Abstract
‘Malindi’ banana (Musa,‘Dwarf Cavendish’) was stored at three storage conditions (11–12°C and 95.5% RH; 20–22°C and 82–85% RH; and cyclic cooling and warming to simulate day/night conditions during the banana harvest season: 28°C and 50% RH/18°C and 70% RH). Fruit storage life was 21, 10, and 8 days in the refrigerated storage, normal room storage, and storage under cyclic day/night conditions, respectively. Fruit ripening, respiration, and ethyelene production were significantly higher at high temperature storage and cyclic day/night conditions leading to faster fruit deterioration. Under these storage conditions, the rate of fruit weight loss, firmness, vitamin C loss, and chlorophyll degradation was also higher compared to refrigeration.
INTRODUCTION
Bananas are one of the most important food products in global trade. They are a climacteric fruit that undergoes several physiological changes after harvest (CitationSimmonds et al., 1987). Postharvest losses occur continuously throughout the transportation chain due to inappropriate handling and storage conditions (CitationOlorunda, 2000; CitationOpara, 2010). Several studies have confirmed that the optimum storage conditions for banana are in the range of 12 to 16°C and 90% to 95% relative humidity (RH), which delayed ripening, maintained shelf-life for marine transport, and prevented chilling injury (CitationBishop, 1996; CitationChauhan et al., 2006; CitationKader, 2003). However, the physiological responses and optimum handling and storage conditions vary among cultivars. For example, CitationSalvador et al. (2007) found gradual changes in color and texture during storage of the Cavendish AAA group, whereas the process was more uneven in Musa paradisiaca AAB group.
‘Malindi’ (Musa acuminate, AAA group Cavendish subgroup, Dwarf Cavendish) is an important banana in the global market, particularly in the Indian subcontinent and the Middle East regions that are dominated by hot and arid climates (CitationOpara et al., 2010). Banana is commonly harvested in the mature-green stage and may experience a wide range of environmental conditions ranging from refrigerated storage to ambient conditions during postharvest handling and marketing. However, there is a dearth of information on the effects of postharvest handling and storage conditions on the fruit quality and shelflife of ‘Malindi’ banana fruit. The objective of this study was to investigate quality and physiological responses of ‘Malindi’ banana during postharvest ripening under different conditions.
MATERIALS AND METHODS
Fruit Supply
Mature green ‘Malindi’ bananas (Musa acuminate, AAA group Cavendish subgroup, Dwarf Cavendish) were packed in cardboard boxes obtained from a local market (Al-Mawllah, Muscat, Oman). Fruits were harvested from banana plantations in Al-Suwaiq, North Al-Batinah, and transported to the Postharvest Technology Research Laboratory at Sultan Qaboos University, Muscat, Oman. Banana fingers were removed from hands, and undamaged fingers were selected and divided randomly into three groups (27 fruits in each group), and each group was used for each of the storage conditions. Within storage conditions, fruit was further divided into three sub-groups (nine fruits each), and were used to determine physical properties (weight loss, color, and firmness,), chemical properties (total soluble solids (TSS), pH, titratable acidity (TA), and vitamin C), and physiological responses (respiration rate (CO2) and ethylene (C2H4) production).
Storage Conditions
The banana fruits were stored under three situations, representing typical environmental conditions under which banana fruits are typically stored. These conditions were: (1) 11–12°C and 95% RH, (2) 20–22°C and 82%–85% RH, and (3) 28°C/50% RH and 18°C/70% RH, simulating cyclic day/night conditions. These conditions were attained using a refrigerator, room with normal air, and an environmental chamber, respectively. Relative humidity (RH) and temperature sensors (model Data Hog, Sky Instruments, Powys, Wales) were used to record RH and temperature at room and refrigerator storage conditions. Verstaile Environmental Test Chamber (model MLR-351 H, SANYO Electric Co. Ltd., Moriguchi, Japan) was set for the desired RH and temperature conditions.
