Abstract
The anthracnose pathogens, Colletotrichum acutatum, C. gloeosporioides, and C. fragariae, have developed resistance to several fungicides. We used a microtiter assay to test in vitro the activity of 16 agrochemicals against 10 isolates of Colletotrichum spp. using a dose-response format. At a concentration of 30 μM, captan, thiram, cyprodinil, chlorothalonil, azoxystrobin, and kelthane provided nearly 100% inhibition of the growth of all 10 Colletotrichum isolates. Iprodione, vinclozolin, metalaxyl, and fosetyl-Al did not inhibit growth of any isolate. Benomyl and thiobendazole inhibited growth of the C. fragariae and C. gloeosporioides isolates, but did not inhibit growth of the six C. acutatum isolates. Footnote Footnote
This article not subject to US copyright law.
Patrick Page is currently affiliated with the School of Pharmacy, University of Southern Mississippi.
INTRODUCTION
Fruit rot diseases of strawberry (Fragaria × ananassa Duch.) represent serious problems for producers in many areas of the world (CitationMaas, 1998), and they are particularly severe in the southeastern United States where diseases are often favored by warm temperatures and frequent rains during the harvest season. Anthracnose diseases caused by Colletotrichum spp. (CitationSmith, 1998) can be especially devastating since, in addition to inciting fruit rot, they can also attack other parts of the strawberry plant. The pathogens, Colletotrichum acutatum Simmonds, C. fragariae Brooks, and C. gloeosporioides (Penz.) Penz. and Sacc., can occur singly or in combination, and can infect flowers, fruit, leaves, petioles, stolons, roots, and crowns. New approaches to anthracnose disease control are necessary as the effectiveness and availability of commercial fungicides decreases. Chemical control of these diseases has become more difficult as several effective fungicides are no longer sold in the United States or have lost their registration for use on strawberries, and some of the pathogens have developed resistance to the more commonly used fungicides. Numerous isolates of Colletotrichum spp. developed benomyl resistance after that fungicide had been used for several years to protect strawberry from various diseases (CitationSmith and Black, 1993a, Citation1993b). In the current study, we evaluated 16 agrochemicals that are currently used or have been used for control of strawberry pests and diseases. Agrochemicals were tested in a micro-dilution broth assay for in vitro activity against isolates of C. acutatum, C. fragariae, and C. gloeosporioides collected from petiole, fruit, and crowns of strawberries grown on farms located in several Gulf coast and Mid-Atlantic states.
MATERIALS AND METHODS
Pathogen Production and Inoculum Preparation
Spores of Colletotrichum spp. were collected from strawberry fruit and petioles by rubbing a sterile cotton swab gently across the surface of typical anthracnose lesions. Fungal cultures were isolated by streaking the surface of acidified potato dextrose agar (A-PDA; Difco, Detroit, MI, USA) with the cotton swabs. Isolates were also obtained from the crowns of symptomatic wilted or dead plants by aseptically plating a small piece of crown tissue displaying a reddish discoloration onto A-PDA. Conidia were picked from resultant Colletotrichum cultures using fine, sterile forceps under a dissecting microscope. Three to four transfers were made for each isolate to achieve an axenic culture. Pure cultures of five isolates of C. acutatum, two of C. fragariae, and two of C. gloeosporioides were selected for further study based on the geographical location from which they were obtained (). An isolate of C. acutatum obtained from an apple fruit (designated ‘CONARTY’) was included in the trials for comparison because the dicarboximide fungicides, vinclozolin and iprodione, which are commonly used on strawberry, are not used on apple. Each fungal isolate was sub-cultured on half-strength PDA and conidia were harvested from axenic cultures at 7–10-day intervals. Conidial concentrations were determined photometrically (CitationEspinel-Ingrof and Kerkering, 1991; CitationWedge and Kuhajek, 1998) from a standard curve and suspensions were then adjusted with sterile distilled water to a concentration of 106 conidia/ml.
