Abstract
Red raspberry (Rubus idaeus) production is a vital component of northwestern Washington's agriculture. The main objectives of this study were to document the occurrence of soilborne pathogens Phytophthora rubi and Pratylenchus penetrans in early stage production fields, relate this information to soil properties, and better understand the individual and combined effect of P. rubi and P. penetrans on raspberry root health. P. rubi was found at each field and P. penetrans population densities were variable (0 to ∼8000 nematodes/g dry root) across locations. In controlled greenhouse studies, P. rubi was very pathogenic to red raspberry ‘Meeker’ at densities >10 oospore/gram soil and there was no interaction between P. rubi and P. penetrans. P. rubi is endemic to raspberry production in this region, and is an aggressive pathogen on raspberry. However, the chronic damage to roots caused by P. penetrans should not be ignored.
Keywords:
INTRODUCTION
The Pacific Northwest region of the United States comprises 92% of the processed red raspberry (Rubus idaeus) acreage nationwide (CitationUSDA, 2009). In northwestern Washington, the raspberry industry generates $59 million in revenue annually and encompasses 3926 hectares of production (USDA, 2009). Over the past few decades the productive lifetime of plantings in this region has decreased from >10 years to ∼5 years due to apparent root health decline. As a consequence, commercial raspberry growers have made understanding the ecology of soilborne pathogens and their effect on plant health and crop yield a top research priority (www.nwsmallfruit.org).
In red raspberry, root damage caused by Phytophthora rubi Wilcox and Duncan and Pratylenchus penetrans (Cobb) Filipjev and Schurmans Stekhoven have been implicated with this decline (CitationMcElroy, 1992; CitationWilcox et al., 1993). Phytophthora rubi is a homothallic oomycete that is considered to be most active from November to March (CitationErwin and Ribeiro, 1996). In cool and saturated conditions, this pathogen causes Phytophthora root rot in most varieties of red raspberry. Root rot symptoms include a blackening discoloration of the roots and crowns and a wilting of leaves and canes in late summer (CitationErwin and Ribiero, 1996). Although raspberry germplasm with resistance to Phytophthora root rot is available, fruit from these plants are not considered commercially suitable because they are not of individually quick frozen (IQF) and other industry processing standards (CitationBristow et al., 1988; CitationPattison et al., 2004).
Pratylenchus penetrans, the root lesion nematode, is a migratory endoparasite with over 400 host plant species, including commercial crops, cover crops, and weeds (CitationDavis and MacGuidwin, 2005). Pratylenchus penetrans can complete several generations in a single growing season depending upon soil temperature. All stages of the nematode move between soil and roots and feed on and migrate in root cortical cells. Damage caused by P. penetrans generally includes a reduction in fine root abundance and the wounding of root tissue, which appear as necrotic lesions on the roots (CitationMcElroy, 1992). Raspberry germplasm resistant to P. penetrans is yet to be found and newly developed cultivars would be subject to the same constraints over IQF standards and yield parameters that exist for those resistant to Phytophthora root rot (CitationVrain and Daubeny, 1986).
While soil fumigation reduces soilborne pathogen and plant-parasitic nematode populations initially, replanted acreage in western Washington is consistently less productive than ground that does not have a history of raspberry production (R. Honcoop, personal communication). Many growers attest that the effect is most noticeable on marginal soil with poor drainage. While there has not been a typical replant disease characterized on raspberry, current research in British Columbia (S. Sabaratnam, personal communication) and Michigan (A.M. Schilder, personal communication) suggests that several soilborne pathogens may be involved. This research focused on P. rubi and P. penetrans, both well-known pathogens in the region.
CitationVrain and Pepin (1989) found that over time the presence of both P. rubi and P. penetrans caused severe stunting of canes, poor emergence of primocanes, and death of floricanes and primocanes. However, further information on the combined effect of P. rubi and P. penetrans on raspberry establishment and productivity is needed in order to develop effective management strategies. In other cropping systems, the presence of both a nematode and fungal pathogen has been found to intensify disease severity compared to when either is present alone. Examples include sudden death syndrome on soybean (Glycine max) where Heterodera glycines increases the severity of root rot caused by Phytophthora sojae (CitationXing and Wesphal, 2006).
