Abstract
Experiments were initiated to identify how and when a reasonably accurate assessment of initial fruit retention could be made and to identify a measurement method to allow early assessment of thinner efficacy. An estimate of initial fruit retention could be made when fruit enters the exponential growth stage, which is generally 6 to 9 days after petal fall and the receptacle has expanded to approximately 6 mm. There was a very good correlation between the slowing of fruit growth 4 to 7 days after thinner application and final fruit set, which led to the conclusion that following fruit growth during this critical time was a good and viable method to predict and quantify thinner response.
INTRODUCTION
Chemical thinning is a critical management activity that is done in all apple growing regions in the world to increase fruit size, elevate fruit quality, and to assure return bloom for sustained cropping. Growers have traditionally depended upon an application of a thinning spray when fruit grows to 7 to 12 mm in diameter (generally 1–3 weeks after bloom). At this stage of fruit development chemical thinners are most effective in reducing fruit numbers, stimulating fruit size, and encouraging return bloom (CitationWilliams & Edgerton, 1981; CitationForshey, 1986).
In recent years, thinning has emerged as an even more important practice because of increasing market demand for larger size fruit and the introduction of new, better-tasting cultivars that are more difficult to thin and may have a greater tendency for biennial bearing. The negative economic consequences of insufficient thinning have forced most orchardists to reappraise their standard thinning strategy that was based previously upon a single thinner application.
Increasingly, local thinning recommendations suggest using multiple thinner applications, starting as early as bloom and extending through the 20-mm stage of fruit development (CitationGreene, 2002; Schwallier, 1996; CitationWilliams and Fallahi, 1999). Although rates of individual thinners may be reduced in any one spray, increased thinning activity is often achieved because thinner applications have a greater probability of coinciding with weather that is favorable for thinner activity, and thinners are in place over a longer period of time.
The use of blossom thinners followed by a postbloom thinner application has been practiced by growers in the Pacific Northwest for many years (CitationWilliams & Edgerton, 1981). However, this thinning approach is less desirable and is more difficult to achieve in regions, such as the east and midwest of the United States, where humid conditions and less predictable weather during bloom may lead to variable pollination and initial set. Growers are now encouraged to evaluate the bloom period for favorable pollination, to assess initial set, and observe responses to early thinner application before making decisions on initial or subsequent thinner applications (CitationGreene, 2002).
A problem with this multiple application, observe, and decide approach to chemical thinning, is that no guidelines have been provided to aid in assessing initial set or timely evaluation of a thinner response. Therefore, growers have little information to help in deciding what thinning action, if any, to take. What is needed is a rapid assay based on one objective measurement of tree behavior that can integrate all the variables of spray uptake, weather, initial crop load, environmental stresses, and tree physiological sensitivity to give a useful early reading of thinner effectiveness.
Persistence of young fruit through the drop period when fruit are exposed to numerous internal and external stresses and competition is associated with continued fruit growth (CitationZucconi, 1981; CitationFukui et al., 1984; CitationLakso et al., 2001). Since fruit growth rate integrates the various positive and negative factors involved in fruit growth and abscission, measuring fruit growth after thinner application may give an accurate reading of final crop load and thinner response.
The first objective in this study was to identify the earliest time and the best method to determine initial set. The second objective was to compare fruit drop and fruit growth as potential predictors of thinner response or effectiveness. The third objective was to establish a basis for developing a grower-friendly system that could be used to predict the thinning response to postbloom thinners early enough to allow supplemental thinning action while fruit were still susceptible to chemical thinner application.
MATERIALS AND METHODS
Growth and Fruit Abscission Relationship and Cultivar Comparison
Within adjacent orchards with different cultivars, six uniform mature cropping trees of ‘Royal Gala’/M.9 and ‘Ace Delicious’/M.26 with comparable bloom density and appearance were selected. When king fruit diameters were approximately 12 mm, thinning sprays of 7.5 mg·L−1 of naphthaleneacetic acid (NAA Fruitone N) plus 600 mg·L−1 carbaryl (Sevin XLR) were applied with an airblast sprayer. On each tree, 15 king fruit of similar size were chosen in representative sites around the tree. All 90 fruits were marked in each cultivar for monitoring of maximum fruit diameter measured with a digital caliper at -5, 0, 4, 7, 10, and 17 days after thinner application. Final fruit set was determined by counting fruit on marked spurs 40 days after thinner application.