TABLE 1 Source and Identification of 11 Colletotrichum spp. Isolates
Microtiter Assay
A standardized 96-well microtiter plate assay developed for discovery of natural product fungicidal agents (CitationWedge and Kuhajek, 1998; CitationWedge et. al., 2000) was used to establish sensitivity profiles of 10 isolates of the three Colletotrichum spp. to a variety of agrochemicals used to control strawberry pests and diseases. Each test well in a microtiter plate (Nunc MicroWell, untreated; Roskilde, Denmark) received 80 μL of Roswell Park Memorial Institute mycological broth 1640 (RPMI 1640; Life Technologies, Grand Island, NY, USA) and 3[N-morpholino] propanesulfonic acid (MOPS, Sigma Chemical Co., St. Louis, MO, USA) buffered broth, and 100 μL of 104 conidia/ml. Twenty μL of agrichemical solution were added to the appropriate wells according to the method of CitationWedge and Kuhajek (1998). The 16 agrochemicals tested are currently, previously, or anticipated to be, registered for use on strawberry (). Technical grade pesticide standards were obtained from Chem Service, Inc. (West Chester, PA, USA) for all compounds except azoxystrobin, which was obtained from Zeneca Agrochemicals (Richmond, CA, USA). Metalaxyl and fosetyl-Al were used as negative validation controls. Captan is a multisite inhibitor with no history of pathogen resistance and a long history of excellent anthracnose disease control on strawberry. Therefore, it was used as an internal fungicide standard. The microtiter plates were covered with plastic wrap and incubated in a growth chamber at 24 ± 1°C and 12 hr photoperiod under 60 ± 5 μmol·m2·s−1 light. Then the fungal growth was evaluated by measuring absorbance of each well at 620 nm using a microplate photometer (Packard Spectra Count, Packard Instrument Co., Downers Grove, IL, USA).
TABLE 2 Pesticides Used in Microtiter Assay
Each chemical was evaluated in duplicate in a dose-response format where the final concentrations were 0.3, 3.0, and 30.0 μM. Sixteen wells containing broth and inoculum served as positive growth control wells used to normalize fungal growth means and eight wells containing broth without inoculum served as negative growth control wells to indicate possible contamination of the assay. Each fungus was challenged in duplicate and data presented were pooled from two independent experiments. Mean absorbance values and standard errors were used to evaluate fungal growth at 48 and 72 hr. The SAS system analysis of variance procedure (Statistical Analysis System, Cary, NC, USA) was used to identify significant factors, and Fisher's protected LSD was used to separate means. The area under the curve was calculated using the trapezoidal rule. The PROC GLM procedure in SAS was used to fit general linear models where concentrations 0.3, 3.0, and 30.0 μM were pooled and alpha was set at 0.05.
RESULTS AND DISCUSSION
Mean percent inhibition (−) or stimulation (+) of growth of each isolate relative to its growth in the non-amended media is reported for each of the 16 agrochemicals after 48 hr at 3 and 30 μM (). The growth of all isolates except C. gloeosporioides isolate ARKP1 was significantly reduced by captan and thiram at a concentration of 0.3 μM compared to their growth on the non-amended medium. The growth of all 10 isolates was significantly reduced after 48 hr on the media amended with the 3 μM concentration of quinomethionate, cyprodinil, chlorothalonil, and azoxystrobin. At the highest concentration tested (30 μM), the growth of all 10 isolates was significantly reduced by captan, thiram, cyprodinil, chlorothalonil, azoxystrobin, kelthane, and butrizol. The growth of nine isolates was reduced when grown on the medium amended with dodine and quinomethionate at a 30-μM concentration.
Growth of the isolates on the media amended with the two benzimidazole fungicides, benomyl and thiobendazole, was variable. At a concentration of 30 μM, the growth of the two C. fragariae isolates and C. gloeosporioides isolate ARKP1 was reduced by 76% to 98%, but that of the other C. gloeosporioides isolate, CG162, was not significantly different from its growth on the non-amended medium.
Three protective fungicides (chlorothalonil, captan, and thiram) provided nearly 100% growth inhibition of all ten Colletotrichum isolates at 72 hr (data not shown). Azoxystrobin (a natural product-based strobilurin fungicide), quinomethionate (an acaricide), and cyprodinil (>3.0 μM) provided nearly 100% growth inhibition of all 10 Colletotrichum sp. isolates at 48 hr with little loss of effectiveness at 72 hr.