Weight Loss
Fruit mass (g) was measured daily using a digital balance (model GX-4000, A & D Company, Tokyo, Japan). The daily change in fruit mass was expressed as percentage of weight loss.
Color
The banana peel color was measured with a chromameter (model CR-400, Konica Minolta, Chiyoda, Japan) on opposite sides of the fruit (mid-section). The CIE L*, a*, and b* system using a D65 illuminant and a visual angle of 10° was used to measure color values (CitationSalvador et al., 2007). The dark spots were avoided while evaluating the banana fruit. Results were obtained, as average of individual values of L* (lightness: 0 (black) to 100 (white)), a* (redness to greenness, +a* is redness, −a* is greenness), and b* (yellowness to blueness, +b* is yellowness, −b* is blueness) (CitationSalvador et al., 2007). Chroma (C*ab) is a quantitative color value used to determine the difference between each hue in comparison with grey color with identical lightness. Hue angle (h*ab) is the feature in which color has been traditionally identified as reddish, greenish, and so on (CitationSimmonds et al., 1987).
C*ab chromaticity (saturation) and hue angle (h*ab) were expressed by EquationEqs. (1) and Equation(2):
Texture
Fruit firmness was measured daily using a non-destructive instrument (model Durofel, Agro-Technology Co., Taracon, France). The penetration on the surface texture was 0.25 cm2. The firmness results were reported on a 100-point unit scale (0 = soft and 100 = firm).
Chemical and Nutritional Properties
Three ripening stages: unripe (stage 1), ripe (stage 6), and overripe (stage 7), were identified using the guidelines given by CitationKader (2005). The chemical analysis was conducted at these three stages for all treatments. Three fruits were used for chemical analysis in each ripening stage and treatment.
Sample Preparation
The banana pulp tissue (15 g) was cut into small cubes, blended, and homogenized with 50 ml of distilled water using a kitchen blender for 2 min to prepare a diluted banana juice. Another 15 g of banana pulp tissue from the same sample was taken and blended to use for vitamin C analysis (CitationAOAC, 2000) by adding 3% metaphoshoric acid (MPA) into a 50-ml volumetric flask under dim light, and covered with aluminum foil to protect from color change due to oxidation. For vitamin C content, dye was prepared by dissolving 0.042 g of NaHCO3 + 0.050 g of 2,6-dichlorophynol indophenol in distilled water (DW) to give a final volume of 200 ml. Also, 30 g of MPA was dissolved in DW to give a final volume of 1 L to prepare 3% MPA, and 0.01 g of Standard (std) ascorbic acid (analar grade) was dissolved in 3% MPA to get a final volume of 100 ml of standard of ascorbic acid. Samples were centrifuged (model J.25I, Beckman, AvantiTM, Palo Alto, CA, USA) at 6000 m/s for 10 min at 4°C. Subsequently, the diluted banana juice was also used to measure total soluble solids (TSS) concentration, pulp pH, and pulp titratable acidity (TA).
pH, TSS, and TA
pH was determined using 10 ml of the diluted banana juice from the previous step using a bench-top pH meter (model Jenway 3520, Barloworld Scientific Ltd., Staffordshire, UK). Total soluble solid contents (TSS) was measured using a digital refractometer (model PR-32α (alpha), Atago Co. Ltd., Minato-ku, Japan) at 20°C. The banana juice was titrated with 0.1 N NaOH up to pH 8.1 using phenolphthalein as an indicator (CitationAOAC, 2000). The results were reported as percentage of malic acid. In general, (TSS–TA):TA and TSS:TA ratios are used to evaluate the flavor index of the fruit (CitationChamara et al., 2000; CitationMostafa, 2005). In the present study, the TSS:TA ratio was determined.
Vitamin C
Vitamin C was measured by titration with 2,6-dichloroindophenol in acidic solution (CitationAOAC, 2000) and expressed as % malic acid.
