Abstract
Most reports on fruit antioxidant capacities are based on extraction of antioxidants using polar solvents. Very little is known about the fate of bioactive compounds of papaya during the digestion process, and particularly in the food matrix under the gastric and intestinal conditions. Thus, an in vitro gastrointestinal digestion method was used to simulate physiological conditions of the stomach and small intestine to evaluate the actual antioxidant capacity of papaya. Results clearly showed a significant increase in antioxidant capacity after digestion. Therefore, the chemical extraction may not relate the actual antioxidant capacity of papaya.
INTRODUCTION
Highly reactive free radicals and oxygen species are continually produced in biological systems from a wide variety of sources (Prior et al., Citation1999). Though naturally existing biological antioxidant mechanisms combat oxidative stress, natural antioxidants from diet strengthen the endogenous antioxidant defenses (Benzie, Citation2003). An increasing number of epidemiological studies have shown an inverse correlation among the consumption of dietary antioxidants and incidence of various diseases including cancer and heart disease (Kris Etherton et al., Citation2002). India is the third largest producer of papaya in the world after Brazil and Nigeria (FAOSTAT, Citation2010). Papaya is rich in carotenes, especially lycopene as well as xanthophylls, lutein, zeaxanthin, and cryptoxanthin (Ben-Amotz and Fishler, Citation1998). It is also a good source of vitamin B1, B2, C, calcium, and proteins (Sankat and Maharaj, Citation1997). Thus, papaya is an excellent source of antioxidants. Ong and Chytil (Citation1983) showed an inverse relationship between cancer and dietary intake of β-carotene, while anti-ulcer properties of β-carotene and β-cryptoxanthin were reported by Moszik et al. (Citation1983), and anti-aging effects of carotenoids were demonstrated by Cutler (Citation1984). Though many studies have reported the beneficial effects of carotenoids and lycopene with regard to chronic diseases, antioxidant effects of papaya have been least reported. Most reports on fruit antioxidant capacity are based on extraction of antioxidants using polar solvents with or without addition of water. Very little is known about the fate of bioactive compounds of papaya during the digestion process, and particularly their fate in the food matrix under gastric and intestinal conditions and the effect of these conditions on the stability of these compounds. Perez-Jimenez and Saura-Calixto (Citation2005) have stated that the antioxidant capacity of foods may be underestimated in the literature because the extraction solvents usually used do not allow a complete release of antioxidant compounds and additionally non-extractable polyphenols with a high antioxidant capacity are ignored. Therefore, an in vitro gastrointestinal digestion method was used in order to simulate physiological conditions of the stomach and small intestine (pH, temperature, and enzyme conditions) to evaluate the actual antioxidant capacity of papaya.
MATERIALS AND METHODS
Chemicals
Pepsin (P-7000), Pancreatin (P-1750), Lipase (L-3126), Bile Extract Porcine (B-8631), α-Amylase (A-3176), Amyloglucosidase (A-7095), 2,2 Azinobis(3–ethylbenzothiazolin-6-sulfonic acid) diammonium salt (ABTS) (A-1888), 2,2-Diphenyl-1-picryl-hydrazyl (DPPH) (D-9132), Catechin (C-1251), Vanillin (V-2375), Rutin (R-5143), Gallic acid (G-7384), and 2,4,6–Tris(2-pyridyl)–s–triazine (TPTZ) (T-1253) were purchased from Sigma Aldrich-Germany and 6-Hydroxy-578-tetra methyl-chromane-2 carboxylic acid (Trolox)–56510 was purchased from Fluka (Sigma Aldrich, Bangalore, India).
Methodology
Sample preparation
Papaya was purchased from the local market. Fresh, medium sized, ripe but firm papayas were selected in both of the purchases of the study. They were then thoroughly washed, peeled, and deseeded to get the edible part. This part was immediately homogenized and used for extraction.
Extraction and Enzymatic Digestion
Chemical extraction
Initially, a 900 mg papaya sample was extracted twice in 80% aqueous methanol (pH set 2.0 with HCl) by shaking at room temperature for 45 min. Supernatants were filtered and centrifuged and volume was made up to 30 ml with the solvent using a measuring cylinder. All samples were stored at −20°C for antioxidant determination.
Extraction by in vitro gastrointestinal digestion
The sample was used for in vitro gastrointestinal digestion. The digestive enzymatic extraction was carried out by using the in vitro procedure previously described by Serrano et al. (Citation2007). Samples were successively incubated with digestive enzymes to simulate digestion in the small intestine. A control of sample was also incubated similarly with buffers without addition of enzymes.
Sample was incubated with pepsin (0.6 ml of a 300 mg/ml solution in a buffer of 0.2 M HCl−KCl, pH 1.5, 40°C, 1 h), pancreatin (3 ml of a 5 mg/ml solution in 0.1 M phosphate buffer, pH 7.5, 37°C, 6 h), lipase (6 ml of a 7 mg/ml solution in 0.1 M phosphate buffer, pH 7.5, 37°C, 6 h), bile extract porcine (6 ml of a 17.5 mg/ml solution in 0.1 M phosphate buffer, pH 7.5, 37°C, 6 h), and α-amylase (3 ml of a 120 mg/ml solution in 0.1 M tris-maleate buffer, pH 6.9, 37°C, 16 h). Then the samples were centrifuged (15 min, 6000 rpm) and supernatants were collected. Residues were washed twice with 5 ml of distilled water, and all supernatants were combined. Each supernatant was incubated with 300 μl of amyloglucosidase for 45 min at 60°C. Volume of all samples was made up to 30 ml with the use of a measuring cylinder. All samples were transferred to Eppendorf tubes and stored at −20°C for antioxidant determination.
