Abstract
Fungicidal sprays are widely used for control of Botrytis fruit rot; however, the pathogen often develops resistance to frequently used fungicides. A 96-well plate micro-dilution broth bioassay developed for fungicide discovery was used to provide strawberry growers with a rapid assessment of the fungicide sensitivity of Botrytis isolates against 16 fungicides. Three sensitivity phenotypes were identified: benzimidazole and dicarboximide resistant, benzimidazole resistant and dicarboximide sensitive, and an intermediate response to both fungicides. Codon at position 198 in the β-tubulin gene confirmed benomyl resistance. This bioassay rapidly identifies fungicide resistance and allows growers to quickly adjust their disease management strategy.
Keywords:
INTRODUCTION
The Louisiana strawberry industry is based in southeastern Louisiana in Tangipahoa and Livingston Parishes on approximately 160 hectares with a farm value of around $9.8 million per year (USDA, 2008). In recent years, strawberry growers reported a failure of their fungicide spray program to control Botrytis fruit rot, which resulted in serious financial losses. This disease was especially severe in years when warm wet weather coincided with harvest. In 2012, strawberry growers in central Florida experienced a severe Botrytis outbreak possibly due to the pathogen, Botrytis cinerea, becoming resistant to multiple fungicides (CitationAmiri et al., 2012).
Botrytis fruit rot (also known as gray mold) is one of the most destructive diseases of strawberry. The causal fungus, Botrytis cinerea Pers.:Fr., may initially infect the petals, stamens, or pistils to cause blossom blight and also may infect leaves and petioles (CitationSutton, 1998). Fruit infection occurs when mycelium grows from the infected flower parts into the receptacle or when it is invaded by mycelium from an adjacent infected fruit. Fruit that are infected while green may remain symptomless until just prior to harvest. Postharvest rot develops rapidly and is the most devastating phase of this disease.
Botrytis cinerea has a history of becoming resistant to commonly used fungicides including those in the benzimidazole and dicarboximide classes. Benzimidazoles (e.g., benomyl [until it was removed from the market in 2001], thiophanate-methyl, and thiabendazole), dicarboximides (e.g., iprodione), and captan are the primary fungicides that were used to control Botrytis on strawberry in the past two decades (CitationSutton, 1990; CitationWedge et al., 2007). More recently, several newer fungicides have become available and are very effective for control of Botrytis diseases (i.e., strobilurins, anilides, and pyrroles; CitationAmiri et al., 2012); however, the development of isolates of B. cinerea resistant to these new chemicals is a major concern for growers.
Traditional assays to establish changes in resistance of fungal populations based on in vitro evaluation of fungicides with IC50 concentrations generated from 6–12 point dose-response curves are tedious and time consuming. In addition, the results are not available in a useful time frame for the grower or the extension specialist to make suggested changes in chemical applications. The rationale for our study was to use a miniaturized, 96-well micro-dilution broth bioassay (microtiter assay) and a 3-point curve protocol developed for natural product fungicide discovery to quickly characterize fungicide resistance in B. cinerea isolates provided by extension personnel from farms where Botrytis fruit rot control was poor. Fungicide sensitivity profiles for 13 B. cinerea isolates against 16 fungicides were tested for in vitro activity. Molecular techniques were then used to corroborate the accuracy of the chemical study by evaluating the amino acid substitutions at codon position 198 in the β-tubulin gene to confirm benomyl fungicide resistance and sensitivity profiles generated from the microtiter assays.