Respiration and Ethylene Production
Respiration rate of the individual banana fruit was measured using a CO2/O2 dual gas analyzer (ICA15, International Controlled Atmosphere (ICA) Ltd., Kent, UK). Fruit ethylene (C2H4) production rate was measured using Ethylene Analyzer (ICA56, International Controlled Atmosphere (ICA) Ltd.). The procedure explained by Dadzie and CitationOrchard (1997) was used in this study.
Respiration and ethylene production rates for nine banana fruit samples were measured daily for each storage condition. Each sample was stored in an airtight 9 L plastic container with a lid. The two analyzers were connected to the container to measure CO2 (%), O2 (%), and C2H4 (ppm) at steady state. Fruit respiration rate (RCO2) and C2H4 rate (RC2H4) were calculated according to CitationBanks et al. (1995) and Dadzie and CitationOrchard (1997).
All measurements of fruit quality attributes and physiological responses were carried out from mature green stage to the final stage of ripening (CitationChen and Ramaswamy, 2002; CitationSimmonds et al., 1987).
Data Analysis
Data were statistically analyzed using the General Linear Midel (GLM) procedure of the SAS statistical software (SAS Institute, Cary, NC) to determine the mean difference between storage conditions, ripening stages, and their interaction. The method of Duncan Multiple Range Test was used to differentiate treatment means at p ≤ 0.05.
RESULTS AND DISCUSSION
Based on the recommended quality incentives of banana (Kader, 2005), the shelf life (days from mature green to overripe stages) of fruit stored under refrigerator storage, environmental chamber, and room condition was 21, 10, and 8 days, respectively. Fruit became fully ripe on the 4th day under room and environmental chamber conditions and at the 16th day under refrigerated storage. Similarly, fruit attained the overripe stage on the 10th and 8th day of storage under room and environmental chamber, respectively, and after 21 days under refrigerated storage.
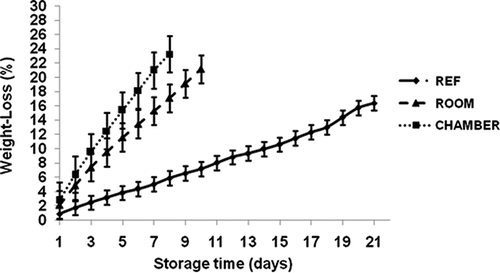
Weight Loss
The weight loss of banana during storage under different conditions is given in The fruit weight loss gradually increased over time at all three storage conditions, but differences among these conditions were observed. At the overripe stage, the weight loss was 24%, 21%, and 16% for environmental chamber, room, and refrigerator storage, respectively. The weight loss in banana during storage is perhaps due to water movement from fruit pulp to peel, release of volatiles containing ethylene, and carbon dioxide by respiration, and also evaporation of water from the peel during ripening (CitationLodh et al., 1971; Loesecke, 1950; CitationWills et al., 1984).
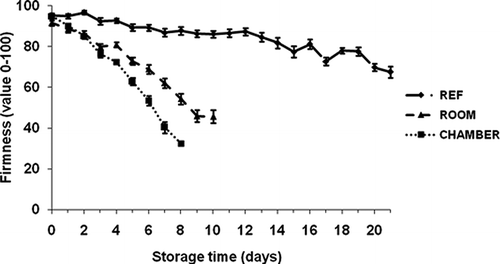
Fruit Firmness
Fruit firmness declined rapidly under both cyclic day/night storage (environmental chamber) and warm temperature (room conditions) from the 3rd day of storage. But under refrigeration, the change in texture was gradual from firm to soft during storage (). The firmness of banana stored in the environmental chamber was reduced from 95 to 32 on a 100-point scale in 8 days. But under refrigeration, the fruit firmness decreased from 95 to 68 in 21 days. The changes in the amounts of structural polysaccharides, starch, and pectic substances found in the banana flesh might have contributed for the textural changes of banana (CitationCano et al., 1997).