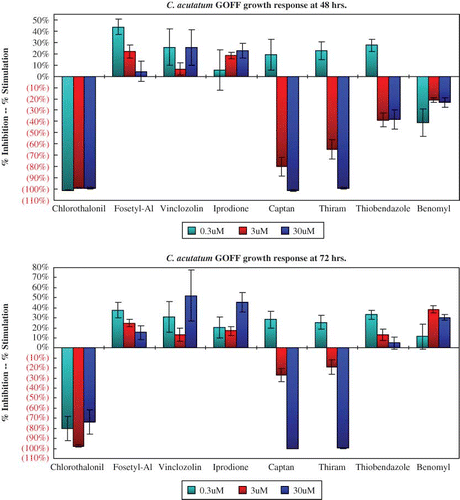
The growth response of C. acutatum isolate GOFF to eight of the chemicals tested varied (). Chlorothalonil almost completely inhibited growth of this isolate at all three concentrations tested after 48 and 72 hr. Fosetyl-Al, vinclozolin, and iprodione slightly stimulated growth compared to that of the non-amended media, and captan and thiram totally inhibited growth at 30 μM. The response of isolate GOFF to thiobendazole and benomyl demonstrates the typical response of an insensitive isolate, i.e., its growth is somewhat inhibited after 48 hr but after 72 hr its growth is greater than on the non-amended media.
FIGURE 1 Colletotrichum acutatum isolate GOFF is sensitive to benomyl and thiobendazole at 48 hr but shows well known insensitivity at 72 hr (color figure available online).
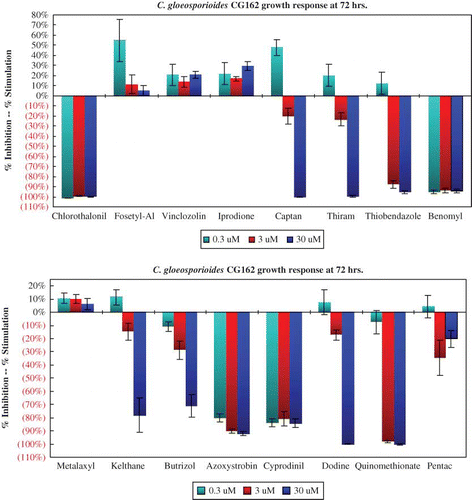
The growth response of C. acutatum isolate LA27 after 72 hr is typical of all the C. acutatum isolates to each of the 16 agrochemicals (). All six C. acutatum isolates were insensitive to benomyl, thiobendazole, vinclozolin, and iprodione at 72 hr. Both C. fragariae isolates responded in a similar manner to all 16 chemicals. The growth of isolate CF63 was inhibited by more than 90% by the higher rates of benomyl and thiobendazole (). Growth of C. gloeosporioides isolate CG162 is similar to that of the two C. fragariae isolates (). Benomyl and thiobendazole provided nearly 90% growth inhibition of both C. fragariae isolates and one of the two C. gloeosporioides isolates.
FIGURE 3 Benomyl and thiobendazole provided near 90% growth inhibition of two isolates of Colletotrichum fragariae at 72 hr (color figure available online).
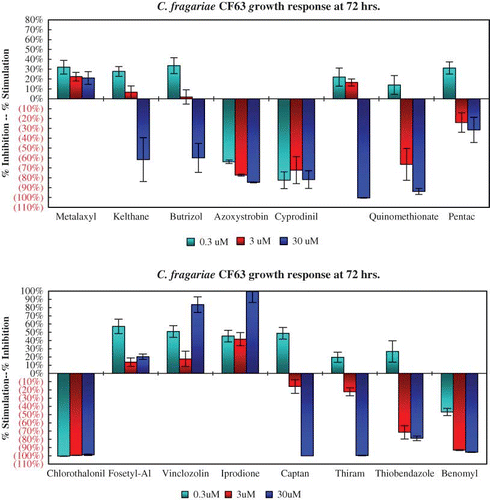
FIGURE 2 Colletotrichum acutatum isolate LA27 response is representative of all eight isolates of C. acutatum that were insensitive to benomyl, thiobendazole, and vinclozolin (color figure available online).