The main objective of this study was to perform a survey in order to document the occurrence of P. rubi and P. penetrans in early production age fields (3–5 years) in northwestern Washington raspberry growing regions and to relate this information to soil properties. In addition, controlled experiments were conducted to better understand the interaction of P. rubi and P. penetrans and their effect on root growth and development. Understanding soil properties or management conditions that support or promote either or both of these organisms will help to address the long-term chronic problem of root health that is endemic to this production system.
MATERIALS AND METHODS
Commercial Red Raspberry Field Survey
Ten raspberry fields, representative of production fields in Skagit (5 fields) and Whatcom (5 fields) counties in northwestern Washington, were sampled. These fields had histories of root rot or were experiencing either above-ground root rot or replant disease symptoms and were identified with guidance from the Skagit County Extension and Whatcom County Raspberry IPM programs. Field history, variety, age of planting, and soil type were recorded. Root and soil samples were collected from 10 random sites (4.6 m long × 0.9 m wide) within each field that had varying above-ground symptoms of root rot (yellowing leaves, poor growth, and stand establishment). At each site, 10 soil cores (15 cm deep × 5 cm diameter) were collected in the root zone of established plants in October 2008. The soil cores from a site were combined and then the soil was passed through a 4-mm-diameter Sieve, and root fragments retained on the sieve were collected and reserved for isolation of Phytophthora spp. and extraction of P. penetrans (see below). The sieved soil samples were subdivided for subsequent nematode extraction and soil chemical and physical analyses. Pratylenchus penetrans were extracted from soil by placing 50 g of soil on a Baermann funnel for 5 days (Ingham, 1994). Extracted nematodes were collected and the number of nematodes was determined using a dissecting microscope at 40× magnification; soil nematode populations are expressed as P. penetrans/100 g soil. Soil chemical and physical analyses were conducted by A&L Laboratories (Portland, OR) and included % organic matter (OM), nitrate-N (NO3 −), sulfur, potassium, phosphorus, calcium, cation exchange capacity (CEC), pH, and soil texture (% sand, silt, and clay).
Root sections (1 cm long) that were symptomatic (discolored with obvious lesions) were surface-sterilized (Sabaratnum, personal communication) for 3 min with 0.02% Tween 20, rinsed with sterile distilled water (repeated three times), and cultured on PARP medium to recover Phytophthora spp. (CitationDuncan and Kennedy, 1989). A 0.3 g subsample of root tissue from each site/field location was evaluated for Phytophthora spp. by enzyme-linked immunosorbent assay (ELISA) using the Phytophthora Complete Kit (AgDia, Elkhart, IN). Polymerase chain reaction (PCR) testing was also performed based on a protocol by CitationBonants et al. (1997) through the Whatcom County Phytophthora spp. survey lab (WSU Puyallup Research and Extension Center, Puyallup, WA), and was used to detect the presence of P. rubi in root samples (3 sites per field). The remaining roots were washed free of soil, and P. penetrans was extracted by intermittent mist for 1 week (Ingham, 1994). Roots from which nematodes were extracted were dried at 72°C for 24 h and then dry weights determined. Extracted nematodes were collected, population densities were determined using a dissecting microscope at 40× magnification, and the density expressed as number of P. penetrans/g dry root.
Infested Field Soil Bioassay
In order to assess disease potential of field soil with varying frequency of Phytophthora spp. inoculums and P. penetrans population densities, bioassays using tissue culture ‘Meeker’ red raspberry plants were performed. Six fields (1, 2, 3, 6, 7, and 8) from the fall 2008 survey were revisited in spring 2009 and 2010 (). In 2010, field 8 was not included (grower had removed raspberry plants) in the bioassay. Soil and roots were collected with a shovel from one site per field (4.6 m long × 0.9 m wide), placed in four 19-liter buckets, passed through a 2-mm-diameter sieve, and root fragments retained on the sieve were collected. Prior to bioassay set-up, root and soil samples were analyzed for P. penetrans in both years, as described above. Randomly selected root samples were also tested to confirm the presence of P. rubi using conventional PCR at WSU-Puyallup, as described above, in 2009 only. The same soil chemical and physical analyses as described above, except textural analysis, were performed by A&L Laboratories during both years on collected soil.