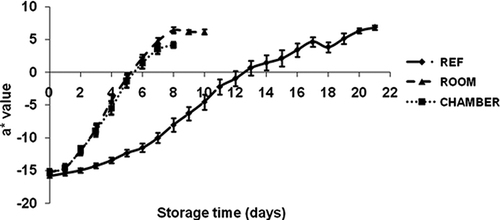
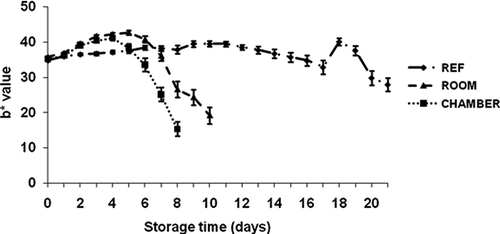
Color
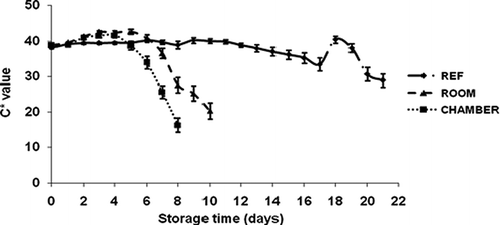
The color changes in the banana peel during the three storage conditions are shown in – The peel color altered from green to yellow during storage time. The color indicator a* did not vary significantly (p ≤ 0.05) during all three treatments. The b*, L*, and C* values slightly increased between day 0 and day 4 for the peel color of banana at environmental chamber condition; then decreased rapidly after day 4. The color indicator b* on the banana peel of refrigerator storage significantly differed from environmental chamber storage. The fruits were less yellow on day 0, and b* rose on day 1 and continued until day 17 at 11–12°C, then dropped considerably.
FIGURE 5 Color parameter L* (lightness, black-to-white) of ‘Malindi’ banana at different storage conditions.
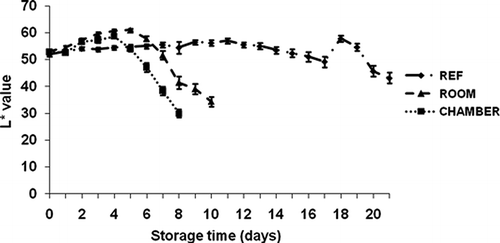
FIGURE 6 Color parameter (Cab*) of ‘Malindi’ banana at different storage conditions: Environmental chamber (50% RH and 18°C during night, 50% RH and 28°C during day); Room (82 to 85% RH and 20 to 22°C); Refrigerator (∼95% RH and 11 to 12°C).
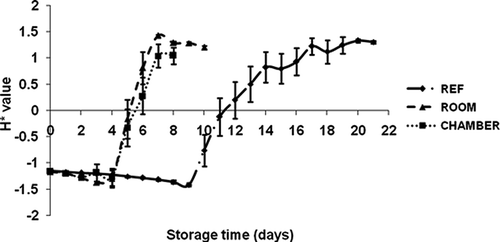
The H* value increased during the initial period of storage and then decreased sharply on the 4th day (H* = −1.29) and 5th day (H* = −0.32) at room and environmental chamber storage, respectively, and on the 10th day at refrigerator storage (H* value = −0.75). Similar trends were observed by other researchers regarding banana ripening at different temperatures for various storage periods (CitationChen and Ramaswamy, 2002; CitationMendoza and Aguilera, 2004; CitationSalvador et al., 2007).
These physical changes could be attributed to the breakdown of chlorophyll in the banana peel throughout the storage duration (CitationSalvador et al., 2007). CitationSeymour et al. (1987) found that degreening in plantains was accelerated by higher temperatures (≥24°C); however, in Cavendish banana, a desirable sweet taste and soft pulp developed without degreening fully (“ripen green” fruits with poor quality). The chlorophyll pigment was retained in the peel of Cavendish banana fruits when stored at higher temperatures (CitationThomas and Janave, 1992).
Chemical and Nutritional Properties
Total soluble solid contents (TSS), pH, titratable acidity (TA), vitamin C, and TSS:TA ratio of ‘Malindi’ banana fruit stored under different conditions increased during the three ripening stages ().