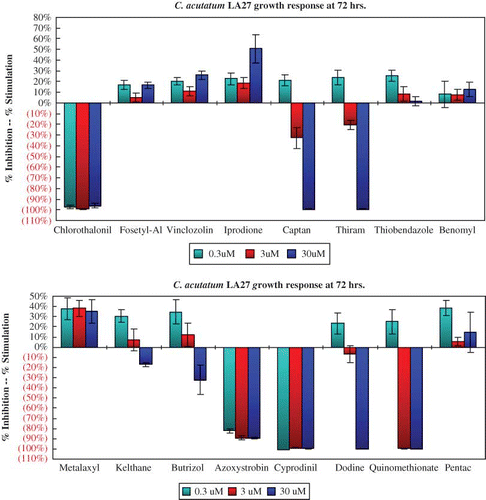
FIGURE 4 Benomyl and thiobendazole provided near 90% growth inhibition of one of two isolates of Colletotrichum gloeosporioides (color figure available online).
“Area under the curve” analyses of the growth response of the 10 isolates to the 16 agrochemicals were pooled over time and concentrated to separate the response of the isolates into either “sensitive” (growth inhibited) or “insensitive” (growth same or greater than that on the non-amended media) responses (). The response of both C. fragariae isolates and the C. gloeosporioides isolate CG162 to each chemical was the same, i.e., sensitive to all agrochemicals except iprodione, vinclozolin, and fosetyl-Al. The C. gloeosporioides isolate ARKP1 was sensitive to five chemicals: chlorothalonil, azoxystrobin, cyprodinil, captan, and quinomethionate. The response of the C. acutatum isolates was more variable. Isolates GOFF and CONARTY were only sensitive to four chemicals, chlorothalonil, azoxystrobin, cyprodinil and quinomethionate, while isolate MD1 was sensitive to all chemicals except benomyl, thiobendazole, iprodione, vinclozolin, fosetyl-Al, and metalaxyl.
CONCLUSION
In the microtiter assay at a concentration of 3.0 μM, the two dicarboximide fungicides, vinclozolin and iprodione, separated together with the internal negative standards, metalaxal and fosetyl-Al ( and ). These negative standards were chosen as known ineffective fungicides since they are active against only the oomycetes (Pythium and Phytophthora spp.). At a concentration of 30 μM, benomyl separated next to metalaxal and fosetyl-Al, with vinclozolin and iprodione immediately below. At this concentration, fungal growth is no longer in the negative range (inhibited) but is in the positive range (stimulated or hormesis, CitationCantrell et al., 2008). These data demonstrate that the benzimidazole fungicides (benomyl and thiabendazole) and the dicarboximide fungicide (iprodione) no longer provide control of these fungi (CitationWedge et al., 2007, and also support the removal of benomyl from the fungicide market in the United States in 2001.
TABLE 3 Growth Response of 10 Isolates of 3 Colletotrichum spp. to 16 Agrochemicals at 3 and 30 Micromolar Concentrations after 48 hr in Micro-Titer AssayFootnote z
At a concentration of 3.0 μM, captan and thiram segregated in the middle of below what appear to be the four most effective chemicals: quinomethionate, cyprodinil, chlorothalonil, and azoxystrobin. However, at the 30.0 μM concentration, captan and thiram exceeded the previous four chemicals, and inhibited growth in the 90% range.
When isolate GOFF was challenged with chlorothalonil, the results were inconsistent compared to the other isolates and this could be due to precipitation occurring in one of the test wells that skewed the results. The strobilurin fungicide, azoxystrobin, has a novel mode of action. It is in the class of fungicides known as quinone outside inhibitors (QoI) that interrupt energy production during respiration. This particular class of chemicals affects all energy-intensive metabolic events of the fungus, including spore germination and mycelial growth, which renders the fungus less capable of causing infection and disease development. The 96-well microtiter assay is very sensitive, but it does not measure that part of the QoI mode of action and, therefore, azoxystrobin appears slightly less active than other compounds.