MATERIALS AND METHODS
Inoculum Preparation
Botrytis cinerea spores were collected from several strawberry farms in southeastern Louisiana by touching the tip of a sterile cotton swab to an infected fruit. The swabs were placed in sealed plastic bags and mailed to the USDA Agricultural Research Service (Thad Cochran Southern Horticultural Laboratory, Poplarville, MS), where fungal cultures were isolated by streaking the surface of acidified potato dextrose agar with the cotton swabs. Conidia were picked from resultant Botrytis cultures using fine, sterile forceps under a dissecting microscope. Three to four transfers were made for each isolate to achieve an axenic culture. Pure cultures of 11 isolates of B. cinerea were evaluated for fungicide resistance. In addition, an isolate obtained from grape (identified as “GR”) and one obtained from blueberry fruit (identified as “BB”) were included in trials for comparison because the dicarboximides (vinclozolin and iprodione) commonly used on strawberry are not used for disease control on blueberry and grape. Each of the 13 fungal isolates was sub-cultured on half strength potato-dextrose agar, and conidia were harvested from axenic cultures at 7- to 10-day intervals. Conidial concentrations were determined photometrically (CitationEspinel-Ingrof & Kerkering, 1991; CitationWedge & Kuhajek, 1998) from a standard curve and suspensions were then adjusted with sterile distilled water to a concentration of 1.0 × 106 conidia/ml.
Microtiter Assay
A standardized 96-well micro-dilution broth assay developed for discovery of natural product fungicidal agents (CitationWedge & Kuhajek, 1998; CitationWedge et al., 2000) was used to evaluate sensitivity profiles of the 13 B. cinerea isolates to a variety of chemicals used to control strawberry pests and diseases. Each microtiter test well received 80 μL of RPMI 1640 (Roswell Park Memorial Institute mycological broth 1640; Life Technologies, Grand Island, NY, USA) and 3[N-morpholino]propanesulfonic acid (MOPS; Sigma Chemical Co., St. Louis, MO, USA) buffered broth at pH 7.0, 100 μL of conidia at 1.0 × 104 conidia per ml, and 20 μL of antifungal solution (). The 16 commercial compounds tested are currently, previously, or anticipated to be, registered for use on strawberry (). Technical grade pesticide standards were obtained from Chem Service, Inc. (West Chester, PA, USA). Fosetyl-Al and metylaxyl were used as negative fungicide standard, and captan was used as a positive fungicide standard in all microtiter assays. Each fungus was challenged in a dose-response format using test compounds where the final treatment concentrations were 0.3, 3.0, and 30.0 μM. Microtiter plates (Nunc MicroWell, untreated; Roskilde, Denmark) were covered with a plastic lid and incubated in a growth chamber at 24 ± 1°C and 12 h photoperiod under 60 ± 5 μmol·m2·s−1 light. Growth was then evaluated by measuring absorbance of each well at 620 nm using a microplate photometer (Packard Spectra Count, Packard Instrument Co., Downers Grove, IL, USA).
FIGURE 1 Experimental set up for 96-well plate microtiter assay in which columns contain 0, 0.3, 3.0, and 30.0 concentrations of test compounds. Rows (A–H) contain fungicides and internal standards for comparison and validation. Sixteen positive growth control wells are used to establish the fungal growth mean and eight negative control wells without inoculum and test compounds serve as sterility checks. Blank reagent well indicated with stippling are used to negate the effects that colored molecules have on the turbidity measurements at 620 nM.
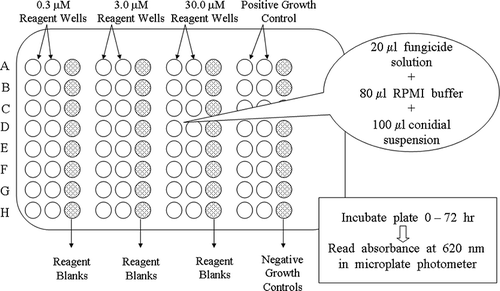
Microtiter Assay Experimental Design
Using the 96-well plate microtiter assay format, each chemical was evaluated in duplicate at three concentrations. Sixteen wells containing broth and inoculum served as positive growth controls, while eight wells containing solvent at the appropriate concentration and broth without inoculum were used as negative growth controls. Mean absorbance values and standard errors were used to evaluate fungal growth at 48 and 72 h. Analysis of variance of means for percent inhibition/stimulation of each fungus at each dose of test compound relative to the untreated positive growth controls was used to evaluate fungal growth. Treatments were arranged as a split-plot design repeated four times within each experiment. Whole-plots were fungal isolates and sub-plots were chemicals. Each dose level and response time was analyzed separately. The experiments were repeated three times. SAS analysis of variance procedure (Statistical Analysis System, Cary, NC, USA) was used to identify significant factors, and Fisher's protected least significant difference (LSD) was used to separate means.