TABLE 1 Changes in Chemical and Nutritional Properties on ‘Malindi’ Banana at Different Storage Conditions and Ripening Stages
pH
Fruit pH value gradually increased from the unripe stage (5.05, 4.84) to overripe stage (5.36, 5.64) under cyclic and warm temperature, respectively. However, it followed an irregular pattern with the ripening stages in refrigerator storage (increseaing to 5.43, and then declining to 5.24). The changes were similar to the results reported by Dadzie and CitationOrchard (1997) who found that the pH level of the fruit pulp depends on the ripening level being high at mature green stage harvest, and decline with ripening. In another study, CitationMustaffa et al. (1998) reported that pH values of fruits increased at early stages and decreased slowly throughout ripening stages.
Total Soluble Solid Contents (TSS)
TSS under environmental chamber, room, and refrigerator storage increased from 7.4, 9.9, and 8.1 °Brix at the unripe stage to 21.2, 19.6, and 20.7 °Brix at the ripe stage to 23.4, 18.0, 18.5 °Brix at the overripe stage. The highest total soluble solid content was 23.3 °Brix in the environmental chamber when the fruit reached the overripe stage. There was a considerable increase in TSS contents as the fruit ripens under all three storage conditions. However, the rate of change in TSS over time varies among cultivars (CitationDadize and Orchard, 1997; CitationMustaffa et al., 1998). Previous studies reported that variation in TSS was more related to banana cultivars than to maturity stages.
Titratable Acidity (TA)
The TA ranged from 0.24% to 0.41% under all storage conditions and ripening stages. There was no difference (p ≤ 0.05) in TA of the fruit in the environmental chamber at all three ripening stages. Under warm conditions, TA decreased steadily from 0.41% to 0.24%, whereas under refrigeration, the titratable acidity increased until the banana fruits became fully ripe (0.38%–0.41%) and declined (0.32%) at the overripe stage after 3 weeks of storage. These results are similar to the findings of CitationWills et al. (1984) who reported that the TA% (malic acid) of the fruit increased from 0.19 g acids/100 g at the unripe stage to 0.41 g acid/100 g at the beginning of ripening, but when the fruit advanced in the ripening process, the TA% declined to 0.25 g acids/100 g at fully ripe at 20°C.
Total Soluble Solid to Titratable Acidity Ratio (TSS:TA Ratio)
Total soluble solid to titrability acidity ratio of ‘Malindi’ banana differed significantly (p ≤ 0.05) at the three storage conditions. The banana TSS:TA ratio changed from low (20.6, 24.2, 21.6) at the unripe stage to high (72.3, 76.5, 58.9) at the overripe stage under environmental chamber, room, and refrigerator storages, respectively. The highest ratio was observed when the banana reached the overripe stage with a maximum ratio of 76.5 under room condition. This might be due to the differences in temperature and relative humidity between the conditions during fruit ripening. These changes are linked to sugar concentration in the peel and pulp tissues and also the water losses of the peel both by transportation to atmosphere and to the pulp by osmotic pressure that causes the fresh weight of the pulp increase as the fruit ripens (CitationDadize and Orchard, 1997).
Vitamin C (Ascorbic Acid)
The vitamin C (ascorbic acid) in the banana was significantly affected by the ripening stages and storage conditions. It declined from 6.1 mg/100 g fresh weight (FW) in unripe fruit to 4.1 mg/100 g (FW) in overripe fruit under the environmental chamber. On the other hand, the ascorbic acid values increased regularly at the two stages of ripening (unripe and fully ripe) and then decreased at a later stage (overripe) in room and refrigerator storages. Similar variations in vitamin C were observed by CitationWills et al. (1984). It was reported that the vitamin C level in the Cavendish banana (Musa acuminata, AAA group) altered from 18 mg/100 g in unripe or green stage to 19 mg/100 g at green and yellow color stage, but then declined rapidly to 6 mg/100 g at the last stage of ripening. This change could have resulted from exposure of the fruit to changes in temperature, RH, physical damage, and chilling injury when it is stored for longer periods (CitationLee and Kader, 2000; CitationWills et al., 1984). It was also reported that it is difficult to specify the amount of vitamin C content in the banana fruit because variety, cultivation, speed of ripening, storage, and seasons can influence the ascorbic acid content in this crop (CitationLeverton, 1937).
