmit the virus for life. Because of those facts rhabdoviruses can be very important in commercial production settings if there is no active pest management program, but they are easy to control with targeted spraying regimes. SCV is better studied and several detection protocols have been developed (CitationPosthuma et al., 2002; CitationThompson et al., 2003). The virus is a major component of disease complexes that have caused significant losses in the last decade in the northern latitudes of North America (CitationMartin and Tzanetakis, 2006). SLCV is less well-studied and there are no laboratory detection protocols available, thus detection relies on grafting on the F. vesca indicator UC-5 or EMC. Several experiments have indicated that the virus does not cause symptoms in single infections in modern cultivars but it does have a synergistic effect in disease severity when found in complexes with other aphid-borne viruses (CitationMiller, 1960).
Strawberry mild yellow edge virus (SMYEV) is the only potexvirus known to be transmitted by a vector, members of the genus Chaetosiphon. The disease associated with the virus was first described in the 1920s by CitationPlakidas (1927) when transmission experiments with Chaetosiphon fragaefolii were performed. In the early days of mild yellow edge research it was observed that affected plants were infected with a spherical virus, very different from the flexuous particle of a potexvirus (CitationMartin and Converse, 1985). After the development of an infectious clone of the potexvirus it became apparent that it can cause mild yellow edge disease symptoms on indicators but was not aphid transmissible (CitationLamprecht and Jelkmann, 1997). The combination of the infectious clone experiments and the spherical virus observations indicate that mild yellow edge is a complex disease where the infectious agent, the potexvirus, may be assisted by a helper virus that functions as a vehicle for aphid transmission. Transmission is persistent but not propagative (the virus does not replicate in the vector) and can last for several weeks after acquisition. The control scheme implemented for rhabdoviruses would also minimize movement of SMYEV and its vector in the field. As one of the most important strawberry viruses, SMYEV has been studied in great detail. It has been shown that there are several distinct isolates around the world that can make molecular detection rather challenging (CitationThompson and Jelkmann, 2003).
Strawberry mottle virus (SMoV) is probably the most common virus in strawberry. It was first described in the 1940s and given the name mild crinkle (CitationPrentice and Harris, 1946). SMoV is found in all areas where its vectors, Aphis gossypii (cotton aphid) and Chaetosiphon species, are present. SMoV is a member of the genus Sadwavirus (CitationThompson et al., 2002) in the newly formed family Secoviridae, is transmitted in a semi-persistent mode, and normally acquisition and transmission require a few hours. Transmissibility of the virus can be well over 50% when a large number of aphids feed on strawberry (CitationEulense, 1981). This virus can cause up to 30% yield losses in sensitive cultivars (CitationFreeman and Mellor, 1962) although almost all modern cultivars do not show any obvious symptoms in single infections. Notwithstanding, the virus gives symptoms on F. vesca indicators ranging from mild mottling to severe epinasty depending on the indicator, the strain, and the presence of additional viruses in the source plant (CitationMellor and Krczal, 1987; ). Given that the time between acquisition and efficient transmission is about 1 hr, chemical control can be an effective way to minimize spread of the virus in the field.
FIGURE 1 Fragaria vesca infected with Strawberry mottle virus. Notice the malformation and yellowing along the veins (color figure available online).
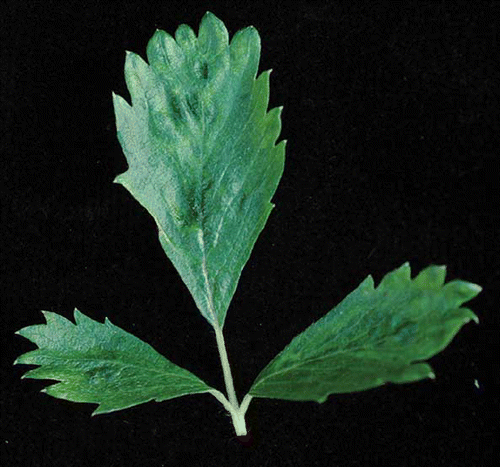
Strawberry vein banding virus (SVBV) was discovered in the 1950s by CitationFrazier (1955) and is the first DNA virus described in the crop, belonging to the genus Caulimovirus (CitationPetrzik et al., 1998). The virus is transmitted in a semi-persistent manner by at least three Chaetosiphon species (C. fragaefolii, C. thomasi, and C. jacobi) (CitationFrazier, 1960). SVBV causes some of the most pathognomonic symptoms among strawberry viruses when grafted to F. vesca and F. virginiana indicators ranging from leaf curling and vein banding to leaf necrosis (CitationFrazier and Morris, 1987; ), although today there are sensitive molecular tests for the detection of the virus (CitationThompson et al., 2003). As in the case of most viruses presented here, SVBV does not cause severe symptoms in single infections in modern cultivars but has a synergistic effect when found in plants infected with other strawberry viruses.
