Abstract
Accurate quantitative assessment of greasy spot of grapefruit, caused by Mycosphaerella citri Whiteside, is crucial for designing appropriate disease management strategies and estimating yield losses. A visual rating scale was developed by measuring the total leaf area of 143 ‘Rio Red’ grapefruit leaf samples, and calculating the percent diseased area. The mean severity of the entire sample was 23.1%, but many leaves on the orchard floor exhibited symptoms on more than 50.0% of the leaf area. This rating scale should be highly useful to growers and researchers for field assessments of greasy spot severity.
INTRODUCTION
Citrus greasy spot is a fungal disease caused by Mycosphaerella citri Whiteside, which is an important pathogen of citrus in the Americas and parts of Asia (CitationTimmer & Gottwald, 2000). This disease is most severe on grapefruit and its hybrids (CitationMondal et al., 2003). Infected leaves develop yellow spots on the abaxial surface, which subsequently develop into lesions with a greasy appearance, before senescing prematurely. Infection and premature defoliation reduces the quantity and quality of fruit. M. citri also infects the fruit and causes it to be less marketable due to rind blotching (CitationMondal et al., 2003).
For various regions of the citrus industry, epidemiology studies have yielded much information about the environmental conditions required for infection by the pathogen, the onset of symptoms, and disease control (CitationTimmer et al., 1980, 2000). For instance, the knowledge of the predicted time of pseudothecial development and ascospore release has allowed for optimal timing of fungicide treatments (CitationTimmer et al., 2000).
One of the key factors to regulate the life cycle of M. citri is the presence of pseudothecia in leaf litter, and ascospore counts in the air as well as the underside of leaves. In Texas, ascospore production is low during spring and high in late August and early September (CitationTimmer et al., 1980). In Florida, however, ascospore production is extremely high from March to July (CitationTimmer et al., 2000), and the production of pseudothecia is rapid from June to September (CitationMondal & Timmer, 2002). This difference in timing of the ascospore production is correlated with the frequency of rainfall in the two regions. Greasy spot can be prevented with appropriate chemical control, if spraying occurs before ascospores germinate and epiphytic growth ensues (CitationMondal & Timmer, 2006). The life cycle stage or degree of infection influences the control measures that are necessary. The mycelium of the fungus initially grows epiphytically on the leaf surface. Later, slow and continued growth of the fungus into the mesophyll results in chlorotic and necrotic spots (CitationMondal et al., 2003).
The fungus has an ability to survive and grow for several weeks with an increased number of mycelial penetrations through nearby stomata before the leaf develops visible lesions (CitationTimmer et al., 2001) and symptoms of greasy spot develop slowly (CitationMondal & Timmer, 2006). A severity scale that provides a quantitative assessment of infection would be beneficial for growers to decide whether to spray or not to spray. It would also provide scientists with an unbiased and reproducible rating system. A pictorial scale provides a quick reference guide in the field for estimating disease severity on leaves. Furthermore, investigators in the laboratory could replace ambiguous terms, such as low, medium, or high infection, with objective numeric values that could correlate to the level of infection (pseudothecia and ascospore development) in the disease process. CitationBock et al. (2009) reported on the quantification of citrus canker on leaves using image analysis software. A review of plant disease assessment using visual, photography, image analysis, and hyperspectral imaging with the various advantages and disadvantages of these approaches was recently published (CitationBock et al., 2010). The objective of this study is to develop a visual rating scale for a quick and easy field assessment of greasy spot disease severity in grapefruit.
MATERIALS AND METHODS
For this study, WinFolia 2004 imaging software (www.regentininstruments.com) was used to calculate the area of citrus leaves affected by greasy spot. Leaves were grouped into categories according to the percentage of infected leaf area (). One hundred and forty-three leaves from three ‘Rio Red’ grapefruit trees (C. x paradisi Macf.) were collected over a 5-day period in late June of 2005. For this study, three grapefruit trees (3 m high) on the edge of an orchard were chosen in the South Research Farm of TAMU-Kingsville Citrus Center (Weslaco, Texas). Leaves showing various levels of greasy spot infection were collected from the middle of the trees between approximate heights of 0.5 to 1.8 m.
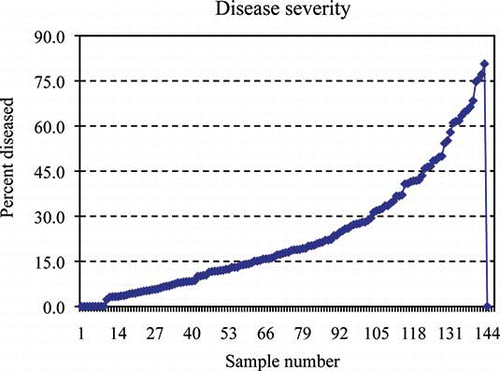
FIGURE 1 Greasy spot disease severity for each grapefruit leaf expressed as percent of diseased area (color figure available online).