Respiration Rate
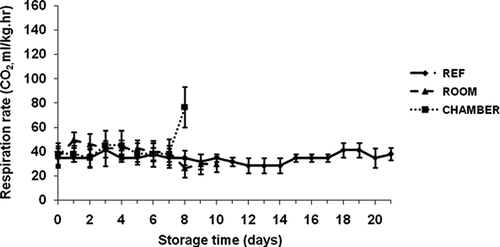
The respiration rates (i.e., CO2 production) of the ‘Malindi’ banana fruit under three storage conditions are shown in Respiration was uneven when stored under all conditions and at each of the ripening stages. Respiration rate ranged from 26.4 ml kg−1 h−1 to 76.6 ml kg−1 h−1 between the first day and the 8th day of storage at three conditions. The respiration rate was higher (∼49.5 ml kg−1 h−1) at the first day under room storage than that of cyclic and refrigerator storage. The respiration rate dramatically increased to 76.6 ml kg−1 h−1 on the 8th day of storage when the fruit became overripe inside the environmental chamber. The respiration rate of the fruit fluctuated from 28.5 to 41.2 ml kg−1 h−1 within 21 days of storage in the refrigerator. The variations in respiration could be due to water loss percentage in the banana, which caused reduction of preclimacteric period, stimulation in ethylene production, and respiration at preclimacteric stage and changes in the quality (CitationFinger et al., 1995). CitationBroughton and Wu (1979) reported that at higher temperatures, the rate of respiration was higher and the fruits ripen and deteriorate faster. In our study, at lower storage temperatures, the respiration rate was lower and, thus, delayed the ripening of the fruit.
Ethylene (C2H4) Production Rate
Ethylene (C2H4) production of ‘Malindi’ banana during three storage conditions are given in Under refrigeration, the ethylene production was uneven and ranged from 0.00098 to 0.0072 ml kg−1 h−1 between day 0 and day 14 of storage, then started rising after day 15 and continued until the last day of storage.
FIGURE 9 Ethylene production rate ‘Malindi’ banana at different storage conditions: Environmental chamber (50% RH and 18°C during night, 50% RH and 28°C during day); Room (82 to 85% RH and 20 to 22°C); Refrigerator (∼95% RH and 11 to 12°C).
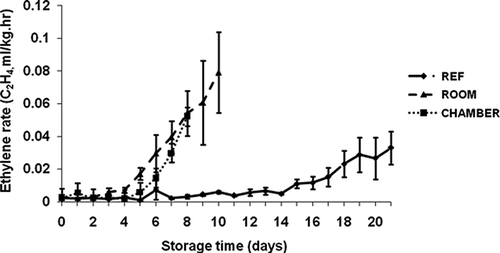
The ethylene production rate was 0.0059 ml kg−1 h−1 under warm-temperature storage (i.e. room) on the third day in comparison with 0.0028 and 0.0016 ml kg−1 h−1 under cyclic and refrigeration, respectively. The respiration rate (28.53–41.22 ml kg−1 h−1) and ethylene production (0.00098–0.033 ml kg−1 h−1) of banana stored in the refrigerator are in agreement with Kader (2005) who found that respiration rates and ethylene production of banana at 13°C were 10 to 30 ml kg−1 h−1 and 0.0001 to 0.02 ml kg−1 h−1, respectively. The present findings revealed that the increased ethylene production accelerated ripening in the first 5 days of storage under cyclic day/night storage conditions.
These variations in ethylene production may be due to storage temperature and relative humidity at the three different conditions and may also result from the changes in the metabolic process throughout fruit ripening (CitationWills et al., 1998). Such alteration is the sequence of the ripening process of the fruit causing a large increase in the rate of ethylene production. Storage temperature, age of fruit, and cultivar control the rate of respiration and ethylene production (CitationBiale et al., 1954; CitationDadize and Orchard, 1997; CitationKader, 1987; CitationLohani et al., 2004).