FIGURE 2 Fragaria × anannassa infected with Strawberry vein banding virus. Notice the yellowing of the main veins (color figure available online).

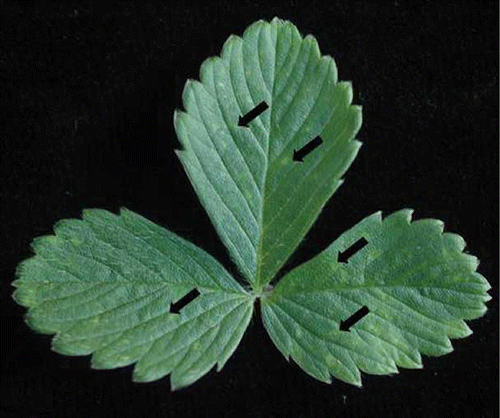
A new closterovirus identified recently has been associated with chlorotic fleck disease (CitationTzanetakis and Martin, 2007). The disease named after the chlorotic flecks on the F. vesca indicators () was first described in the 1960s. It was reported that the disease could cause up to 70% runner reduction and that the causal agent was transmitted with the cotton aphid (CitationHorn and Carver, 1962). Strawberry chlorotic fleck associated virus (SCFaV) does not appear to be widespread in strawberry production fields in the United States as it has been only detected in California (Martin and Tzanetakis, unpublished), whereas there is an unpublished report of the virus in Italy (Ratti, personal communication). As a typical closterovirus, SCFaV is expected to be transmitted in a semi-persistent manner.
FIGURE 3 Fragaria vesca infected with Strawberry chlorotic fleck associated virus. Arrows point to the chlorotic flecks associated with the disease (color figure available online).
Strawberry pseudo mild yellow edge virus is a carlavirus that is semi-persistently transmitted by Chaetosiphon sp. and A. gossypii (CitationYoshikawa and Inouye, 1986). It is symptomless in single infections and its importance as a potential component of virus complexes is unknown. The virus has been reported in the United States and Japan and detection is based on F. vesca and F. virginiana indicators where it causes red discoloration and even necrosis on older leaves. At this point there are no commercial detection tests available for the virus (CitationYoshikawa and Inouye, 1988).
WHITEFLY-TRANSMITTED VIRUSES
In the last decade there have been several whitefly-transmitted viruses discovered in strawberry. Four of them are criniviruses associated with pallidosis, a disease first described in the 1950s, and the fifth is a new geminivirus recently discovered in Egypt. Pallidosis is one of the most interesting strawberry disorders as it gives only mild symptoms on F. virginiana indicators () and remains symptomless in F. vesca indicators and F. × ananassa cultivars. Two of the pallidosis-associated viruses, Strawberry pallidosis associated virus (SPaV) and Beet pseudo-yellows virus (BPYV), are fully characterized whereas the other two are incomplete. SPaV and BPYV are transmitted solely by the greenhouse whitefly (T. vaporiorum) in a semi-persistent manner (CitationTzanetakis et al., 2006b). The vectors need to feed for a prolonged period of time (∼6 hr) on infected material before they acquire the viruses. Transmission does not happen until after 12 hr after acquisition. Both viruses are very widespread where the vector is present and have been detected in North America in California, the eastern seaboard, and Canada (Martin and Tzanetakis, unpublished). Both viruses have also been detected in South America (CitationWintermantel et al., 2006b) and the Middle East (CitationRagab et al., 2009). SPaV has a narrow host range that extends to only a few plant species outside of the genus Fragaria, whereas BPYV can infect a wide range of plant species, making its management more difficult (CitationTzanetakis et al., 2006). Neither virus causes any visual symptoms in single infections in modern strawberry cultivars but they appear to play a major role in symptom development when found in mixed infections with the majority of the viruses presented here (CitationMartin and Tzanetakis, 2006). Geminiviruses are transmitted by Bemisia tabaci whiteflies and Strawberry leaf curl virus (StLCV) is the first known to infect strawberry. It has been associated with leaf curling and cupping but its effect on yield or in mixed infections is yet to be studied (CitationEl-gaied et al., 2008).