TABLE 1 Occurrence of Phytophthora rubi and Phytophthora spp. and Population Densities of Pratylenchus penetrans in Commercial Red Raspberry (Rubus idaeus) Fields during a Survey Conducted in Northwestern Washington in 2008
Aliquots of sieved soil were loaded into 15-cm-wide Deepots (D40H; Stuewe and Sons Inc., Tangent, OR) and one tissue cultured red raspberry ‘Meeker’ plant (Sakuma Bros Farm, Burlington, WA) was planted into the soil. Tissue culture plants were 6 months and 3 months old prior to transplanting in 2009 and 2010, respectively. Plants were grown in a greenhouse maintained at 16°C with a 12 h day/night light cycle. Plants were watered regularly using a combination of below (immersion of Deepots in 473 ml (12-cm-high) cups for 6 to 8 h) and overhead watering to maintain field capacity and fertigated with 20-20-20 NPK (Plant Marvel Laboratories, Chicago Heights, IL) as needed. Each trial was arranged in a randomized, complete block design with four replications of four plants per field soil.
Plants were harvested approximately 8 weeks after planting. The aerial portions of the plants was removed and dried at 72°C for 24 h and dry weights were then determined. Adhering soil was gently removed from roots that were then washed with tap water to remove excess soil and roots were set in trays by replication and survey field. Root rot was evaluated using a standardized, 0 to 9 (0 = healthy, 9 = dead), continuous, visual rating scale (CitationWalters and Pinkerton, 2008). A subsample of symptomatic root material was collected from one plant per survey location and viewed under the microscope for presence of Phytophthora spp. oospores by cutting roots with a sterilized scalpel and observing the roots with a squash mount technique. Surface sterilized (see above) root fragments were plated onto selective media (PARP) for verification of Phytophthora spp. Pratylenchus penetrans was extracted and quantified from a handful of the root as described above. The remaining roots were dried at 72°C for 24 h and dry weights then determined.
Co-inoculation of raspberry with P. rubi and P. penetrans
P. rubi (strain ATCC 16184) inoculum was produced using a method adapted from CitationDissanayake et al. (1997). P. rubi was grown on a mixture of vermiculite, V-8 broth, and oats in a 900-ml glass jar. The mixture was autoclaved twice over a 24-h period and then inoculated with five 5-mm plugs from the margin of a 3-week-old P. rubi culture. After 4 weeks, jars were inspected for contamination after which the inoculum mixture was removed from the jar and dried in a fume hood for 1 week. The inoculum mixture was then ground into a powder using a grain grinder (Kitchen Aid, St. Joseph, MI). Oospore density of the inoculum was evaluated by taking 6 g of inoculum and homogenizing the material in 60 ml of water in a Janke & Kunkel Labortechnik Ultra-turrax T25 (IKA, Wilmington, NC). Four 10-μl aliquots of this mixture were distributed onto glass slides and the total number of oospores was determined. The inoculum contained approximately 17,000 oopspores/ml. Pratylenchus penetrans, originally collected from a raspberry field in Lynden, WA and maintained on peppermint (Mentha piperita), was used in this experiment. Nematodes were extracted from roots by intermittent mist and suspensions of nematodes were adjusted to approximately 100 P. penetrans/ml.
Prior to planting, field soil (Skagit silt loam) was collected at WSU-Mount Vernon, passed through a 2-mm-diameter sieve, and autoclaved twice (121°C, 15 min). Soil was then mixed with fine vermiculite (Steubers, Snohomish, WA) at a 1:2 ratio. P. rubi inoculum was added to the soil:vermiculite mixture to attain oospore densities of 0, 10, 100, and 1,000 oospores/g soil. Raspberry ‘Meeker’ tissue culture plants were planted into the P. rubi infested mixture (400 ml P. rubi inoculum/150 g soil mixture per container) in 15-cm-diameter Deepots. Phytophthora rubi infested soil was either inoculated or not inoculated with P. penetrans at all oospore densities. To inoculate with P. penetrans, small holes (2.5 cm deep) were created on two sides of the tissue culture plant and the nematode solution (1.5 ml) was pipetted into each hole to obtain 1 P. penetrans/g soil and then covered. This experiment was a 4 × 2 factorial design (4 oospore levels × 2 nematode densities) and was arranged as a randomized block design with each treatment combination replicated six times; the experiment was conducted twice.
