Abstract
Forcing solution experiments were conducted on ‘Edelweiss’ dormant canes. In 2009, canes were treated with naphthaleneacetic acid (NAA) at 500, 750, and 1000 mg/l, Amigo Oil 10% v/v, and the non-treated control. In 2010, the treatments were NAA (500, 1000, and 1500 mg/l), Amigo Oil, and the control. In both years, results showed a month, bud position, and treatment interaction. In 2009, NAA at 1000 ppm in April significantly delayed bud break 7 days. In 2010, bud break was significantly delayed 4 and 5 days using NAA at 1000 and 1500 ppm, respectively, in March. Shoot length was not affected.
INTRODUCTION
One of the chemicals studied and used by many researchers to extend life of many cut flowers is 8-hydroxyquinoline citrate (8-HQC) (CitationLarsen & Scholes, 1965, 1966; CitationMarousky, 1969b). The mechanism of prolonging life by 8-HQC was due to decreasing vascular blockage in stems and increasing water absorption and stomatal closure. Regarding sucrose, it was found to reduce the stomatal opening. The same mechanisms were found to be the reasons of extending vase-life and improving quality of gladiolus (CitationMarousky, 1969a). CitationPaulin (1986) added that sugar addition delays the loss of membrane integrity and degradation of phospholipids. Other studies that showed an increase in cut flower life and improvement in quality include: CitationAbdel-Kader and Rogers (1986) in gerbera, CitationKofranek (1986) in Triteleia laxa, and CitationNooh et al. (1986) in Ruscus hypoglossum and Nephrolepis exaltata. After the applications of forcing solutions to extend vase-life of many cut flowers, investigators studied the possibility of forcing solution applications on bud break and rooting of woody plants, especially for micropropagation purposes. The same forcing solution employed for vase-life extension was suggested by CitationRead et al. (1984), to be used to produce explants by soaking cut stem ends in the solution to force new growth. Since the availability of plant materials for in vitro purposes is limited to a short period of time during early spring (CitationYang & Read, 1991), forcing solution and plant growth regulators have been investigated to accelerate bud break and enhance rooting of cuttings. CitationRead and Yang (1989) found that infusion of plant growth regulators via forcing solutions enhanced in vitro performance. CitationRead and Yang (1992) concluded that indolebutyric acid (IBA) delivered via the forcing solution increased root numbers per cutting and promoted root elongation, while gibberellic acid (GA3) inhibited rooting of softwood cuttings taken from forced dormant stems. Similarly, CitationHamooh (2001) found that GA3 decreased rooting ability of some softwood cuttings. In vitro shoot proliferation in Vanhoutte spirea was stimulated by adding benzyladenine (BA) in the forcing solution, while GA3 resulted in less in vitro shoot proliferation (CitationYang & Read, 1993). The use of forcing solution and some wetting agents hastened bud break in lilac, privet, and Vanhoutte spirea (CitationYang & Read, 1992). In another study, CitationYang and Read (1997b) found that GA3 hastened bud break while BA and IBA delayed break. CitationHamooh (2001) found that adding silver thiosulfate to the forcing solution hastens bud break and shoot elongation. Less time to bud break and longer shoots were achieved when GA3 was combined with silver thiosulfate in the forcing solution. An advantage of the use of forcing solution technique was demonstrated by the reduction of time from culture to potted plants of 5-leaf aralia (CitationYang & Read, 1997a). It can be concluded that the forcing solution technique could be a useful method for micropropagation purposes to enhance bud break as well as a method for studying bud break dormancy in woody plants.
According to CitationGu and Read (2004), there are many studies on bud dormancy of grapevines, but none of the studies used forcing solutions. CitationGu (2003) studied the effects of forcing solution and some plant growth regulators: GA3, BA, indoleactic acid (IAA), and abscistic acid (ABA) on bud break of ‘Lacrosse’, own rooted ‘Chambourcin’, and grafted ‘Chambourcin’ on the ‘3309 Couderc’ rootstock. Forcing solution did not accelerate bud break compared to water treatments, which is contrary to the previous studies on other woody plants. All of the added plant growth regulators delayed bud break with GA3 delaying the most. Furthermore, interactions between cultivar, month, and the plant growth regulators were present.