POLLEN-BORNE VIRUSES
Pollen-borne viruses could be important to any crop and strawberry is no exception. The pollen-borne viruses affecting strawberry are members of the genus Ilarvirus, namely Strawberry necrotic shock virus (SNSV), Tobacco streak virus (TSV), Fragaria chiloensis latent virus (FClLV), and Apple mosaic virus (ApMV). The host range of each of these viruses include several rosaceous hosts and the fact that thrips can move infected pollen grains and transmit viruses makes control even more complex (CitationSdoodee and Teakle, 1987). Until recently, it was thought that TSV is the only ilarvirus that infects strawberry in North America, but we now know that this virus is rarely present in strawberry plants. There are laboratory tests for the four ilarviruses that have been used to determine their incidence in production fields in North American and elsewhere. The most widespread of the ilarviruses in strawberry is SNSV, once thought to be a strain of TSV (CitationTzanetakis et al., 2004). FClLV is widespread in South America and a few positive plants have been found in Canada (Martin and Tzanetakis, unpublished). ApMV has been associated with the leafroll disease and is present in the field, again in a small number of samples (CitationTzanetakis and Martin, 2005). The role of the four viruses in yield losses is unknown but they are believed to be significant players in mixed infections with other strawberry viruses as is the case with other ilarviruses infecting rosaceous hosts. The only known control measure known for ilarviruses is exclusion.
NEMATODE-TRANSMITTED VIRUSES
Nematodes have been known to transmit several strawberry viruses since the early 1960s. The use of methyl bromide and other soil fumigants have diminished the importance of the five strawberry nematode-transmitted viruses, which are now very rarely detected in strawberry fields. The elimination of methyl bromide as a soil fumigant and potential loss of other fumigants may reverse this trend, and these viruses could reemerge as a major problem in the crop. The nematode-transmitted viruses in strawberry are Strawberry latent ringspot virus (SLRSV), Arabis mosaic virus (ArMV), Raspberry ringspot virus (RpRSV), Tomato black ring virus (TBRV), and Tomato ringspot virus (ToRSV). All but SLRSV are members of the genus Nepovirus in the family Secoviridae. SLRSV is an unassigned member of the family (CitationTzanetakis et al., 2006a). All but Tomato ringspot are considered European viruses as they have never or rarely been found established in New World soils. Tomato ringspot, on the other hand, is a New World virus. The vectors of SLRSV, ArMV, and ToRSV belong to the genus Xiphinema, whereas TBRV and RpRSV are transmitted by members of the genus Longidorus (CitationMartin and Tzanetakis, 2006). There is a single report of RpRSV also being transmitted by Paratrichodorus and Xiphinema species (CitationTrudgill et al., 1983). All nematode-transmitted viruses have an extensive host range to hundreds of species of both monocots and dicots. Although all strawberry nematode-transmitted viruses are well studied and several detection protocols have been developed, their management is a difficult undertaking once established in a field. For this reason the best management strategy is to avoid introduction by minimizing soil movement. Non-chemical treatment requires long fallow periods or crop rotation with a nonhost of the virus (CitationPinkerton and Martin, 2005).
MINOR VIRUSES
There are a few additional viruses known to infect strawberry, namely Tobacco necrosis virus D (CitationFránová-Honetšlegrová et al., 1998), Fragaria chiloensis cryptic virus (CitationTzanetakis et al., 2008), and Strawberry latent virus (CitationTzanetakis and Martin, 2008). These viruses are not well studied in strawberry, but their nature suggests that they are probably not causing any symptoms nor would they affect disease development in mixed infections.
DISCUSSION
The expansion of strawberry production into areas without history of the crop will certainly lead to the introduction of new viruses into strawberry as is the case of the newly identified geminivirus. Geminiviruses are normally associated with plants grown in tropical and subtropical environments, but today with the expansion of the production and global warming their vector has expanded its geographic range and with it the virus host range to include plants that grow in temperate climates like strawberry (CitationTzanetakis et al., 2006b).
Today, where the major strawberry production is carried out on raised beds in an annual cropping system, virus importance should be diminishing. This, of course, depends on the production of plants free of known viruses for planting these fields. If nursery stocks are infected, severe virus problems can occur in annual production fields. Since plants are propagated in nurseries for several years to obtain the numbers needed to plant production fields, care must be taken to prevent virus introduction and spread in the nurseries. For this reason, management practices in propagation nurseries, is the single most important measure to minimize virus problems in the future. In areas that produce fruit in a perennial system for fruit production, care must be taken to control viruses in the fruiting fields depending on the vector and virus pressure in the region.