The plants were watered and fertigated as needed similar to above. In addition, Deepots were flooded to encourage P. rubi infection by placing Deepots in 946 ml (17 cm high) cups for 48 h every 2 weeks (Walters and Pinkerton, 2008). Greenhouse conditions were set at 16°C, 12-h day/night light cycle. At experiment termination (∼6 weeks), root rot ratings, P. penetrans population densities in roots, and aerial and root biomass were evaluated using methods described above.
Data Analysis
Pearson correlation coefficients were calculated to examine the relationship between fall soil properties (n = 100) and number of P. penetrans/g root (PROC CORR) and frequency of Phytophthora spp. detection from ELISA (KENDAL) in the field survey. All parameters in the infested field soil bioassay and co-inoculation experiment were analyzed using a general linear model (PROC GLM; SAS Institute, Cary, NC). Trials from the co-inoculation experiment were analyzed separately because there was a significant interaction between trials (p < 0.001). Means were separated by Fisher's protected least significant difference test (p < 0.05).
RESULTS
Commercial Red Raspberry Field Survey
In the fall of 2008, P. rubi and Phytophthora spp. were detected in roots at all locations using PCR- and ELISA-based methods, respectively (). The frequency of P. rubi detection by PCR in each location ranged from 33% to 100% while the frequency of Phytophthoraspp. detection by ELISA ranged from 30% to 100%. P. penetrans was recovered from soil and root samples at all ten survey locations (). Average P. penetrans population densities varied greatly within and between survey locations from 0 to approximately 8,000 nematodes/g root tissue (). Average soil population densities of P. penetrans were much lower than densities detected in corresponding roots and ranged from 0 to 242 P. penetrans/100 g soil.
Dominant soil types for each location are listed in . In fall 2008, NO3 − levels in surveyed fields were variable, but were very high, >80 PPM, in locations 1, 6, and 7 (data not shown). Across locations, OM ranged from 3.3% to 8.7% and pH values ranged from 4.2 to 6.8. Percentage organic matter was positively, but only moderately correlated (r = 0.34; p < 0.01) with P. penetrans population densities in roots while percentage clay was negatively, but only moderately correlated (r = −0.33; p < 0.01) with P. penetrans population densities in roots. The frequency of Phytophthora spp. identified in grower fields as determined by ELISA was negatively, but only moderately correlated (r = −0.25; p < 0.01) with the textural analysis for percentage silt. No other soil properties showed correlations with Phytophthora spp. frequency or P. penentrans population densities in roots.
TABLE 2 Predominant Soil Types within Each Survey Field in Skagit and Whatcom Counties, WashingtonFootnote z
Infested Field Soil Bioassay
In spring 2009 and 2010, P. rubi was detected by PCR at all six survey locations sampled. P. penetrans population densities in soil were lower in spring 2009 and 2010 (data not shown) compared to samples collected in the fall of 2008 (). In 2009, P. penetrans were not detected in fields 1, 3, or 6 and there were only 2 P. penetrans/100 g soil detected at field 8. Similar to 2008, fields 2 and 7 had the highest P. penetrans population densities with 88 and 40 P. penetrans/100 g soil, respectively. In 2010, there were still no nematodes detected in soil samples from fields 1, 3, or 6, while 198 and 226 P. penetrans/100 g soil were found in soils from survey locations 2 and 7, respectively.
In the 2009 bioassay, raspberry plants planted into soil from field 6 had the lowest root rot ratings, and these ratings were lower than those observed on plants from fields 2, 4, and 5 (). Root rot ratings of plants planted into soil from the other survey locations were similar. In contrast to 2009 bioassay results, raspberry plants planted in soil from location 5 had significantly greater root rot ratings compared to plants planted into soils from other survey fields. Fields 2 and 4 had significantly higher root rot than fields 3 and 6. Oospores of P. rubi in bioassay plant roots were observed in both years (data not shown), although no clear pattern of colonization across fields or replications was discernable. P. penetrans population densities were significantly higher in plant roots grown in soil from survey fields 2 and 5 during both years (). In 2010, P. penetrans population densities in bioassay plant roots were higher than in 2009 (). In 2009, aerial biomass of red raspberry grown in soil from field 4 was lower than the other survey fields except location 5 (). There was no significant difference in root biomass across survey fields in 2009. In 2010, plants grown in soil from field 5 had the lowest aerial and root biomass among the survey locations.