TABLE 1 Chemical and Trade Names, Chemical Class, Mode of Action, FRAC Group, Systemic Activity, Range of Controlled Pathogens, and Commercial Rate Range of Fungicides Used in This Study Based on Fungicide Labels and Fungicide Resistance Action Committee (FRAC) Website (http://www.frac.info)z
A Priori Assumption
Comparison or validation of various in vitro bioassays is a long and tedious process because various fungi and fungicidal compounds behave differently due to oxygen availability, solubility of the test compounds, fungal growth characteristics, and spore germination. Therefore, we made the a priori assumptions to utilize our existing 96-well micro-dilution broth assay with a 3-point dose-response format and to include metalaxyl and fosetyl-Al (active against oomycetes) as internal negative standards and captan as the internal positive standard. Captan is the primary commercial fungicide used for disease control on strawberry. Therefore, the B. cinerea isolates were placed into three categories based on their growth response to the test compounds. These categories were defined within each concentration as follows: resistant (R), fungal growth the same as the metalaxyl (i.e., the test chemical performed the same as the ineffective control) as indicated by the LSD (α = 0.05); intermediate (I), fungal growth was less than the growth on metalaxyl but more than the growth on captan as indicated by the LSD (α = 0.05); and sensitive (S), fungal growth was the same as that on captan (e.g., the chemical caused the same or more inhibition as the effective positive control) as indicated by the LSD (α = 0.05). These assumptions were confirmed using molecular techniques.
Collection and Analysis of DNA for B-Tubulin Gene Structure
Isolates of Botrytis spp. were grown on Difco™ potato dextrose agar (Becton, Dickinson and Company, Sparks, MD, USA) for 7 days at 15°C in the dark. Total genomic DNA was extracted from mycelium following the procedure of CitationYourman et al. (2000). The polymerase chain reaction (PCR) was used to amplify a portion of the β-tubulin gene with the BCF and BCR primers of CitationLuck and Gillings (1995). This region of the gene contains codons 198 and 200, where point mutations leading to amino acid substitutions have been correlated with benomyl resistance (CitationYarden & Katan, 1993). PCR amplifications were conducted in a total volume of 50 μl using 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 0.01% gelatin, 2 mM MgCl2, 200 μM dNTPs, 0.5 units Taq polymerase (Promega Co., Madison, WI, USA), 0.3 μM of each primer, approximately 100 ng template DNA, and water to the final volume. PCR cycling conditions consisted of an initial 1 min denaturing step at 95°C followed by 30 cycles of 1 min at 95°C, 1 min at 50°C, and 1 min at 72°C. A final elongation step of 7 min at 72°C completed the cycle. PCR products were cleaned with the ExoSAP-IT system (USB Co., Cleveland, OH, USA) and were then used as the template in a cycle sequencing reaction with an ABI BigDye Terminator cycle sequencing kit (Foster City, CA, USA) using the primers described above. All sequencing reactions were cleaned with sephadex (Princeton Separations, Adelphia, NJ, USA) prior to gel runs at the Iowa State University DNA Sequencing and Synthesis Facility. Sequence data were edited and aligned using Sequencher v. 4.1 (GeneCodes Co., Ann Arbor, MI, USA).