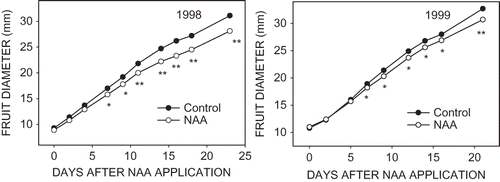
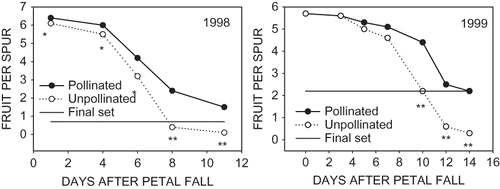
Six uniform trees were selected in a block of mature ‘More-Spur McIntosh’/M.7 growing at the University of Massachusetts Horticultural Research Center (Belchertown, MA). On 28 Apr., at the pink stage of flowers development, 3 uniform limbs per tree were selected and 10 flowering spurs on each limb were tagged. One limb on each tree was designated to be the open-pollinated control and received no treatment. Pollination was aided by having a cluster of bee hives positioned approximately 30 m from the test trees. Lack of pollination was simulated on a second limb by covering tagged spurs with a 20 × 20 cm piece of Gardens Alive Super-Light Insect Barrier (Gardens Alive, Lawrenceburg, IN, USA) and then securing the barrier fabric at the base of the spur with a plastic tie. The barrier allowed light penetration to the spurs while preventing bee visitations. The insect barriers were removed from all spurs on 8 May, one day after petal fall (PF). Starting on 8 May, and then again on 11, 13, 15, and 18 May, all fruit on the tagged spurs on open pollinated control and pollination deficient spurs were measured with a digital caliper and recorded. Fruit set was calculated as persisting fruit per spur, while fruit size was calculated by averaging the fruit diameter of all persisting fruit.
On 18 May all persisting fruit on the 10 tagged spurs on the control limb and 10 spurs on the third selected limb were marked individually at the equator using an indelible marker. Individual fruit were identified in each spur by placing between one and six dots on fruit in individual spurs depending upon the number of fruit persisting on the spur. The diameter of each fruit was measured with a digital caliper at the location of the dots on 18 May and then NAA at 8 mg·L−1 was applied as a dilute spray to the limb designated to receive the thinning spray. On 20 May and at subsequent 2- to 3-day intervals, individual fruit were measured on the tagged spurs at the location on a fruit where they were marked. Measurements continued until 10 June when fruit diameter averaged over 30 mm. Fruit size was recorded on each date so that the growth rate of fruit could be followed over the measurement period. A final set of individual fruit was taken on 29 June.
Pollination, Thinner, Fruit Growth Fruit Set Studies, 1999
Six uniform trees were again selected in the same block of mature ‘More-Spur McIntosh’/M.7 used in 1998. At the pink stage of flower development, 5 May, three limbs 12 to 15 cm in circumference were selected. Ten spurs per limb were tagged on each of the selected limbs designated to be the control and the unpollinated limbs. Insect barrier netting was applied at the pink stage on 8 May on spurs on the limb designated as unpollinated, as previously described. The insect barrier was removed from spurs at PF on 14 May. Fruit diameter and fruit set were then determined on all fruit on the tagged spurs on the control and unpollinated limbs at 2- to 3-day intervals until 26 May. Individual fruit persisting on tagged spurs on the limbs designated as control and NAA-treated were identified and measured on 26 May as previously described, when fruit size averaged 10.1 mm. Immediately following fruit numbering and measurement, NAA was applied to the designated limb at 8 mg·L−1. Measurement of the diameter of persisting fruit on tagged spurs continued at 2- to 3-day intervals until 16 June when fruit size averaged over 30 mm. Final fruit set on tagged spurs was recorded, and all persisting fruit on tagged portions of the control and NAA treated limbs were counted on 22 July.