Leaves were pressed by placing them in between two thin wooden boards overnight to ensure a flat surface with no curling or shadows that might be misinterpreted by the imaging software. For comparison, some leaves were kept non-pressed. All the leaves were scanned within one day of collection. However, when preliminary scans were taken of both pressed and non-pressed leaves, there was no detection of shadows because the program used a simple color classification (green = healthy, brown = diseased, gray = shadows). Thus, the remaining leaves were not pressed, but scanned within 3 h of collection. The 143 leaves scanned included duplicate leaf scans of 35 adaxial sides, and 5 non-symptomatic leaves designated as the Control. On the 5 healthy leaves, the software detected >0 <1% diseased areas (). Diseased areas that were detected on the Control leaves could have been due to a number of non-visible discolorations since the software classifies the true color at 24 bits color space. Portions of the leaves that were designated as diseased were reviewed by the investigators. A tracing feature of the imaging software allowed discolored areas, which were not consistent with the disease patterns of greasy spot, to be excluded from calculation.
TABLE 1 Calculated Diseased Areas (cm2) of Non-Symptomatic (Control) Grapefruit Leaves Using WinFolia Imaging Software Assessing Greasy Spot Disease
TABLE 2 The Range of Percentages for Each Disease Severity Class of Greasy Spot Infection and the Mean for the Respective Ranges
RESULTS AND DISCUSSION
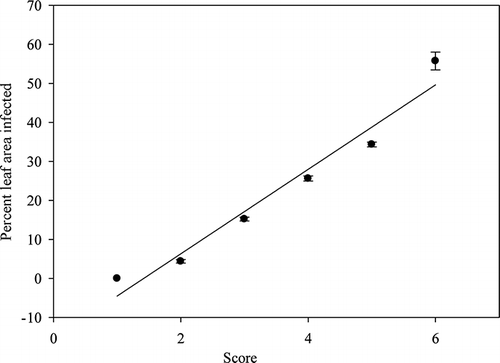
The chlorotic regions surrounding the greasy spot lesions were included in the calculation of diseased areas since they are diseased tissues. For this reason, a color scale is best so the user may take the yellow discoloration into account during disease assessment. reveals slow development of greasy spot lesions until the frequency of severity reaches 20%, where infection appears to spread rapidly. Lesion development increased after 40% and 60%, showing a proportionality curve. ANOVA revealed mutually exclusive classes as shown in . A t-test was run to compare the mean of the small class, with 0 yielding a t-value of 9.77, with p < 0.0001. Many leaves on the orchard floor showed symptoms above 50% surface area. There being very few leaves with stage 5 severity (, ), it may be reasoned that leaf drop occurs shortly after 50% visible infection of the surface area. This supports having an open range of 40% plus for stage 6. The leaves selected for the severity scale were near the mean for their respective ranges (). The mean severity of the entire sample size was 23.1%, which falls just below the mid-point 4 on the scale. The progression of disease severity fits the scale shown in The 10% range for lesser measures of severity provides flexibility for the investigator.
FIGURE 3 Visual scale of greasy spot severity (percentage of diseased leaf area) in grapefruit leaves. Top lane: lower surface; Bottom lane: upper surface. Score 1 = 0%, 2 = >0–10%, 3 = 10–20%, 4 = 20–30%, 5 = 30–40%, 6 = >40%. Leaves on this scale display a percentage near the median of the score range for infection, i.e., leaf 2 has 4.85% diseased area (color figure available online).

FIGURE 2 The mean value of mutually exclusive classes with standard errors of 0.449, 0.490, 0.655, 0.624, and 2.291 for respective classes 1–6.
CitationGodoy et al. (2006) reported that human estimates of disease severity without a scale is less precise than scaled estimates, and that the use of a scale improved accuracy of visual assessments. Moreover, humans vary in their ability to assess disease severity since it is influenced by several factors, such as psychological stimuli, fatigue, ability to concentrate, sampling size, shape, color, number, and size of lesions (CitationKranz, 1988).