Grapes in the Midwest states are greatly influenced by frost injury. A strategy has been adopted to apply chemicals, such as growth regulators (CitationNigond, 1960) and dormant oils (CitationDami & Beam, 2004), to delay bud break until the frost risk period passes and reduce frost injury damage. The objective of this study was to examine the possibility of delaying bud break on ‘Edelweiss’ single-bud cuttings placed in forcing solution after treating the cuttings with NAA and Amigo Oil.
MATERIALS AND METHODS
Plant Material
Dormant canes of ‘Edelweiss’ grapevines were collected from James Arthur Vineyards in Raymond, Nebraska on four dates: 6 Jan., 3 Feb., 3 Mar., and 1 Apr. 2009. For each date, 20 canes were randomly selected, similar in diameter and length, and headed back to the first five buds. Each cane was then cut into five single-bud cuttings. A 5 × 5 Latin Square design was used for this experiment. The single-bud cuttings were soaked in a solution containing 10% Clorox for 15 s and then rinsed with tap water. The identity of the bud position was retained using four different colored tapes and the control without any tape. The single-bud cuttings with tags were placed in plastic storage bags and placed in the cooler at 4–5°C prior to treatment the following day.
Treatments
A stock of the forcing solution developed by CitationRead et al. (1984) containing 200 mg 8-hydroxyquinoline citrate (8-HQC)/l and 2% sucrose was prepared and placed in autoclaved GA7 containers.
The 2009 experiment consisted of five treatments: NAA (500, 750, and 1000 mg/l) purchased from Phyto Technology Laboratories (Shawnee Mission, KS, USA), Amigo Oil (9.3% oil and 0.7% emulsifier, Loveland Industries, Greely, CO) applied at 10% v/v and the non-treated control. Treatments were applied on buds by adding one drop per bud using a sterile transfer pipette at the time of placement of cuttings in the GA7 containers. After treatment, the single-bud canes were placed vertically (proximal ends down) in GA7 containers containing 100 ml of forcing solution as shown in The solutions were replaced with 100 ml of freshly prepared forcing solution every 4 days and the basal 0.2 cm ends of the cuttings were cut off each time the solutions were changed. The GA7 containers were placed in a room where the temperature was 25°C. Days to bud break starting from the date of treatment and shoot length after 1 week of bud break were recorded throughout the study. Bud break was determined as stage five of the CitationEichhorn and Lorenz (1977) scale of grapevine development. Stage five indicates that the bud scales have expanded to the point at which a green shoot is visible. Buds that did not show bud break were cut into longitudinal sections and examined under a stereomicroscope to examine the viability of the bud and any phytotoxity effect of any of the treatments.
FIGURE 1 ‘Edelweiss’ single-bud cuttings placed in GA7 containers containing forcing solution (color figure available online).

The same procedures were used in the 2010 experiment. Differences were in the collection dates, which were 28 Jan., 25 Feb., and 25 Mar. 2010, and the NAA concentrations, which were 500, 1000, and 1500 mg/l. All statistical analyses were performed using SAS/STAT Version 9.2 (SAS Institute Inc., Cary, NC, USA) and ANOVA was conducted by the PROC GLIMMIX procedure.
RESULTS AND DISCUSSION
Bud Break and Shoot Length in Forcing Solution Experiment 2009
A total of 400 single-bud cuttings were used in this experiment. Only the single-bud cuttings treated with oil showed missing data values. Among the 20 single-bud cuttings in January, February, March, and April (a total of 80), only 12 showed bud break at stage five in April. Some of the single-bud cuttings showed only bud swelling and others showed no growth symptoms at all ().
FIGURE 2 ‘Edelweiss’ single-bud cuttings treated with oil showing no bud break (left) and some bud swelling (right) (color figure available online).

CitationDami and Beam (2004) reported 6–10% bud injury with Prime Oil and 4–5% with Amigo Oil. They found no differences between Amigo Oil treated vines and the controls, which led them to conclude that only Prime Oil was phytotoxic to the grapevines studied. Furthermore, they noticed that November treated vines showed the most injury. In addition, a bud position effect in ‘Chambourcin’ was detected showing that basal buds had more injury than apical buds (CitationDami & Beam, 2004). They considered buds one to five as basal buds, the same bud positions used in this study. No explanation was given for their results.