The experiments were arranged as a randomized block design with trees representing replications, and treatments were applied to individual limbs on a tree. Analysis of variance was done in evaluating fruit set effects and initial growth of fruit. Data were analyzed using SAS (SAS Institute, Cary, NC, USA) with repeated measures analysis. Means were separated using Duncan's new multiple range test. In measurement of fruit growth, fruit were considered to be experimental units.
There were no frost events in either 1998 or 1999 and there were no prolonged periods of rain and/or cloudy weather during the evaluation period. Warm temperatures followed NAA application in both years, which is favorable for effective thinning activity.
RESULTS
Assessment of Initial Set
Abscission of the majority of unpollinated flowers started approximately 4 to 5 days after PF and continued until 8 to 14 days after PF (). The period over which fruit abscission on open pollinated spurs occurred was essentially the same as that for unpollinated flowers, except that fewer fruit abscised. In 1998, estimation of initial fruit retention by counting fruit could be made with reasonable accuracy between 8 and 11 days after PF, and in 1999 this estimate could be made approximately 12 to 14 days after PF. The diameter of fruit on open pollinated and unpollinated spurs was about 3.5 mm at PF in both years (). The fruit on unpollinated spurs did not grow and remained about the same diameter until abscission occurred. Diameter of fruit on open pollinated spurs increased very slowly as expected with early cell division growth. It was not until 6 to 7 days after PF that growth rates started to accelerate. Although statistical differences in fruit diameter between open pollinated and unpollinated fruit were documented 4 or 5 days after PF, it was not until 7 to 10 days after PF when fruit diameter on open pollinated spurs increased to between 5 and 6 mm that it was possible to determine visually and with conviction which fruit were pollinated and likely to persist and continue to develop.
FIGURE 2 Initial fruit growth of ‘McIntosh’ apples on open pollinated spurs and on spurs that were covered between pink and petal fall with insect barrier. Values represent means of all fruit on all spurs.
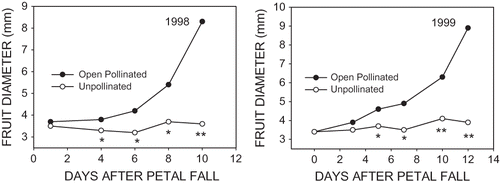
FIGURE 1 Initial fruit retention of ‘McIntosh’ apples on open pollinated spurs and spurs that were covered between pink and petal fall with insect barrier covering. Values represent means of all fruit on all spurs.
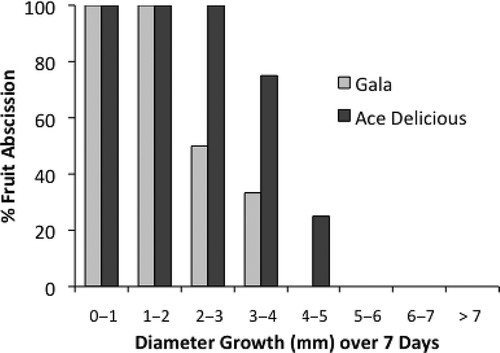
To evaluate the relationship of fruit growth rates to the likelihood of abscission and possible cultivar differences in fruit drop in relation to fruit growth, the growth rates of fruit over the first 7 days after a thinner application were sorted into growth classes and the % final drop of each growth class calculated. There was a clear relationship between reduced growth rate of ‘Ace Delicious’ and ‘Gala’ fruit during the first 7 days after NAA application and final drop of measured fruit (). However, ‘Gala’ showed less fruit drop for the same reduction in fruit growth rate. Although this basic relationship of fruit growth to drop has been found in many of our studies (CitationLakso et al., 2001) there has also been variation in the key absolute values (for example, % drop at 50% growth reduction) observed from year to year or among cultivars. The sources of variation are not fully understood (possibly environmental conditions, tree vigor, stage of fruit development, etc.), and will require more research to determine how consistent the key values determining the curve and how the cultivar differences may be.