CONCLUSIONS
The least reduction in fruit mass and highest firmness of the fruit were achieved by refrigeration storage compared to other storages. Higher weight loss of fruit under the cyclic day/night conditions than under the normal warm storage condition could be due to the lower relative humidity and cyclic warming of fruit. The change of peel color was delayed in refrigerator condition, whereas in other storages, the color changed rapidly in a shorter period of time. TSS:TA ratio increased rapidly under all three conditions during ripening. Vitamin C content in this cultivar is low at all stages of ripening. ‘Malindi’ had higher respiration rates and ethylene production, particularly at room temperature storage. Refrigerated storage offered significant benefits in postharvest handling of the ‘Malindi’ banana by reduced fruit weight loss, extended storage life, and enhanced vitamin C retention in fruit during ripening.
ACKNOWLEDGMENT
This work is based upon research supported by His Majesty's Strategic Research Project at Sultan Qaboos University awarded to Prof UL Opara and the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation.
LITERATURE CITED
- AOAC . 2000 . “ Official methods of analysis of AOAC International ” . In AOAC International. 17th Edition , Edited by: Horwitz , W. 16 – 20 . Washington , D.C : Office of the Federal Register, U.S. Government . (ed.).
- Banks , N.H. , Cleland , D.J. , Cameron , A.C. , Beaudry , R.M. and Kader , A.A. 1995 . Proposal for a rationalized system of units for postharvest research in gas exchange . HortScience , 30 : 1129 – 1131 .
- Biale , J.B. , Young , R.E. and Olmstead , A.L. 1954 . Fruit respiration and ethylene production . Plant Physiol , : 168 – 174 .
- Bishop , D. 1996 . Controlled atmosphere storage , Edited by: Dellino , C.J.V. Blackie, London : Cold and chilled storage technology . (ed.).
- Broughton , W.J. and Wu , K.F. 1979 . Storage conditions and ripening of two cultivars of banana . Scientia Hort , 10 : 83 – 93 .
- Cano , M.P. , de Ancos , B. , Matallana , M.C. , Camara , M. , Reglero , G. and Tabera , J. 1997 . Differences among Spanish and Latin-American banana cultivars: Morphological, chemical and sensory characteristics . Food Chem , 59 : 411 – 419 .
- Chamara , D. , Illeperuma , K. , Galappatty , P.T. and Sarananda , K.H. 2000 . Modified atmosphere packaging of ‘Kolikuttu’ bananas at low temperature . J. Hort. Sci. Biotechnol , 75 : 92 – 96 .
- Chauhan , O.P. , Raju , P.S. , Dasgupta , D.K. and Bawa , A.S. 2006 . Instrumental textural changes in banana (var. Pachbale) during ripening under active and passive modified atmosphere . Intl. J. Food Proper , 9 : 237 – 253 .
- Chen , C.R. and Ramaswamy , H.S. 2002 . Color and texture change kinetics in ripening bananas . Leb. Wiss. Technol , 35 : 415 – 419 .
- Dadzie , B.K. and Orchard , J.E. 1997 . Routine post-harvest screening of banana/plantain hybrids: Criteria and methods, p , 3 – 56 . INIBAP Tech. Guid. 2 .
- Finger , F.L. , Puschmann , R. and Barros , R.S. 1995 . Effects of water loss on respiration, ethylene production and ripening of banana fruit. Rev . Bras. Fis. Veg , 7 : 115 – 118 .
- Kader , A.A. 1987 . “ Respiration and gas exchange of vegetables ” . In Post-harvest physiology of vegetables , Edited by: Weichmann , J. 27 – 30 . New York : Marcel Dekker . (ed.).
- Kader , A.A. 2003 . A summary of CA requirements and recommendations for fruits other than apples and pears . Acta Hort , 600 : 737 – 740 .