RESULTS
Mean percent inhibition (-) or stimulation (+) of growth of each isolate relative to the growth in untreated wells is reported for each of the 16 pesticides at 0.3, 3.0, and 30 μM concentration after 72 h (, , and , respectively). On each table, fungicides are listed in order of those causing the most growth inhibition on average across all 13 isolates to those causing the most stimulation. The effect of experimental design factors (e.g., whole plot and subplot) was found to be non-significant (α = 0.05) in almost all cases; therefore, these factors were pooled with residual error. Minute differences over time among 96-well plates, standard operating procedures, and growth chambers are responsible for the small variance associated with the pooled factors of the statistical model used in this research project. Isolates are listed in order of those most sensitive overall to the 16 fungicides to those most resistant. Five fungicides inhibited the growth of all 13 isolates by 70% or more after 72 h at the 3 μM concentration (). The mean percent growth on each of these fungicide-amended media across all isolates was dodine −94%, fenhexamide −93%, butrizol −93%, cyprodinil −92%, and chlorothalonil −90%. Two fungicides inhibited the growth of all isolates except isolate DD: kresoxim-methyl by −89% and azoxystrobin by −84%. In contrast, three fungicides either stimulated or inhibited growth by less than 10% of all except one, three, or five isolates: metalaxyl by +14%, fosetyl-Al by −3%, and thiram by −8%, respectively. The three fungicides in the benzimidazole class stimulated the growth of some isolates and inhibited the growth of others. The mean percent growth on each of these fungicide media after 72 h at 3.0 μM was benomyl −27%, thiophanate-methyl −26%, and thiabendazole −37%. The dicarboximide class fungicides also produced mixed results. The growth of five isolates in vinclozolin-amended medium was inhibited by 70% or more at the 3 μM concentration after 72 h, but this medium induced hormesis (stimulated growth) or had no effect on the growth of two isolates. Such stimulatory effects of fungicidal compounds to fungi at sub-fungitoxic doses are not uncommon (Olivia et al., 2003). Iprodione-amended medium inhibited the growth of the 12 other isolates by 12 to 61% and stimulated growth of one isolate by 4.3% at the concentration of 3 μM ().
TABLE 2 In Vitro Sensitivity of 13 Botrytis cinerea Isolates to 16 Antifungal Chemicals Was Determined Using a Standardized 96-Well Microtiter Assay. Fungal Growth Is Reported as Mean Percent Inhibition (-) or Stimulation (+) for Each Fungicide at 0.3 μM Concentration after 72 h of Growth Compared to the Growth of Each Isolate in Unamended RPMI Broth
TABLE 3 In vitro sensitivity of 13 Botrytis cinerea isolates to 16 antifungal chemicals was determined using a standardized 96-well microtiter assay. Fungal growth is reported as mean percent inhibition (-) or stimulation (+) for each fungicide at 3 μM concentration after 72 h of growth compared to the growth of each isolate in unamended RPMI broth
TABLE 4 In Vitro Sensitivity of 13 Botrytis cinerea Isolates to 16 Antifungal Chemicals Was Determined Using a Standardized 96-Well Microtiter Assay. Fungal Growth Is Reported as Mean Percent Inhibition (-) or Stimulation (+) for Each Fungicide at 30 μM Concentration after 72 h of Growth Compared to the Growth of Each Isolate in Unamended RPMI Broth
LSD values are of limited use in evaluating fungal growth means containing mixed populations of chemically sensitive and insensitive isolates. While the use of non-single spored isolates better represents the real field populations, it also complicates the in vitro evaluation of fungal growth because the culture is not in synchronous growth. However, chemical classes that produce more uniformly lower LSD values, such as azoxystrobin, appear to have a less variable population of fungicide sensitive isolates. Large LSD values often appear to occur in chemical classes in which the fungi have become insensitive or there are both sensitive and insensitive isolates as seen in isolates challenged by benomyl and iprodione. There are also other factors, such as compound solubility in the aqueous based RPMI medium, its polarity, overall activity, and whether it is a multisite or single site inhibitor. Also, the intrinsic pharmacokinetics that affect every molecule (absorption, metabolism, distribution, and excretion) within the fungus can dramatically affect the in vitro activity (CitationWedge & Camper, 2000).