Relationship between Fruit Growth and Fruit Set, ‘Starkrimson Delicious’
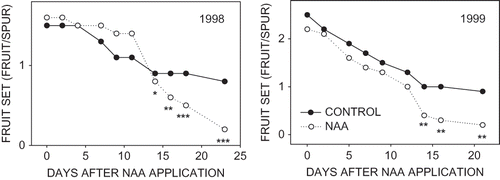
Abscission of fruit on control and NAA-treated branches was followed after NAA application at the 8–10 mm stage for 2 years and final fruit retention was determined at the end of June drop in July (). Statistical differences in fruit retention between controls and NAA-treated trees were found starting 14 days after NAA application. However, it was not possible to get an accurate estimate of the final extent of thinning caused by NAA until 21 to 23 days after NAA application based upon fruit retention, and by this time fruit abscission had essentially ceased.
FIGURE 4 Fruit retention of ‘McIntosh’ apples on untreated spurs and those treated with NAA at the 8- to 10-mm stage of fruit development. At least 95% of the fruit abscission had occurred on the last evaluation date, 23 and 21 days after NAA application in 1998 and 1999, respectively.
Growth of all fruit in tagged clusters was followed after NAA application and from these data growth rates between measurement periods were calculated. The growth rate between measurement periods of NAA-treated fruit that persisted to harvest and those that abscised during the major time of drop, 14 to 21 days after application, diverged soon after application (). In 1999, a significant difference in growth rate between abscising and persisting fruit was detected between 2 and 5 days after application, whereas in 1998 the first statistically significant reduction in growth was between these two classes of fruit at 4 to 7 days after application. Within 2 to 3 days of detection of slowing of growth, fruit destined to drop stopped growing completely. Slowing of fruit growth preceded actual abscission by 7 to 14 days.
FIGURE 5 Growth of ‘McIntosh’ apple fruit treated with 8 mg·L−1 NAA that abscised and untreated fruit that persisted to harvest. The statistical significance noted is for the growth rate between two adjacent times of measuring fruit.
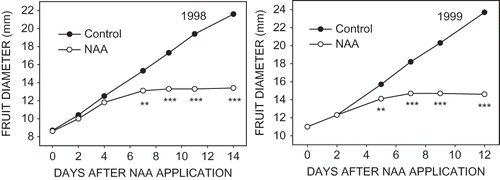
Growth of NAA-treated fruit that persisted to harvest was measurably slowed by 7 days after application, compared with untreated control fruit that persisted (). Fruit size was not determined at harvest in these studies but many of our related studies have shown such early fruit size differences persist to harvest (CitationLakso et al., 1995; Greene and Lakso, unpublished data).
DISCUSSION
The first wave of fruit drop started 4 to 5 days after PF and it was not complete until 8 to 12 days after PF. Since the kinetics and the appearance of fruit in this first wave of drop were similar on unpollinated and open pollinated spurs, it appears that the majority of fruit in this first drop consisted largely of unpollinated flowers. This observation is in agreement with the description of the first drop made initially by CitationMurneek (1933).
At bloom, apple flower growth slows or ceases for a few days (CitationSmith, 1950). Growth of pollinated fruit resumes very slowly in the first few days after PF as described by CitationDenne (1960). In this investigation, this period of slow growth occurred for 6 to 8 days after PF. M. Goffinet (personal communication, 1996) has found that during this first week after bloom that growth was occurring by cell division but no cell expansion occurred until about 7 days after bloom. During this period of time, it was difficult to confirm visually a resumption in receptacle growth, although statistical differences were documented 4 to 5 days after PF. The exponential phase of fruit growth described by CitationDenne (1960) and CitationTetley (1931) started 6 to 8 days after PF and continued through the measurement period. It was only at the initiation of this exponential phase of fruit growth that it was possible to make a qualitative estimate of initial set and crop load potential.