TABLE 3 Aerial and Root Biomass (g) of Red Raspberry (Rubus idaeus) ‘Meeker’ Co-inoculated with Phytophthora rubi and Pratylenchus penetrans Footnote z
Co-inoculation of raspberry with P. rubi and P. penetrans
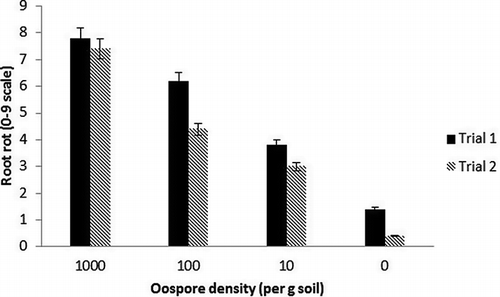
As stated above, results from trials were different (p < 0.01), therefore data were analyzed separately; however, similar trends were observed in both trials. There was no interaction between P. rubi oospore density and the presence or absence of P. penetrans in both trials (p = 0.40 and 0.85); therefore, P. rubi data across nematode treatments were combined for analysis. In both trials, the level of root rot was significantly affected by oospore density, with increasing root rot with increasing levels of oospore inoculum (). The same trend was observed for proportion diseased root (data not shown), which ranged from 5.5 to 6.1 at 1,000 oospores/g soil and <0.8 at 0 oospores/g soil across trials. P. penetrans was recovered from inoculated plants in trial 1 (181.6 + 16.1 nematodes/g dry root) and trial 2 (136 + 14.7 nematodes/g dry root). On average, the highest densities of P. penetrans in root tissues (223.24 + 16.2 P. penetrans/g dry root) were found in plants that had been inoculated with 1,000 P. rubi oospores/g soil, but this difference was not significant. Root biomass was significantly lower for plants inoculated with 1,000 P. rubi oospores/g soil than for plants inoculated with lower densities of P. rubi in both trials (). There was no impact of P. rubi oospore density on aerial biomass in either trial. P. penetrans at an inoculation density of 1 nematode/g soil did not affect root or aerial biomass in either trial (data not shown).
FIGURE 4 Root rot rating (0–9 scale; 0 = healthy, 9 = dead) of raspberry (Rubus ideaus) ‘Meeker’ inoculated with different densities of Phytophthora rubi (0, 10, 100, and 1,000 oospores/g soil) and Pratylenchus penetrans (0 or 200 nematodes/g soil). There was no interaction between P. rubi and P. penetrans so data were combined. Columns identified by the same letter are not significantly (P < 0.05) different according to least significant difference. NSD = No significant difference.
DISCUSSION
Phytophthora spp. and P. penetrans are prominent soilborne pathogens of red raspberry in northwestern Washington, posing a threat to the long-term viability of this industry. Our survey is the first to demonstrate the widespread occurrence, frequency, and population densities of these pathogens in red raspberry. In our production field survey, P. rubi, Phytophthora spp., and P. penetrans were all commonly encountered. While we did not quantify P. rubi propagules in soil, PCR-based tests showed that P. rubi was detectable in >50% of samples/site from 8 out of 10 fields. All surveyed fields were ELISA-positive for Phytophthora spp., while the frequency of detection within a field was >50% in 7 out of 10 fields. Other species of Phytophthora may also be affecting root rot and root health in raspberry (CitationWilcox et al., 1993). The identification and impact of other Phytophthora spp. on raspberry productivity are unknown.