The fundamental concepts to consider when comparing the position of the compounds between 3.0 and 30.0 μM are “therapeutic threshold” and “dose-response.” Growth inhibition of captan and thiram ranges from 60% to 80% at 3.0 μM and from 99% to 100% at 30.0 μM. Any test compound possessing <50% growth inhibition at 30 μM in this bioassay is considered to have weak antifungal activity. The therapeutic threshold for captan and thiram is near 30 μM, while the therapeutic thresholds for quinomethionate, cyprodinil, chlorothalonil, and azoxystrobin are below 3.0 μM. These therapeutic thresholds are reflected in the label rates of captan and thiram in pounds of product per acre (kilograms per hectare) compared to those of quinomethionate, cyprodinil, chlorothalonil, and azoxystrobin in ounces of product per acre (grams per hectare). The dose-response and hormetic effect or positive growth stimulation of several low-dose fungicides (benomyl, vinclozolin, and iprodione) in the in vitro assay illustrate the risk of applying commercial agrochemicals at improper concentrations that can result in ineffective disease control.
TABLE 4 Area under the Curve Analysis of Colletotrichum spp. Isolates versus Agrochemicals Pooled Over Time and Concentration
Older, protective, multi-site fungicides (chlorothalonil, captan, thiram, and dodine) inhibited the growth of isolates of all three Colletotrichum species in this study at the highest concentration tested. Two of the newer fungicides in the study (azoxystrobin and cyprodinil) inhibited the growth of most isolates at the lowest concentration. Two commercial formulations of these newer fungicides, Abound® (azoxystrobin, Syngenta Crop Protection, Greensboro, NC, USA) and Switch® (cyprodonil + fludioxinil, Syngenta Crop Protection, Greensboro, NC, USA), are now labeled for disease control on strawberries (CitationWedge et al., 2007).
Notes
This article not subject to US copyright law.
Patrick Page is currently affiliated with the School of Pharmacy, University of Southern Mississippi.
LITERATURE CITED
- Cantrell , C.L. , Case , B.P. , Mena , E.E. , Kniffin , T.M. , Duke , S.O. and Wedge , D.E. 2008 . Isolation and identification of antifungal fatty acids from the basidiomycete Gomphus floccosus . J. Agric. Food Chem. , 56 : 5062 – 5068 .
- Espinel-Ingrof , A. and Kerkering , T.M. 1991 . Spectrophotometric method of inoculum preparation for the in vitro susceptibility testing of filamentous fungi . J. Clin. Microbiol. , 29 : 393 – 394 .
- Mass , J.L. 1998 . Compendium of strawberry diseases , 2nd , St. Paul , MN : American Phytopathological Society .
- Smith , B.J. 1998 . “ Anthracnose fruit rot (black spot), p ” . In Compendium of strawberry diseases , 2nd , Edited by: Maas , J.L. St. Paul , MN : American Phytopathological Society . 31–33. In
- Smith , B.J. and Black , L.L. 1990 . Morphological, cultural, and pathogenic variation among Colletotrichum species isolated from strawberry . Plant Dis. , 74 : 69 – 76 .
- Smith , B.J. and Black , L.L. 1993a . In vitro activity of fungicides against Colletotrichum fragariae . Acta Hort. , 348 : 509 – 512 .
- Smith , B.J. and Black , L.L. 1993b . In vitro fungicide studies show the occurrence of benomyl-resistant Colletotrichum spp . from strawberry. Adv. Strawberry Res. , 12 : 42 – 48 .
- Wedge , D.E. and Kuhajek , J.M. 1998 . A microbioassay for fungicide discovery . SAAS Bull. Biochem. Biotech. , 11 : 1 – 7 .
- Wedge , D.E. , Galindo , J.C. and Marcias , F.A. 2000 . Fungicidal activity of natural and synthetic sesquiterpene lactone analogs . Phytochemistry , 53 : 747 – 757 .
- Wedge , D.E. , Quebedeaux , J.P. , Constantine , R.J. and Smith , B.J. 2007 . Fungicide management strategies for control of strawberry fruit rot diseases in Louisiana and Mississippi . Crop Protect. , 26 : 1449 – 1458 .