‘Edelweiss’ buds that did not show bud break were longitudinally sectioned and examined under a stereomicroscope to determine bud viability and any treatment phytotoxity. Total number of buds, position, and the cane number from which they were taken are presented in . Total number of buds that were treated with oil was 80. Phytotoxity damage as shown in is 10%, which may be because these cuttings were placed inside with no environmental factors to help break down the phytotoxic effects of the oil. The remaining buds showed no phytotoxity symptoms and a live primary bud as shown in
TABLE 1 Month, Cane Number, Bud Position on Cane, and Total Number of ‘Edelweiss’ Single-Buds That Showed Oil Phytotoxicity Damage in 2009
TABLE 2 Analysis of Variance Table for Number of Days to Show Stage Five Bud Break and Shoot Length (SL) after One Week of Bud Break of ‘Edelweiss’ Single-Bud Cuttings in the 2009 Forcing Solution Experiment for ‘Edelweiss’ Single-Bud Cuttings
FIGURE 3 Transverse sections of buds of ‘Edelweiss’ single-bud cuttings treated with oil showing phytotoxity and primary bud damage (color figure available online).
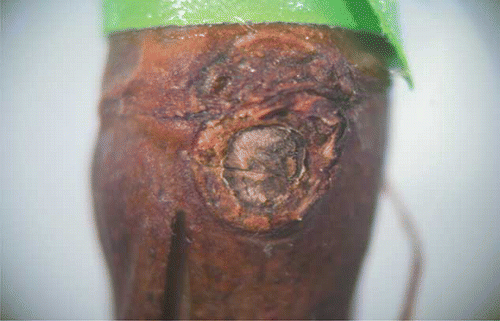
FIGURE 4 Transverse section of buds of ‘Edelweiss’ single-bud cuttings treated with oil showing no phytotoxity and a live primary bud (color figure available online).
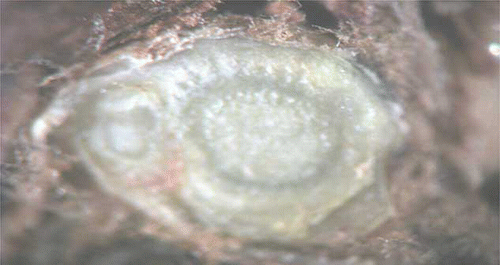
CitationLavee and May (1997) mentioned that reasons for no growth could be explained by physical or chemical conditions external to the bud, restriction by enclosing bract tissues, or correlative inhibition. Our expectation is that single-bud cuttings were taken too early because grapevines are in the endodormant stage in January (CitationGu, 2003). Another contributing factor is that these cuttings probably did not receive the adequate chilling hours especially considering that average monthly temperatures in January and February were 0.7 and 6.4°C, respectively.
Analysis of variance for number of days to show bud break and shoot length are shown in . A significant month by position by treatment interaction was detected at (P ≤ 0.05) as shown in . Single-bud cuttings at bud position five treated with NAA at 1000 ppm clearly showed a delay in bud break ().
FIGURE 5 ‘Edelweiss’ single-bud cuttings at bud position five showing a delay in bud break by using NAA at 1000 ppm (color figure available online).

An objective of this experiment was to examine the capability of treatments to delay bud break mainly at bud position five in the presence of a forcing solution. Grape buds normally show bud break starting from distal toward proximal ends. A comparison between NAA at 1000 ppm and the control at bud position five is shown in
FIGURE 6 Number of days required by ‘Edelweiss’ single-bud cuttings to show bud break at bud position five treated with NAA at 1000 ppm and the control in 2009.
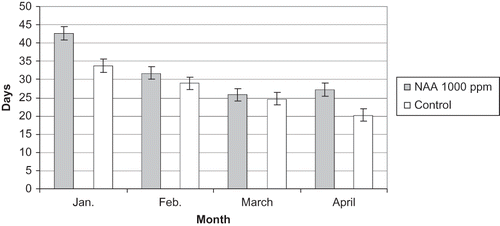
Figure 6 shows that there was a significant difference in bud break delay of 9 days in January and 7 days in April at (P ≤ 0.05). Obtaining such results in January could be because the treatment was most effective in the deepest endodormant stage as shown in other studies (CitationKubota & Miyamuki, 1992; CitationKurooka et al., 1990) or the delay was perhaps because the cuttings were taken too early and that they did not receive enough chilling hours.
Regarding shoot length, average shoot lengths from January, February, March, and April treatments were 2.64, 6.07, 4.86, and 3.74 cm, respectively (CitationQrunfleh, 2010). Shorter shoot lengths were obtained from single-bud cuttings taken early in January treatments. This is also an indication that the vines were at the endodormant stage and had not received adequate chilling hours as mentioned previously. Overall winter temperatures in 2009 were warmer than normal (CitationQrunfleh, 2010). Most importantly, there was no treatment effect which also gives support that such applications seem to have no effect on the vegetative growth.