For most Phytophthora diseases, propagule recovery and quantification is problematic with dilution plating methods and oospore germination from plant roots and in culture being unpredictable (CitationErwin and Ribeiro, 1996). It was reported that P .rubi was most active from November to March, which may also affect how easily P. rubi is detected during other times of the year and how inoculum levels are assessed in soil. Examples of the difficulty in propagule recovery and quantification can be drawn from P. fragariae, which is closely related to P. rubi (formerly P. fragariae var. rubi; CitationMan and Willem, 2007). In strawberry, the amount of P. fragariae sporulation varied by host genotype with susceptible plants supporting a faster rate and greater amount of sporulation by the pathogen compared to the traditionally resistant germplasm (CitationMilholland and Daykon, 1993). An inspection of strawberry roots over 2 years found that oospores of P. fragariae formed in roots during the months when temperatures were over 16°C, with the maximum recovery occurring in March of both years (CitationForge et al., 1998). Similar to P. fragariae, the activity and ability to recover P. rubi may be related to sporulation levels and germination rates of dormant spore structures. More work is needed on isolate recovery techniques and monitoring to determine the activity period of P. rubi in northwestern Washington.
The majority of the fields sampled in fall 2008 had P. penetrans root population densities >500 nematodes/g root and population densities in soil <100 nematodes/g soil. CitationMcElroy (1992) suggested that P. penetrans population densities exceeding 1 nematode/cm3 soil affected stand establishment and 2 to 8 nematodes/cm3 soil decreased plant growth in established plantings. We rarely detected P. penetrans soil population densities greater than 1 nematode/g soil; therefore, according to CitationMcElroy (1992) treatment would not be warranted in the majority of the survey locations. However, population densities of P. penetrans detected in root samples were higher, and in three locations exceeded 1,000 nematodes/g dry root. The relationship between P. penetrans population densities in roots on raspberry productivity has not been investigated. This survey demonstrated that raspberries in northwestern Washington are grown on a wide range of soil types. Skagit County soils generally were fine to sandy loams, while the predominant Kickerville loam of Whatcom County is characterized by high organic matter content and a low sand content. Phytophthora root rots have historically been associated with high water content in soil (CitationDuncan and Cowan, 1980; CitationDuncan and Kennedy, 1989). Following this reasoning, it was assumed that soils in our survey with higher clay contents would be associated with higher moisture levels, which could also exacerbate Phytophthora root rot. However, it was found that Phytophthora spp. frequency in raspberry soils was negatively correlated with silt content and there was no correlation with any other soil property. CitationKlironomos et al. (1999) documented spatial variability in soil properties and suggested that in-field heterogeneity makes it difficult to detect relationships between soil properties and pathogens. While host genotype and relative resistance of roots to P. rubi can mitigate pathogen sporulation duration and number of oospores formed in roots (CitationLaun and Zinkernagel, 1997), increasing soil moisture content can encourage persistence and infectivity of Phytophthora spp. over time (CitationDuncan and Cowan, 1980). Interestingly, P. cryptogea zoospore movement in soil columns was measured to be 8–12 mm in a clay loam soil and 40 mm in a coarse soil mix (CitationDuniway, 1992). It was concluded that pore space between primary particles of finer textured soils are too small to permit significant zoospore movement. Other, still unknown, factors in the soil biosphere may be affecting P. rubi persistence in northwestern Washington soils.
Pratylenchus penetrans population densities did increase with decreasing clay content in our survey. Population densities of this nematode also increased as percentage organic matter increased. A strong relationship between sand content and high nematode population densities has been reported (CitationTownshend, 1972), however, other researchers found varying results when investigating other soil properties on plant-parasitic nematode distributions, such as pH and organic matter content (CitationNoe and Barker, 1985; CitationWyse-Pester et al., 2002). Relationships between nematodes and soil properties vary by species, and in a study of soil properties and nematode diversity, less than 30% of the variability could be explained by edaphic factors (CitationRobertson and Freckman, 1995). The addition of organic matter to the soil will also influence nematode population densities, especially if a field is under management that employs cultural practices like cover cropping and manure additions that can cause temporal augmentation of different nematode populations (CitationWidmer et al., 2002).