There are a number of weather-related conditions, bloom quantity and quality, and bee activity that may influence pollination effectiveness. Thinners are also applied at bloom and petal fall that may influence pollination or the ability of flowers to resume growth and development. The decision to apply a thinner or a subsequent thinner during the traditional postbloom period (7–12 mm) is often made only after an assessment can be made of the number of fruit that initially persist. In this investigation, the earliest an assessment of pollination effectiveness could be made was by measuring fruit growth and this was 6 to 8 days after petal fall, whereas an assessment based upon counting persisting fruit required 8–12 days. Therefore, determining the relative number of fruit entering the log phase of growth by fruit growth measurement or observation appears to be the most rapid and reliable way to assess initial fruit retention.
In this investigation, it required 23 days in 1998 and 21 days in 1998 to get a reasonably accurate assessment of final fruit retention following NAA application based upon counting the number of persisting fruit. Further, NAA has been reported to retard abscission of young fruit even though they do ultimately abscise (CitationHartman & Howlett, 1962; CitationLuckwill, 1953; CitationMurneek & Tuebner, 1953; CitationStruckmeyer & Roberts, 1950). No statistically significant delay in abscission was observed in this investigation although it appears that abscission was delayed for up to 10 days after application. If a supplemental thinner application is postponed 2 to 3 weeks until actual fruit set is determined by observing drop, the probability of effective and satisfactory thinning would be low (CitationGreene, 2002). Therefore, observation of set following thinner application based upon the number of fruit present is not a viable way to assess thinner response, especially if supplemental thinning is required.
CitationByers et al. (1991) artificially shaded apple trees, which promoted abscission 7 to 12 days later. They noted that fruit destined to drop stopped growing several days before dropping. CitationWard and Marini (1999) evaluated several ways to assess thinner response, including dry weight, fruit diameter, water potential, starch index, respiration rate, and cellulose activity. They concluded that fruit growth measurement is the only practical method to assess results of initial thinning treatments in time to apply additional treatments in commercial orchards. CitationJankovic et al. (2009) reported that following growth rate of fruit 5 to 25 days after PF can be used as a relatively reliable method for early detection of natural fruit drop. CitationMarini (1998) described a system based upon fruit growth measurements to evaluate the effects of a thinner. CitationLakso et al. (2001) showed the increase of fruit drop related to reduction in growth rate to be consistent in pattern, though not identical, in several cases. The results from this investigation confirm that measuring a slowing in fruit growth rate is a predictable and very accurate method to identify and quantify fruit that will ultimately abscise.
Growth of fruit following thinner application reported in represents an average of many fruit. A steady or near steady increase in fruit size characterizes the growth pattern of fruit that will persist through the ‘June drop’ period. Fruit destined to drop following thinner application share a very similar growth pattern. Fruit growth slows 2 to 5 days after NAA application and it stops growth 7 to 10 days after application, but actual fruit abscission may not occur for 14 to 18 days after application. We propose that this slowing of fruit growth soon after application is an excellent predictor of fruit that will ultimately abscise. Details to document this as an accurate and early method to predict final fruit set are the primary focus in a subsequent publication (Greene et al., in press).
It has been recognized for many years that NAA has the potential to reduce fruit size (CitationLuckwill, 1953), or to have no influence on fruit size even though postbloom thinning was done (CitationJones et al., 1989). In this investigation, NAA not only reduced growth on fruit destined to drop, it also reduced growth on fruit that persisted through the June drop period. Therefore, it appears prudent to select NAA concentrations for thinning that have the potential for appropriate thinning, yet are not so high that they may jeopardize losing the size increase benefits normally associated with a reduction in crop load. Late application, high rates, and hot weather following application of NAA are all conditions associated with reduced fruit size following NAA application (CitationGreene, 2002), while earlier applications give better final fruit size or the same yield (CitationLakso et al., 2001). Multiple thinner applications is rapidly becoming the preferred approach for thinning apples in the United States (CitationGreene, 2002; Schwallier, 1996; CitationWilliams & Fallahi, 1999). Including NAA in this thinning program would allow lower rates to be used that would either reduce or eliminate any negative effects NAA may have on fruit size.
LITERATURE CITED
- Byers , R.E. , Carbaugh , D.H. , Presley , C.N. and Wolf , T.K. 1991 . The influence of low light on apple fruit abscission . J. Hort. Sci. , 66 : 7 – 17 .