Bud Break and Shoot Length in Forcing Solution Experiment 2010
Similarly to 2009, analysis of variance for bud break shows a significant month by position by treatment interaction at (P ≤ 0.05) as shown in . For this study, a total of 300 single-bud cuttings were used. Only the single-bud cuttings treated with oil showed missing values. Among the 20 single-bud cuttings collected and treated in January, February, and March, only 11 showed bud break stage five from February treatments and 10 in March. No bud break was observed in January treatments. Similarly, buds that did not show bud break were longitudinally sectioned and examined under a stereomicroscope. Total number of buds, position, and the cane number from which they were taken are presented in .
TABLE 3 Analysis of Variance Table for Number of Days to Reach Stage Five Bud Break and Shoot Length (SL) after One Week of Bud Break of ‘Edelweiss’ Single-Bud Cuttings in the 2010 Forcing Solution Experiment
TABLE 4 Month, Cane Number, Bud Position on Cane, and Total Number of ‘Edelweiss’ Single-Bud That Showed Oil Phytotoxicity Damage in 2010
Total number of buds that were treated with oil was 60. The phytotoxity damage was also 10%. In order to explain failure of a single-bud treated with oil, xylem, and phloem, tissues were examined under a stereomicroscope. Possible failure of single-buds treated with oil could be attributed to problems in the conducting tissues. However, examination showed normal xylem and phloem tissues as shown in
FIGURE 7 A cross section of one of the ‘Edelweiss’ single-bud cuttings treated with oil showing normal xylem and phloem tissues (color figure available online).

Single-bud cuttings treated with oil that did not show bud break in 2009 and 2010 could be explained by bud scale and oil effects on bud respiration and cell membranes. Bud scales were shown to be involved in dormancy (CitationIwasaki, 1980; CitationIwasaki and Weaver, 1977). Respiration was shown to be decreased with oil applications (CitationDami & Beam, 2004). Furthermore, there is also a possibility that oil applications are affecting cell membranes, since membrane lipids were also found to be involved in dormancy (CitationErez, 2000; CitationIzadyar & Wang, 1999; CitationWang & Faust, 1990). Further studies are required to prove this interpretation.
Single-bud cuttings treated with oil only showed a 10% phytotoxity damage in both years, which is probably explained by the indoor placement of the cuttings without any environmental factors that could help in breaking down oil effects. On the other hand, the remaining buds showed an active primary living bud, which is a critical issue when applying chemicals on vines to delay bud break. shows a delay in bud break at bud position five by using oil applications. NAA at 1500 ppm was also effective in delaying bud break at bud position five () similarly to that observed with NAA at 1000 ppm in the 2009 study. Similarly, a comparison between NAA at 1000 ppm and the control at bud position five is shown in and a similar comparison between NAA at 1500 ppm is shown in
FIGURE 11 Number of days required by single-bud cuttings to show bud break at bud position five treated with NAA at 1500 ppm and the control in 2010.
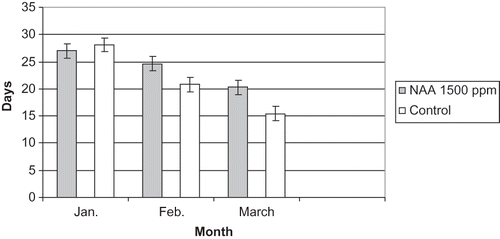
FIGURE 10 Number of days required by single-bud cuttings to show bud break at bud position five treated with NAA at 1000 ppm and the control in 2010.
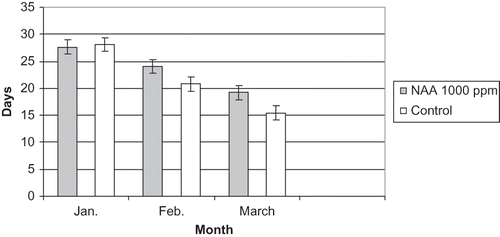
FIGURE 9 ‘Edelweiss’ single-bud cuttings at bud position five showing a delay in bud break by using NAA at 1500 ppm compared to the non-treated control (color figure available online).