Greenhouse experiments were conducted to evaluate the disease response of young raspberry plants to infested soil from grower's fields where mature raspberry plants where demonstrating yield decline and above-ground plant symptoms (i.e., wilt, yellowing, poor growth and establishment, etc.). Due to the constraint of working in production fields where root rot ratings are not possible without using destructive methods, field soil was imported into a greenhouse setting. In these experiments, only field soils for which P. rubi was detected with the PCR assay were used (). Pratylenchus spp. population densities varied between fields, with fields 2 and 5 having the highest densities in both years. These fields also had high root rot ratings in the bioassay. We observed minimal P. rubi colonization of roots due to unsaturated conditions in the assay (i.e., no flooding as in co-inoculation trial). However, despite the potential inoculum in the soil and oospore formation in plant roots, the amount of root damage caused by P. penetrans alone was interesting and may be an indication of pre-plant issues for raspberry growers. In 2010, the degree of root rot was greater than in 2009. In this year, tissue culture raspberry plants were 3 months younger and this may have affected their susceptibility to soilborne pathogens.
A controlled greenhouse study was performed to further assess the effect and interaction of P. rubi and P. penetrans. In the co-inoculation study, which included flooding to encourage P. rubi infection, P. rubi inoculation levels above 10 oospores/g soil caused substantial disease on raspberry roots, regardless of the presence of P. penetrans. These results are in accordance with previous work by CitationVrain and Pepin (1989). Although P. penetrans did not significantly affect the dry weight of raspberry roots, the amount of fine roots was observed to be less on plants that had been inoculated with P. penetrans. On the roots with the most root rot that had been inoculated with 1,000 oospores/g soil, high population densities of P. penetrans were still detected. As an aggressive soilborne pathogen, at higher inoculum levels, P. rubi can destroy raspberry roots. While P. penetrans do not necessarily exacerbate Phytophthora root rot symptoms, P. penetrans presence in all diseased roots is interesting and suggests that P. penetrans might contribute to long-term root health decline especially at the lower P. rubi inoculum levels.
CONCLUSIONS
While P. rubi, Phytophthora spp., and P. penetrans are present in raspberry systems in western Washington, it seems that population densities of both pathogens vary and are affected differently by various confounding factors like management and soil type. In this study, the dynamics of two main pathogens on raspberry in northwestern Washington have been explored. While it is obvious that P. rubi is common in raspberry production in this region, and is an aggressive pathogen on raspberry, the chronic damage to roots caused by P. penetrans should not be ignored. Furthermore, investigation into additional indigenous soil pathogen communities is warranted.
ACKNOWLEDGMENTS
This research was partially funded by the WSU-BIOAg program. We would like to thank Jerry Weiland for his critical review of this manuscript and Mike Partika for his support in all greenhouse and field work.
LITERATURE CITED
- Bonants , P. , Hagenaar-de Weerdt , M. , Gent-Pelzet , M. , Lacourt , I. , Cooke , D. and Duncan , J. 1997 . Detection and identification of Phytophthora fragariae Hickman by polymerase chain reaction . Eur. J. Plant Pathol , 103 : 345 – 355 .
- Bristow , P.R. , Daubany , H.A. , Sjulin , T.M. , Pepin , H.S. , Nesby , R. and Windom , G.E. 1988 . Evaluation of Rubus germplasm for reaction to root rot caused by Phytophthora erythroseptica . HortScience , 113 : 588 – 591 .
- Davis , E.L. and MacGuidwin , A.E. 2005 . Lesion nematode disease . The Plant Health Instructor , DOI: 10.1094/PHI-I-2000-1030-02
- Dissanayake , J.W. , Hoy , N. and Griffin , J.L. 1997 . Weed hosts of the sugarcane root rot pathogen Pythium arrhenomanes . Plant Dis , 81 : 587 – 591 .
- Duncan , J.M. and Kennedy , D.M. 1989 . The effect of waterlogging on Phytophthora root rot of red raspberry . Plant Pathol , 38 : 161 – 168 .
- Duncan , J.M. and Cowan , J.B. 1980 . Effect of temperature and soil moisture content on persistence of infectivity of Phytophthora fragariae in naturally infested field soil . T. Brit. Mycol. Soc , 75 : 133 – 139 .
- Duniway , J.M. 1992 . Movement of zoospores of Phytophthora crytogea in soils of various textures and matric potentials . Phytopathology , 66 : 877 – 882 .
- Erwin , D. and Ribeiro , O. 1996 . Phytophthora diseases worldwide , St. Paul, MN : APS Press .