Sensitivity/resistance profiles were established from fungal growth for each of the 13 Botrytis isolates to 11 fungicides based on mean percent inhibition/stimulation values obtained using the 96-well microtiter system (). Isolate categories were defined as follows: resistant (R) = chemical performed the same as metalaxyl; intermediate (I) = chemical caused more inhibition than metalaxyl but less than captan; and sensitive (S) = chemical caused the same or more inhibition than captan. After 72 h at 3 and 30 μM, all isolates were sensitive to dodine, fenhexamide, butrizol, azoxystrobin, and cyprodinil (data not shown). The variable response of the isolates to the other six fungicides is shown in . Botrytis cinerea growth response to the three benzimidazole fungicides (benomyl, thiabenadzole, and thiophanate methyl) was uniform at 3 μM within each isolate, except isolates P1, HR, and TG. Three isolates were sensitive to these three fungicides (isolates GH, BH, and NW), four were resistant (isolates GC, BB, DD, and LB), and three were rated chemically intermediate (isolates GR, DW, and FP). Three isolates (NS, GC, and BB) were sensitive to the two fungicides of the dicarboxymide class (iprodione and vinclozolin), and two isolates (isolates P1and DD) were not sensitive to these two fungicides at 3 μM.
TABLE 5 In vitro growth response of 13 Botrytis cinerea isolatesz to 6 fungicides. All isolates were obtained from strawberry except isolate GR, which was obtained from grape, and isolate BB, which was obtained from blueberry. Fungal growth to each chemical using an in vitro 96-well microtiter assay is reported as sensitivey (S), intermediatey (I), or resistanty (R). The 72 h growth response to antifungal compounds at 3 μM concentrations identified chemically sensitive B. cinerea isolates and the growth response at 30 μM concentrations identified chemically resistant B. cinerea isolates
TABLE 6 Benomyl Resistance Profile and the Identity of Codon Position 198 in the β-Tubulin Gene for the 11 Strawberry Isolates of Botrytis cinerea
When we combined the resistant and intermediate B. cinerea isolates obtained from commercial strawberry farms receiving standard spray protocols, our data supported that of CitationYourman et al. (2000), who reported that the most common phenotype (67% of 56 of their South Carolina Botrytis isolates) were resistant to both benzimidazoles and dicarboximides. In our study, 7 of 13 isolates (54%) were either resistant or intermediate in response to fungicides in these two classes at 3 μM (). Isolates DD, LB, P1, TG, HR, FP, and GR possessed resistant or intermediate response to both the benzimidazoles and dicarboximides. Isolate NW was sensitive to both fungicide classes. Isolates BB and GC were sensitive to dicarbamates and resistant to benzimidazoles.
Thiram is an older fungicide once used to control strawberry anthracnose fruit rot (Colletotrichum spp.; CitationMiller, 1992). All of our Botrytis isolates were either resistant or intermediate to thiram () at the lower concentrations (0.3 and 3 μM); however, at 30 μM all isolates were sensitive to it. This response is reflected in the application rate recommendations for thiram of 3 to 3.7 kg ai/ha. Dodine inhibited the growth of all 13 isolates by 77% or greater ( and ) at the 3 and 30 μM concentrations; however, at the lowest concentration of 0.3 μM (), it either had no growth inhibitory effect or stimulated the hormetic responses of 8 isolates. It too has a higher recommended rate of 1 to 1.5 kg ai/ha. Newer fungicides evaluated in our study, including azoxystrobin, butrizol, cyprodinil, and fenhexamide, caused significant growth inhibition at the lowest concentration tested (0.3 μM).