ACKNOWLEDGMENTS
The authors acknowledge the North American Strawberry Growers Association and the California Strawberry Commission for funding part of the research performed on whitefly transmitted viruses.
LITERATURE CITED
- Demaree , J.P. and Marcus , C.P. 1951 . Virus diseases of strawberries in the United States with special reference to distribution, indexing and insect vectors in the East . Plant Dis. Reptr. , 35 : 527 – 537 .
- El-gaied , L.F. , Salama , M.I. , Salem , A.M. , Nour El-deen , A.F. and Abdallah , N.A. 2008 . Molecular and serological studies on a plant virus affecting strawberry . Arab J. Biotech. , 11 : 303 – 314 .
- Eulense , G.E. 1981 . Differential transmission of strawberry mottle virus by Chaetosiphon thomas Hille Ris Lambers and Chaetosiphon fragaefolii (Cockerell) , 30 Corvallis , OR : Oregon State University . M.S. Thesis
- Fránová-Honetšlegrová , J. , Erbenova , M. and Martin , R.R. 1998 . Isolation of tobacco necrosis virus from strawberry leaves in the Czech Republic . Acta Virol. , 42 : 325 – 331 .
- Frazier , N.W. and Morris , T.J. 1987 . “ Strawberry vein banding ” . In Virus diseases of small fruits , Edited by: Converse , R.H. 16 – 20 . Washington , D.C. : USDA ARS Agriculture Handbook No. 631 .
- Frazier , N.W. 1955 . Strawberry vein banding virus . Phytopathology , 45 : 307 – 312 .
- Frazier , N.W. 1960 . Differential transmission of strains of strawberry vein banding virus by four aphid vectors . Plant Dis. Reptr , 44 : 436 – 437 .
- Freeman , J.A. and Mellor , F.C. 1962 . Influence of latent viruses on vigor, yield and quality of British Sovereign strawberries . Can. J. Plant Sci. , 42 : 602 – 610 .
- Horn , N.L. and Carver , R.G. 1962 . Effects of three viruses in plant production and yields of strawberries . Plant Dis. Rep. , 46 : 762 – 765 .
- Krczal , H. 1982 . Investigations on the biology of the strawberry aphid (Chaetosiphon fragaefolii), the most important vector ofstrawberry viruses in West Germany . Acta Hort. , 129 : 63 – 68 .
- Lamprecht , S. and Jelkmann , W. 1997 . Infectious cDNA clone used to identify strawberry mild yellow edge associated potexvirus as the causal agent of the disease . J. Gen. Virol. , 73 : 475 – 479 .
- Martin , R.R. and Converse , R.H. 1985 . Purification, properties and serology of strawberry mild yellow-edge virus . Phytopathol. Z. , 114 : 21 – 30 .
- Martin , R.R. and Tzanetakis , I.E. 2006 . Characterization, detection and management of strawberry viruses . Plant Dis. , 90 : 384 – 396 .
- Mellor , F.C. and Krczal , H. 1987 . “ Strawberry mottle ” . In Virus diseases of small fruits , Edited by: Converse , R.H. 10 – 16 . Washington , D.C. : USDA ARS Agriculture Handbook No. 631 .
- Miller , P.W. 1960 . Rapid degeneration of some strawberry varieties and selected seedlings infected with latent C virus in combination with other viruses . Plant Dis. Reptr. , 44 : 796 – 799 .
- Petrzik , K. , Beneš , V. , Mráz , I. , Honetšlegrová-Fránová , J. , Ansorge , W. and Špak , J. 1998 . Strawberry vein banding virus—Definitive member of the Genus Caulimovirus . Virus Genes , 16 : 303 – 305 .
- Pinkerton , J.N. and Martin , R.R. 2005 . Management of Tomato ringspot virus in red raspberry with crop rotation . Int. J. Fruit Sci. , 5 : 55 – 67 .
- Plakidas , A.G. 1927 . Strawberry xanthosis (yellows) a new insect-borne disease . J. Agri. Res. , 35 : 1057 – 1090 .
- Posthuma , K.I. , Adams , A.N. , Hong , Y. and Kirby , M.J. 2002 . Detection of Strawberry crinkle virus in plants and aphids by RT-PCR using conserved L gene sequences . Plant Pathol. , 51 : 266 – 274 .
- Prentice , I.W. and Harris , R.V. 1946 . Resolution of strawberry virus complexes by means of the aphis vector Capitophorus frugariae Tlieob . Ann. Appl. Biol. , 33 : 50 – 53 .