- Forge , T.A. , de Young , R. and Vrain , T.C. 1998 . Temporal changes in the vertical distribution of Pratylenchus penetrans under raspberry . J. Nematol , 30 : 179 – 183 .
- Ingham , R.E. 1994 . “ Nematodes ” . In Methods of soil analysis. Part 2—Microbiological and biochemical properties , Edited by: Michelson , S.H. 459 – 490 . Madison , WI : Soil Science Society of America, Inc .
- Klironomos , J.N. , Rillig , M.C. and Allen , M.F. 1999 . Designing belowground field experiments with the help of semi-variance and power analyses . Appl. Soil Ecol , 12 : 227 – 238 .
- Laun , N. and Zinkernagel , V. 1997 . A comparison of the resistance against Phytopthora fragariae var. rubi, the causal agent of a raspberry root rot . J. Phytopathol , 145 : 197 – 204 .
- Man , V. and Willem , A. 2007 . Gene flow analysis demonstrates that Phytophthora fragariae var. rubi constitutes a distinct species, Phytophthora rubi comb . Mycologia , 99 : 222 – 226 .
- McElroy , F.D. 1992 . A plant health care program for brambles in the Pacific Northwest . J. Nematol , 24 : 457 – 462 .
- Milholland , R.D. and Daykon , M.E. 1993 . Colonization of roots of strawberry cultivars with different levels of susceptibility of Phytophthora fragariae . Phytopathology , 83 : 538 – 542 .
- Noe , J.P. and Barker , K.R. 1985 . Relation of within-field spatial variation of plant-parasitic nematode population densities and edaphic factors . Phytopathology , 75 : 247 – 252 .
- Pattison , J.A. , Wilcox , W.F. and Weber , C.A. 2004 . Assessing the resistance of red raspberry (Rubus idaeus L.) genotypes to Phytophthora fragariae var. rubi hydroponic cultures . Hortscience , 39 : 1553 – 1556 .
- Robertson , G. and Freckman , D. 1995 . The spatial distribution of nematode trophic groups across a cultivated field . Ecology , 76 ( 5 ) : 1425 – 1432 .
- Townshend , J.L. 1972 . Influence of edaphic factors on penetration of corn roots Pratylenchus penetrans and P. minyus in three Ontario soils . Nematologica , 18 : 201 – 212 .
- USDA. 2009. Non–citrus Fruits and Nuts 2008 Summary. Agri. Stat. Board. NASS, USDA. March 2009. < http://usda.mannlib.cornell.edu/usda/current/NoncFruiNu/NoncFruiNu-07-08-2009.pdf (http://usda.mannlib.cornell.edu/usda/current/NoncFruiNu/NoncFruiNu-07-08-2009.pdf)
- Vrain , T.C. and Daubeny , H.A. 1986 . Relative resistance of red raspberry and related genotypes to the root lesion nematode . HortScience , 21 : 1435 – 1437 .
- Vrain , T.C. and Pepin , H.S. 1989 . Effect of Pratylenchus penetrans on root rot of red raspberry caused by Phytophthora erythroseptica . Acta Hort , 262 : 231 – 240 .
- Walters, T.W. and J.N. Pinkerton. 2008. Methyl bromide alternatives for raspberry nursery: First year progress. < http://groups.ucanr.org/pacificmba (http://groups.ucanr.org/pacificmba)
- Widmer , T.L. , Mitkowski , N. and Abawi , G. 2002 . Soil organic matter and management of plant parasitic nematodes . J. Nematol , 34 ( 4 ) : 289 – 295 .
- Wilcox , W.F. , Scott , P.H. , Hamm , P.B. , Kennedy , D.M. , Duncan , J.M. , Brasier , C.M. and Hansen , E.M. 1993 . Identity of a Phytophthora species attacking raspberry in Europe and North America . Mycol. Res , 97 : 817 – 831 .
- Wyse-Pester , D.Y. , Wiles , L.J. and Westra , P. 2002 . The potential for mapping nematode distributions for site-specific management . J. Nematol , 34 : 80 – 87 .
- Xing , L. and Wesphal , A. 2006 . Interaction of Fusarium solani f. sp. glycines and Heterodera glycines in sudden death syndrome of soybean . Phytopathology , 96 : 763 – 770 .