Two B. cinerea isolates obtained from crops other than strawberry were also included in this study because they were isolated from crops that are not registered for use of dicarboximide fungicides, iprodione and vinclozolin. Isolate GR was obtained from grape and isolate BB was obtained from blueberry and neither isolate was expected to be resistant to the dicarboxymide fungicides. At the 0.3 μM concentration, however, isolate BB was not sensitive to either of the dicarboxymide fungicides and isolate GR was not sensitive to vinclozolin and was intermediate to iprodione (). Isolate BB was sensitive to both vinclozolin and iprodione at 3 and 30 μM and isolate GR was intermediate to both at 3 μM and sensitive to iprodione at 30 μM.
PCR with the BCF and BCR primers produced the expected 381 bp fragment from the β-tubulin gene. We detected two alleles differing at base position 101 in the 11 strawberry isolates we sequenced. This polymorphic site was found within codon position 198 when we compared our partial sequences to the more complete sequence reported by CitationYarden and Katan (1993; GenBank Accession number X73133) and corresponded to either the amino acids glutamic acid (GAG) or alanine (GCG). All the strains of B. cinerea characterized by CitationYarden and Katan (1993) as being highly resistant to benomyl possessed either an alanine or lysine (AAG) at codon position 198. The benomyl resistance profiles of 10 of the 11 strawberry isolates in this study matched the predicted codon at position 198 in the β-tubulin gene (). Isolate GH was the only isolate characterized as being benomyl sensitive, yet it possessed the GCG (alanine) codon suggesting that other regions of the β-tubulin gene, or even other genes, may influence sensitivity to benomyl.
DISCUSSION
Our results indicate that Botrytis isolates from strawberry in southeastern Louisiana are resistant to benzimidazole fungicides and are becoming resistant to dicarboximide fungicides. Louisiana Botrytis isolates were of three phenotypes: benzimidazole and dicarboximide resistant, benzimidazole resistant and dicarboximide sensitive, and those with benzimidazole and dicarboximide intermediate resistance. Analysis of fungal growth when challenged by 3 μM test concentrations at 72 h identified chemically sensitive isolates and fungal growth when challenged by 30 μM test concentrations at 72 h identified chemically resistant isolates. These in vitro results corroborate the findings of other researchers that Botrytis spp. has developed resistance to benzimidazole and dicarboximide fungicides (CitationElad et al., 1992; CitationMyresiotis et al., 2007; CitationVallejo et al., 2003). Benomyl (a benzimidazole fungicide) was removed from the market in 2001, and vinclozolin (a dicarboximide fungicide) is no longer approved for use on strawberries. In an effort to slow the development of fungicide resistant strains of B. cinerea, the use of iprodione is restricted to one application per season—just before the first flower. Fungicide resistance profiles obtained in this study of Botrytis from Louisiana strawberry farms fully corroborate these restrictions. Several states have received specific exemption from the U.S. Environmental Protection Agency (EPA) under the provisions of Section 18 of the Federal Insecticide, Fungicide, and Rodenticide Act for new chemistry. Abound® (azoxystrobin) and Switch® (cyprodonil + fludioxinil) are now labeled for disease control on strawberries. Based on the in vitro findings of growth inhibition of the pathogen, our data support the use of these compounds as new Botrytis disease control agents. Since Botrytis fruit rot is controlled primarily by chemical applications during the flowering period, butrizol, azoxystrobin, and cyprodinil should be considered for future disease control of Botrytis fruit rot of strawberry. We believe that the one application of iprodione should now be discontinued and more effective fungicide classes substituted.