Greasy spot is known to infect the abaxial side of leaves more often than the adaxial side as a result of easier ascospore deposition and more stomatal openings. The major source of ascospore originated in the orchard floor from decaying infected leaves on the ground (CitationMondal et al., 2003). However, ascospores can be carried a good distance on windy days and thus the abaxial sides may become infected first. Therefore, including the data for each (abaxial and adaxial) scan, even on the same leaf, provides the most accurate data and score. The goal of this study was not to delineate differences in infection of top versus bottom, but to generate an accurate assessment of disease severity on infected leaves for both the abaxial and adaxial sides. A quantitative visual scale provides representative images on a score card with convenient ranges for disease severity assessment. The leaves chosen for the score card were based on approximate size of the blade, similar size and shape petiole, and are near the mean percentage for the respective range they represent.
Precise disease assessment assists in application of disease management strategies, monitoring yield loss and disease progress, and measuring disease resistance of cultivars (CitationNutter et al., 2006). This visual rating scale allows estimation and quantification of greasy spot disease status of a leaf, tree, or orchard with more precision. With a severity scale, one can ascribe sound reasoning to the weeks that are ideal for fungicidal treatment given a particular level of infection. Drawing a correlation between disease severity on leaves to fungal population density in the leaf litter of the orchard floor and to that of the tree is quantifiable. Previous studies reported that scales such as this one were more accurate in the assessment of disease severity (CitationParker et al., 1995; CitationGodoy et al., 2006).
ACKNOWLEDGMENT
This article is Contribution No. 13-168-J from the Kansas Agricultural Experiment Station, Manhattan.
LITERATURE CITED
- Bock , C.H. , Cook , A.Z. , Parker , P.E. and Gottwald , T.R. 2009 . Automated image analysis of the severity of foliar citrus canker symptoms . Plant Dis. , 93 : 660 – 665 .
- Bock , C.H. , Poole , G.H. , Parker , P.E. and Gottwald , T.R. 2010 . Plant disease severity estimated visually, by digital photography and image analysis, and by hyperspectral imaging . Critical Rev. Plant Sci. , 29 : 59 – 107 .
- Godoy , C.V. , Koga , L.J. and Canteri , M.G. 2006 . Diagrammatic scale for assessment of soybean rust severity . Fitopatol. Brasil. , 31 : 63 – 68 .
- Kranz , J. 1988 . “ Measuring plant disease ” . In Experimental techniques in plant disease epidemiology , Edited by: Kranz , J. and Rotem , J. 35 – 50 . New York : Springer-Verlag .
- Mondal , S.N. and Timmer , L.W. 2002 . Environmental factors affecting pseudothecial development and ascospore production of Mycosphaerella citri, the cause of citrus greasy spot . Phytopathology , 92 : 1267 – 1275 .
- Mondal , S.N. and Timmer , L.W. 2006 . Relationship of the severity of citrus greasy spot, caused by Mycosphaerella citri, to ascospore dose, epiphytic growth, leaf age, and fungicide timing . Plant Dis. , 90 : 220 – 224 .
- Mondal , S.N. , Gottwald , T.R. and Timmer , L.W. 2003 . Environmental factors affecting the release and dispersal of ascospores of Mycosphaerella citri . Phytopathology , 93 : 1031 – 1036 .
- Nutter , F.W. , Esker , P.D. and Coelho Netto , R.A. 2006 . Disease assessment concepts and the advancements made in improving the accuracy and precision of plant disease data . Eur. J. Plant Pathol. , 115 : 95 – 103 .
- Parker , S.R. , Shaw , M.W. and Royle , D.J. 1995 . The reliability of visual estimates of disease severity on cereal leaves . Plant Pathol. , 44 : 856 – 864 .
- Timmer , L.W. and Gottwald , T.R. 2000 . “ Greasy spot and similar diseases ” . In Compendium of citrus diseases , 2nd , Edited by: Timmer , L.W. , Garnsey , S.M. and Graham , J.H. 27 – 28 . St. Paul , Minnesota : American Phytopathological Society .
- Timmer , L.W. , Roberts , P.D. , Darhower , H.M. , Bushong , P.M. , Stover , E.W. , Peever , T.L. and Ibáñez , A.M. 2000 . Epidemiology and control of citrus greasy spot in different citrus-growing areas in Florida . Plant Dis. , 84 : 1294 – 1298 .
- Timmer , L.W. , Roberts , P.D. , Chung , K.R. and Bhatia , A. 2001 . Greasy Spot. Publ. No. SP-154 , Gainesville , Florida : Univ. Florida, IFAS, EDIS .
- Timmer , L.W. , Reeve , R.J. and Davis , R.M. 1980 . Epidemiology and control of citrus greasy spot in Texas . Phytopathology , 70 : 863 – 867 .