- Denne , M.P. 1960 . The growth of apple fruitlets, and the effect of early thinning on fruit development . Ann. Bot. , 24 : 397 – 406 .
- Forshey , C.G. 1986 . Chemical thinning of apples . New York's Food and Life Sci. Bul. , 116
- Fukui , H. , Imakawa , S. and Tamura , T. 1984 . Relationship between early fruit drop of apple fruit, ethylene evolution, and formation of abscission layer (in Japanese) . J. Jap. Soc. Hort. Sci. , 53 : 303 – 307 .
- Greene , D.W. 2002 . Chemicals, timing and environmental factors involved in thinner efficacy of apples . HortScience , 37 : 477 – 481 .
- Greene , D.W. , Lakso , A.N. , Robinson , T.L. and Schwallier , P. Development of a fruit growth model to predict thinner response on apples . HortScience (in press) ,
- Hartman , F.O. and Howlett , F.S. 1962 . Effects of naphthaleneacetic acid on fruit setting and development in the apple . Ohio Agr. Expt. Sta., Res. Bul. , 920 : 66
- Jankovic , D , Jankovic , J. and Blagojevic , R. 2009 . Growth rate of apple fruit as a method of predicting their abscission . Acta Agr. Serbica , 14 : 23 – 30 .
- Jones , K.M. , Koen , T.B. , Oakford , M.J. and Bound , S. 1989 . Thinning Red Fuji apples with ethephon and NAA . J. Hort. Sci. , 64 : 527 – 532 .
- Lakso , A.N. , Corelli Grappadelli , L. , Barnard , J. and Goffinet , M.C. 1995 . An expolinear model of the growth pattern of athe apple fruit . J. Hort. Sci. , 70 ( 4 ) : 389 – 394 .
- Lakso , A.N. , Robinson , T.L. , Goffinet , M.C. and White , M.D. 2001 . Apple fruit growth responses to varying thinning methods and timing . Acta Hort. , 557 : 407 – 412 .
- Luckwill , L.C. 1953 . Studies on fruit development in relation to plant hormones. II. Effect of naphthalene acetic acid on fruit set and fruit development in apples . J. Hort. Sci. , 28 : 25 – 40 .
- Marini , R.P. 1998 . Predicting and assessing effectiveness of apple thinning treatments . Proc. Mass Fruit Growers’ Assoc. , 104 : 28 – 31 .
- Murneek , A.E. 1933 . The nature of shedding of immature apples . Univ. Missouri Agr. Expt. Sta. Bul., Res. Bul. 201, 34 p. ,
- Murneek , A.E. and Teubner , F.G. 1953 . The dual action of naphthaleneacetic acin in thinning apples . Proc. Amer. Soc. Hort. Sci. , 61 : 149 – 154 .
- Schwallier , P.G. 1996 . Apple thinning guide , 13 Sparta , MI : Great Lakes Publishing Company .
- Smith , W.H. 1950 . Cell multiplication and cell enlargement in the development of the flesh of apple fruit . Ann. Bot. , 14 : 23 – 38 .
- Struckmeyer , B.E. and Roberts , R.H. 1950 . A possible explanation of how naphthaleneacetic acid thins apples . Proc. Amer. Soc. Hort. Sci. , 56 : 76 – 78 .
- Tetley , U. 1931 . The morphology and cytology of the apple fruit, with special reference to the Bramley's seedling variety . J. Pomol. , 9 : 278 – 297 .
- Ward , D. and Marini , R.P. 1999 . Growth and development of young apple fruits following applications of ethephon plus carbaryl for thinning . HortScience , 34 ( 6 ) : 1057 – 1059 .
- Williams , K.M. and Fallahi , E. 1999 . The effects of exogenous bioregulants and environment on regular cropping of apples . HortTechnology , 9 ( 3 ) : 323 – 327 .
- Williams , M.W. and Edgerton , L.J. 1981 . “ Fruit thinning of apples and pears with chemicals ” . In U.S. Dept. Agr. Info. Bul. 289
- Zucconi , F. 1981 . Regulation of abscission in growing fruit . Acta Hort. , 120 : 89 – 94 .