Design of effective fungicide spray schedules along with the selection of effective combinations of fungicides as a practice for chemical disease management programs is complicated and often needs to be modified during the season due to changes in disease pressure and environmental factors. CitationLegard et al. (2005) recommended the use of low-rate applications of captan during the early season followed by applications of fenhexamid during the second peak bloom period for Botrytis fruit rot control in winter strawberry production in Florida. In Louisiana field trials, CitationWedge et al. (2007) found Botrytis fruit rot incidence was lowest on fruit receiving applications of cyprodinil + fludioxonil, fenhexamid, fenhexamid + captan, pyraclostrobin + boscalid, captan, pyrimethanil, or azoxystrobin. They recommended the use of a combination or alternation of fungicides during early bloom, which included a fungicide specific for Botrytis spp. As the season progressed, they stressed the importance of including fungicides effective against other fruit rot pathogens, such as Colletotrichum, Gnomonia, and Phytophthora spp. The results of these field trials correlate to the findings of this study. Combining or alternating fungicides is an important management strategy to prevent the development of fungicide resistant pathogens, which is one of the most difficult problems of chemical disease management. Intensive and repeated use of a single fungicide destroys the sensitive portion of the pathogen population and leaves only the resistant portion of that population to survive, multiply, and become dominant.
In addition, incorporating fungicides into spray programs with novel modes of action and lacking cross-resistance with botryticides already in use should certainly assist in overcoming the limitations in disease control caused by fungicide resistance. CitationVallejo et al. (2003) detected three phenotypes with resistance to benzimidazole and carbendazim fungicides among 36 Botrytis cinerea isolates collected from strawberries in southwestern Spain: (i) a phenotype resistant to benomyl and highly resistant to carbendazim; (ii) a phenotype highly resistant to both benomyl and carbendazim; and (iii) a phenotype highly resistant to benomyl and resistant to carbendazim. Strains were either resistant or sensitive to the dicarboximide vinclozolin. CitationMyresiotis et al. (2008) and CitationAvenot et al. (2008) demonstrated that boscalid and pyraclostrobin are effective against Botrytis spp. and Alternaria spp., respectively. In view of these results, we emphasize the need to introduce fungicides with alternate biochemical mechanisms to disease management programs in crops with high disease pressure where resistance to site-specific fungicides is imminent. The strawberry-Botrytis pathosystem represents a good example of this kind of vulnerable situation.
ACKNOWLEDGMENTS
We thank Wanda S. Elliott, J. L. Robertson, and D. W. Harries for their technical assistance in these studies. Mention of a trademark, proprietary product, or vendor does not constitute a guarantee or warranty of the product by the US Department of Agriculture and does not imply its approval to the exclusion of other products or vendors that also may be suitable.
LITERATURE CITED
- Amiri , A. , Peres , N. and Whidden , A. March 6 2012 . Perspective on resistance of Botrytis cinerea from strawberry to multiple fungicides in Florida , March 6 , IFAS Extension Service . 2012Berry/Vegetable Times, Special Alert, University of Florida
- Avenot , H. , Morgan , D.P. and Michailides , T.J. 2008 . Resistance to pyraclostrobin, boscalid, and multiple resistance to Pristine® (pyraclostrobin+ boscalid) fungicide in Alternaria alternate causing Alternaria late blight of pistachios in California . Plant Pathol. , 57 : 135 – 140 .
- Elad , Y. , Yunis , H. and Katan , T. 1992 . Multiple resistance to benzimidazoles, dicarboximides and dicthoxencarb in field isolates of Botrytis cinerea in Israel . Plant Pathol. , 41 : 41 – 46 .
- Espinel-Ingroff , A. and Kerkering , T.M. 1991 . Spectrophotometric method of inoculum preparation for the in vitro susceptibility testing of filamentous fungi . J. Clin. Microbiol. , 29 : 393 – 394 .
- Legard , D.E. , MacKenzie , S.J. , Mertely , J.C. , Chandler , C.K. and Peres , N.A. 2005 . Development of a reduced use fungicide program for control of Botrytis fruit rot on annual winter strawberry . Plant Dis. , 89 : 1353 – 1358 .