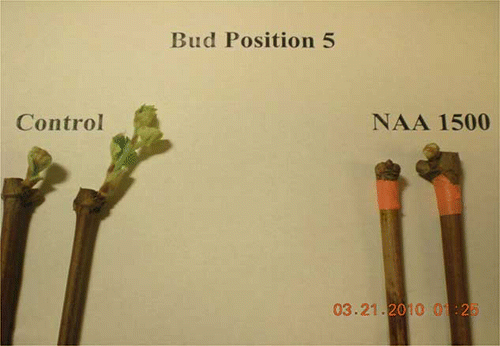
FIGURE 8 ‘Edelweiss’ single-bud cuttings showing no bud break at bud position five by using oil applications (far right) and the non-treated control at bud position one (far left) showing more advanced bud break stage than NAA at the three concentrations applied: 500, 1000, and 1500 ppm (color figure available online).

In January, NAA at 1000 ppm failed to delay bud break at bud position five as shown in This supports the interpretation of January 2009 results where it was assumed that results were related to time of taking cuttings and inadequate chilling hours. However, NAA at 1000 ppm significantly delayed bud break 3 days where cuttings were treated in February and 4 days when treated in March. A similar trend was shown by NAA at 1500 ppm, where it failed to significantly delay bud break in January but significantly delayed bud break 4 days with February treatments and 5 days with March treatments.
Regarding shoot length, analysis of variance shows only a month by treatment interaction at (P ≤ 0.05) (). Average shoot lengths in January, February, and March were 5.01, 4.71, and 4.78 cm, respectively (CitationQrunfleh, 2010). Differences in shoot length results for both years are probably because of different winter weather and the time of taking cuttings (CitationQrunfleh, 2010).
CONCLUSION
Oil and NAA treatments delayed bud break of ‘Edelweiss’ single-bud grapevine cuttings placed in forcing solution. NAA applications at 1000 ppm in April significantly delayed bud break 7 days. In the 2010 forcing solution experiment, bud break was significantly delayed 4 days by NAA at 1000 ppm and 5 days by NAA at 1500 ppm with March applications. One of the possible drawbacks found when using Amigo Oil in the forcing solution studies was phytotoxity observed in the treated buds.
LITERATURE CITED
- Abdel-Kader , B. and Rogers , M. 1986 . Postharvest treatment of Gerbera jamesonii . Acta Hort. , 181 : 169 – 176 .
- Dami , I. and Beam , B. 2004 . Response of grapevines to soybean oil application . Amer. J. Enol. Viticult. , 55 : 269 – 275 .
- Eichhorn , K. and Lorenz , D. 1977 . Phänologische Entwicklungsstadien der Rebe . Nachrichtenbl. Deut. Pflanzenschutz. , 29 : 119 – 120 .
- Erez , A. 2000 . “ Bud dormancy: A suggestion for the control mechanism and its evolution ” . In Dormancy in plants: From whole plant behaviour to cellular control , Edited by: Viémont , J.D. and Crabbé , J. 23 – 34 . New York : CABI Publishing .
- Gu , S. 2003 . Rootstock and mounding effect on growth and cold hardiness of ‘Gewürztraminer’ (Vitis vinifera) and bud dormancy of ‘Lacrosse’ and ‘Chambourcin’ (Vitis Spp.) , Univ. Nebraska , Lincoln : PhD Diss .
- Gu , S. and Read , P. 2004 . The influence of plant growth regulators included in the forcing solution on bud break of dormant grapevines (Vitis spp.) . Acta Hort. , 640 : 225 – 234 .
- Hamooh , B. 2001 . Application of forcing solution technology to micro and macropropagation woody plant species , Univ. Nebraska , Lincoln : PhD Diss .
- Iwasaki , K. 1980 . Effects of bud scale removal, calcium cyanamide, GA3, and ethephon of ‘Muscat of Alexandria’ grape (Vitis vinifera L.) . J. Japan. Soc. Hort. Sci. , 48 : 395 – 398 .
- Iwasaki , K. and Weaver , R. 1977 . Effects of chilling, calcium cyanamide, and bud scale removal on bud break, rooting, and inhibitor content of buds of ‘Zinfandel’ grape (Vitis vinifera L.) . J. Amer. Soc. Hort. Sci. , 102 : 584 – 587 .
- Izadyar , A. and Wang , S. 1999 . Changes of lipid components during dormancy in ‘Hull Thornless’ and ‘Triple Crown Thornless’ blackberry cultivars . Sci. Hort. , 82 : 243 – 254 .