- Ragab , M. , El-Dougdoug , K. , Mousa , S. , Attia , A. , Sobolev , I. , Spiegel , S. , Freeman , S. , Zeidan , M. , Tzanetakis , I.E. and Martin , R.R. 2009 . Detection of strawberry viruses in Egypt . Acta Hort. , 842 : 319 – 322 .
- Richardson , J. , Frazier , N.W. and Sylvester , E. 1972 . Rhabdovirus-like particles associated with strawberry crinkle virus . Phytopathology , 62 : 491 – 492 .
- Sdoodee , R. and Teakle , D.S. 1987 . Transmission of Tobacco streak virus by Thrips tabaci: A new method of plant virus transmission . Plant Pathol. , 36 : 377 – 380 .
- Thompson , J.R. , Wetzel , S. , Klerks , M.M. , Vašková , D. , Schoen , C.D. , Špak , J. and Jelkmann , W. 2003 . Multiplex detection of four aphid-borne viruses in Fragaria spp. in combination with a plant mRNA specific internal control . J. Virol. Methods , 111 : 85 – 95 .
- Thompson , J.R. and Jelkmann , W. 2003 . Strain diversity and conserved genome elements in Strawberry mild yellow edge virus . Arch. Virol. , 149 : 1897 – 1909 .
- Thompson , J.R. , Leone , G. , Lindner , J.L. , Jelkmann , W. and Schoen , C.D. 2002 . Characterization and complete nucleotide sequence of Strawberry mottle virus: A tentative member of a new family of bipartite plant picorna-like viruses . J. Gen. Virol. , 83 : 229 – 239 .
- Trudgill , D.L. , Brown , D.J.F. and McNamara , D.G. 1983 . Methods and criteria for assessing the transmission of plant viruses by longidorid nematodes . Rev. Nematol. , 6 : 133 – 141 .
- Tzanetakis , I.E. and Martin , R.R. 2005 . First report of strawberry as a natural host of Apple mosaic virus . Plant Dis. , 89 : 431
- Tzanetakis , I.E. , Mackey , I.C. and Martin , R.R. 2004 . Strawberry necrotic shock virus is a distinct virus and not a strain of Tobacco streak virus . Arch. Virol. , 149 : 2001 – 2011 .
- Tzanetakis , I.E. and Martin , R.R. 2007 . Strawberry chlorotic fleck: Identification and characterization of a novel Closterovirus associated with the disease . Virus Res. , 124 : 88 – 94 .
- Tzanetakis , I.E. and Martin , R.R. 2008 . How similar are plant and insect viruses? Strawberry latent virus: A case study . Acta Hort. , 780 : 17 – 20 .
- Tzanetakis , I.E. , Postman , J.D. , Gergerich , R.C. and Martin , R.R. 2006a . A virus between families: Nucleotide sequence and evolution of Strawberry latent ringspot virus . Virus Res. , 121 : 199 – 204 .
- Tzanetakis , I.E. , Price , R. and Martin , R.R. 2008 . Nucleotide sequence of the tripartite Fragaria chiloensis cryptic virus and presence of the virus in the Americas . Virus Genes , 36 : 267 – 272 .
- Tzanetakis , I.E. , Wintermantel , W.M. , Cortez , A.A. , Barnes , J.E. , Barrett , S.M. , Bolda , M.P. and Martin , R.R. 2006b . Epidemiology of Strawberry pallidosis associated virus and occurrence of pallidosis disease in North America . Plant Dis. , 90 : 1343 – 1346 .
- Wintermantel , W.M. , Fuentes , S. , Chuquillanqui , C. and Salazar , L.F. 2006 . First report of Beet pseudo-yellows virus and Strawberry pallidosis associated virus in Strawberry in Peru . Plant Dis. , 90 : 1455
- Yoshikawa , N. and Inouye , T. 1986 . Purification, characterization and serology of strawberry pseudo mild yellow-edge virus . Ann. Phytopathol. Soc. Jpn. , 52 : 643 – 652 .
- Yoshikawa , N. and Inouye , T. 1988 . Strawberry viruses occurring in Japan . Acta Hort. , 236 : 59 – 67 .
- Yoshikawa , N. , Inouye , T. and Converse , R.H. 1986 . Two types of rhabdovirus in strawberry . Ann. Phytopathol. Soc. Japn. , 52 : 437 – 444 .
- Zeller , S.M. and Vaughan , E.K. 1932 . Crinkle disease of strawberry . Phytopathology , 22 : 709 – 713 .