- Luck , J.E. and Gillings , M.R. 1995 . Rapid identification of benomyl resistant strains of Botrytis cinerea using the polymerase chain reaction . Mycological Res. , 99 : 1483 – 1488 .
- Miller , R.W. 1992 . Agricultural chemicals handbook , 382 Clemson , SC, p : Clemson University Cooperative Extension Service .
- Myresiotis , C.K. , Bardas , G.A. and Karaoglanidis , G.S. 2008 . Baseline sensitivity of Botrytis cinerea to pyraclostrobin and boscalid and control of anilinopyrimidine- and benzimidazole resistant strains by these fungicides . Plant Dis. , 92 : 1427 – 1431 .
- Myresiotis , C.K. , Karaoglanidis , G.S. and Tzavella-Klonari , K. 2007 . Resistance of Botrytis cinerea isolates from vegetable crops to anilinopyrimidine, phenylpyrrole, hydroxyanilide, benzimidazole, and dicarboximide fungicides . Plant Dis. , 91 : 407 – 413 .
- Oliva , A. , Meepagala , K.M. , Wedge , D.E. , Hale , A.L. , Harries , D. , Aliotta , G. and Duke , S.O. 2003 . Natural fungicides from Ruta graveolens L. leaves, including a new quinolone alkaloid . J. Agr. Food Chem. , 51 : 890 – 896 .
- Sutton , J.C. 1990 . Epidemiology and management of Botrytis leaf blight of onion and gray mold of strawberry: A comparative analysis . Can. J. Plant Pathol. , 12 : 100 – 110 .
- Sutton , J.C. 1998 . “ Fungal diseases of the fruit, p. 28–31. In: Compendium of strawberry diseases ” . In American Phytopathol. Soc , 2nd , Edited by: Maas , J.L. Minnesota : St. Paul .
- Vallejo , I. , Muoz , F. , Carbu , M. , Rebordinos , L. , Fernandez-Acero , F.J. and Cantoral , J.M. 2003 . Study on fungicide resistance of Botrytis cinerea isolates from diseased strawberry plants . Arch. Phytopathol. Plant Protec. , 36 : 1 – 7 .
- Wedge , D.E. and Camper , D.E. 2000 . “ Connections between agrochemicals and pharmaceuticals, p. 1–15 ” . In Biologically active natural products: Agrochemicals and pharmaceuticals , Edited by: Cutler , H.G. and Cutler , S.J. Boca Raton , Florida : CRC Press .
- Wedge , D.E. and Kuhajek , J.M. 1998 . A microbioassay for fungicide discovery. SAAS Bull . Biochem. Biotech. , 11 : 1 – 7 .
- Wedge , D.E. , Smith , B.J. , Quebedeaux , J.P. and Constantin , R.J. 2007 . Fungicide management strategies for control of strawberry fruit rot diseases in Louisiana and Mississippi . Crop Prot. , 26 : 1449 – 1458 .
- Wedge , D.E. , Galindo , J.C.G. and Marcias , F.A. 2000 . Fungicidal activity of natural and synthetic sesquiterpene lactone analogs . Phytochemistry , 53 : 747 – 757 .
- Yarden , O. and Katan , T. 1993 . Mutations leading to substitutions at amino acids 198 and 200 of beta-tubulin that correlate with benomyl-resistance phenotypes of field strains of Botrytis cinerea . Phytopathology , 83 : 1478 – 1483 .
- Yourman , L.F. , Jeffers , S.N. and Dean , R.A. 2000 . Genetic analysis of isolates of Botrytis cinerea sensitive and resistant to benzimidazole and dicarboximide fungicides . Phytopathology , 90 : 851 – 859 .
- USDA Economics, Statistics and Market Information System. 2008. Albert R. Mann Library, Cornell University http://usda.mannlib.cornell.edu/MannUsda/viewDocumentInfo.do?documentID=1381 (http://usda.mannlib.cornell.edu/MannUsda/viewDocumentInfo.do?documentID=1381)