- Kofranek , A. 1986 . Studies on the post harvest handling of Triteleia laxa . Acta Hort. , 181 : 231 – 236 .
- Kubota , N. and Miyamuki , M. 1992 . Breaking dormancy in grapevines with garlic paste . J. Amer. Soc. Hort. Sci. , 117 : 898 – 901 .
- Kurooka , H. , Horiuchi , S. , Fukunga , S. and Yuda , E. 1990 . Effects of electric current on breaking bud dormancy in grapes . Bul. Univ. Osaka Prefecture , 42 : 111 – 119 .
- Larsen , F. and Scholes , J. 1965 . Effects of sucrose, 8-hydroxyquinoline citrate, and N-dimethylamino succinamic acid on vase-life and quality of cut carnations . Proc. Amer. Soc. Hort. Sci. , 87 : 458 – 463 .
- Larsen , F. and Scholes , J. 1966 . Effects of 8-hydroxyquinoline citrate, N-dimethyl amino succinamic acid, and sucrose on vase-life and spike characteristics of cut snapdragons . Proc. Amer. Soc. Hort. Sci. , 89 : 694 – 701 .
- Lavee , S. and May , P. 1997 . Dormancy of grapevine buds—Facts and speculation . Austral. J. Grape Wine Res. , 3 : 31 – 46 .
- Marousky , F. 1969a . Physiological role of 8-hydroxyquinoline citrate and sucrose in extending vase-life and improving quality of cut gladiolus . Florida State Hort. Soc. , 81 : 409 – 414 .
- Marousky , F. 1969b . Vascular blockage, water absorption, stomatal opening, and respiration of cut ‘Better Times’ roses treated with 8-hydroxyquinoline citrate and sucrose . J. Amer. Soc. Hort. Sci. , 94 : 223 – 226 .
- Nigond , J. 1960 . Delaying bud break in vines by the use of α-naphthaleneacetic acid and defense against frost . Compt. Rend. Acad. Agr. France , 46 : 452 – 457 .
- Nooh , A. , El-Kiey , T. and Khattab , M. 1986 . Studies on the keeping quality of cut green Ruscus hypoglossum L. and Nephrolepis exaltata Schott . Acta Hort. , 181 : 223 – 230 .
- Paulin , A. 1986 . Influence of exogenous sugars on the evolution of some senescence parameters of petals . Acta Hort. , 181 : 183 – 194 .
- Qrunfleh , I. 2010 . Delaying bud break in ‘Edelweiss’ grapevines to avoid spring frost injury byNAA and vegetable oil applications , Univ. Nebraska , Lincoln : PhD Diss .
- Read , P. , Economou , A. and Fellman , C. 1984 . “ Manipulating stock plants for improved in vitro mass propagation ” . In Proc. Intl. Symp. plant tissue and cell culture: Application to crop improvement , Edited by: Novak , J. , Havel , L. and Dolezel , J. 467 – 473 . Czech. Acad. Sci., Prague .
- Read , P. and Yang , G. 1989 . Influencing propagation by stock plant PGR treatments . Acta Hort. , 251 : 121 – 128 .
- Read , P. and Yang , G. 1992 . Plant growth regulators effects on rooting of forced softwood cuttings . Acta Hort. , 300 : 197 – 200 .
- Wang , S. and Faust , M. 1990 . Changes of membrane lipids in apple buds during dormancy and budbreak . J. Amer. Soc. Hort. Sci. , 115 : 803 – 808 .
- Yang , G. and Read , P. 1991 . Influence of plant growth regulators and season on growth responses of forced woody stems . Acta Hort. , 300 : 173 – 176 .
- Yang , G. and Read , P. 1992 . Pre-forcing treatments influence bud break and shoot elongation in forced woody species . J. Environ. Hort. , 10 : 101 – 103 .
- Yang , G. and Read , P. 1993 . In vitro culture of Vanhoutte's spirea explants from ‘secondary cultures’ and dormant stems forced in solutions containing plant growth regulators . Plant Cell, Tissue, & Organ Cult. , 33 : 25 – 30 .
- Yang , G. and Read , P. 1997a . In vitro shoot proliferation of 5-leaf aralia explants from field grown plants and forced dormant stems . Plant Cell, Tissue, & Organ Cult. , 47 : 289 – 291 .
- Yang , G. and Read , P. 1997b . Plant growth regulators in the forcing solution influenced bud break and shoot elongation of dormant woody plant species . PGRSA Qrtly. , 25 : 145 – 152 .