- Kader, A.A. 2005. Banana: Recommendations for maintaining postharvest quality. Davis, CA. 27 April 2008. < http://Produce/ProduceFacts/Fruits/banana.shtml (http://Produce/ProduceFacts/Fruits/banana.shtml)
- Lee , S.K. and Kader , A.A. 2000 . Preharvest and postharvest factors influencing vitamin C content of horticultural crops . Post. Bio.Tech , 20 : 207 – 220 .
- Leverton , R.M. 1937 . Ascorbic-acid content of bananas at three stages during ripening . J.Food Sci , 2 : 59 – 63 .
- Lodh , S.B. , Ravel , P. , Selvaraj , Y. and Kohli , R.R. 1971 . Biochemical changes associated with growth and development of ‘Dwarf Cavendish’ banana . Ind. J. Hort , 28 : 38
- Loesecke , H. Von. 1950 . Bananas. 2nd Edition , New York : InterScience .
- Lohani , S. , Trivedi , P.K. and Nath , P. 2004 . Changes in activities of cell wall hydrolases during ethylene-induced ripening in banana: Effect of 1-MCP, ABA, IAA . Post. Bio.Tech , 31 : 119 – 126 .
- Mendoza , F. and Aguilera , J.M. 2004 . Application of image analysis for classification of ripening bananas . J. Food Sci , 69 : 471 – 477 .
- Mostafa , E.A.M. 2005 . Response of Williams banana to different rates of nitrogen and potassium fertilizers . J. Appl. Sci. Res , 1 : 6 – 71 .
- Mustaffa , R. , Osman , A. , Yusof , S. and Mohamed , S. 1998 . Physico-chemical changes in Cavendish banana (Musa cavendishii L. var Montel) at different positions within a bunch during development and maturation . J. Sci. Food Agr , 78 : 201 – 207 .
- Olorunda , A.O. 2000 . Recent advances in postharvest technologies of banana and plantain in Africa . Acta Hort , 450 : 517 – 527 .
- Opara , U.L. 2010 . Editorial: High incidence of postharvest food losses is worsening global food and nutrition security . Int. J. Post. Technol. Innov. 2:1 ,
- Opara , L.U. , Jacobson , D. and Al-Saady , N.A. 2010 . Analysis of genetic diversity in banana cultivars (Musa cvs.) from the South of Oman using AFLP markers and classification by phylogenetic, chemometric and hierarchical clustering . J. Zhej. Uni. Sci , 11 : 332 – 341 .
- Salvador , A. , Sanz , T. and Fiszman , S.M. 2007 . Changes in colour and their relationship with eating quality during storage of two different dessert bananas . Post. Bio. Tech , 43 : 319 – 325 .
- Seymour , G.B. , Thompson , A.K. and John , P. 1987 . Inhibition of degreening in the peel of bananas ripened at tropical temperatures. I. Effect of high temperature on changes in the pulp and peel during ripening . Ann. Appl. Biol , 110 : 145 – 151 .
- Simmonds , N.W. , Stover , R.H. and Harry , R. 1987 . Bananas. 3rd Edition , London : Longmans .
- Thomas , P. and Janave , M.T. 1992 . Effect of temperature on chlorophyllase activity, chlorophyll degradation and carotenoids of Cavendish bananas during ripening . Int. J. Food Sci.Technol , 27 : 57 – 63 .
- Viswanath , P. , Al-Bakry , A.N. and Nadaaf , S.K. 1997 . Performance of banana (Musa spp.) cultivars in Sultanate of Oman . InfoMusa , 6 : 21 – 22 .
- Wills , R.B. , Lim , J.S. and Greenfield , H. 1984 . Changes in chemical composition of ‘Cavendish’ banana (Musa acuminate) during ripening . J. Food Biochem , 8 : 69 – 77 .
- Wills , R. , McGlasson , W.B. , Graham , D. and Joyce , D. 1998 . Postharvest: An introduction to the physiology and handling of fruit, vegetables and ornamentals, p. 262. 4th Edition , Wallingford , , UK : Commonwealth Agricultural Bureaux International (CABI) .