Both chemical and digestive extracts (control and enzymatic) were used to determine the antioxidant capacity.
Determination of Antioxidant Capacity
Total phenol
Folin–Ciocalteu method (Singleton et al., Citation1999) was used to determine the total phenol content of the chemical and physiological extracts. Gallic acid was employed as a standard for comparison.
Flavonoid
Different aliquots of concentrated sample were used for estimation of flavonoid content by the method of Zhishen et al. (Citation1999). Rutin was used as a standard for comparison.
Flavonol
Flavonol content was measured from concentrated samples by the method of Yermakov et al. (Citation1987). Different aliquots of known concentration of catechin were treated as standard.
Total carotenoid
Total carotenoid was extracted in acetone, transferred to the petroleum ether phase, and read at 452 nm using petroleum ether for baseline correction (Gupta and Prakash, Citation2009).
Total antioxidant capacity
Ferric Reducing Antioxidant Power
Total antioxidant capacity of the chemical and physiological extracts was determined by using ferric reducing antioxidant power assay (FRAP) using the method of Benzie and Strain (Citation1999).
Reducing Power Assay
A method described by Oyaizu et al. (Citation1986) was employed to evaluate the reducing power of the chemical and physiological extracts of mango.
ABTS Radical Scavenging Ability
The radical scavenging capacity was determined using the modified ABTS radical decolorization assay as suggested by Re et al. (Citation1999).
DPPH Radical Scavenging Ability
The antioxidant activity of the extracts, on the basis of the scavenging activity of the stable 2,2-diphenyl-1-picryl-hydrazyl (DPPH) free radical, was determined by the method described by Brand-Williams et al. (Citation1995).
For all four methods, Trolox was used as standard.
Statistical Analysis
Experiments were done in duplicate batches with two separate purchases in the same season. Four observations of two different experiments were analyzed statistically. Differences among variables were tested for significance by using a one-way analysis of variance, Duncan using the level significance of p ≤ 0.05 by SPSS.
RESULTS AND DISCUSSION
The total phenol content (TPC) of the chemical extract of papaya was 72.28 mg GAE/100 g, whereas the enzymatically digested papaya had significantly higher (p < 0.05) TPC (117.72 mg GAE/100 g) than the chemical extract. The digestion control had the lowest TPC, i.e., 18.57 mg GAE/100 g. Similar results were found by Reddy et al. (Citation2010). They extracted papaya in 60% methanol containing 0.1% HCl and found 62 mg GAE/100 g.
Flavonoid and flavonol concentrations of different papaya extracts followed similar trend as total phenol. The enzymatic extract had the highest flavonoid and flavonol content, i.e., 52.53 mg rutin eq/100 g and 26.84 mg catechin eq/100 g, respectively. Luximon-Ramma et al. (Citation2003) extracted papaya in acetone/water (70:30 v/v) and found flavonoid concentration of 37.6 mg quercetin/100 g. The chemical extract of our study had flavonoid content of 31.15 mg rutin eq/100 g and 16.46 mg catechin eq/100 g of flavonol content, whereas the digestion control had the lowest flavonoid and flavonol concentration (9.45 mg rutin eq/100 g and 4.82 mg catechin eq/100 g) respectively. The total carotenoid content in the chemical extract of papaya was 10.02 and 5.60 mg/100 g in the digestion control. Total carotenoid content increased significantly after enzymatic digestion, which was 19.55 mg/100 g. Isabelle et al. (2010) found 11.82 mg total carotenoid/100 g. Epriliati et al., (Citation2009) showed a marked release of carotenoids after intestinal digestion. shows the total phenol, flavonoid, and flavonol content of different papaya extracts.
TABLE 1 Biochemical Quality Characteristics Showing Antioxidant Potential of Different Papaya Extracts
Total antioxidant capacity as measured by FRAP was 183.86 and 133.8 mg TE/100 g in the enzymatically digested and chemically extracted papaya samples, respectively, whereas the digestion control had significantly lower concentration, i.e., 34 mg TE/100 g. Luximon-Ramma et al. (Citation2003) extracted papaya in acetone/water (70:30 v/v) and found 200 μmol Fe (II)/100 g fresh weight. Reducing power of the three extracts of papaya were significantly different where the enzymatically digested extract had 550.44 mg TE/100 g of papaya. The chemical extract had significantly lower (p < 0.05) reducing power (224.41 mg TE/100 g) than the enzymatic extract and the digestion control had the lowest reducing power, i.e., 81.87 mg TE/100 g. FRAP and reducing power assay (RPA) of different papaya extracts is depicted in .
The highest DPPH radical scavenging ability (DPPHRSA) was shown by the enzymatically digested extract of papaya, which was 70.24 mg TE/100 g. The chemically extracted papaya sample showed a significant reduction (p < 0.05) in DPPHRSA, which was found to be 51.58 mg TE/100 g. Reddy et al. (Citation2010) extracted papaya in 60% methanol containing 0.1% HCl and showed 46 mg TE/100 g of DPPHRSA. The digestion control had the lowest antioxidant activity among the three which was 27.47 mg TE/100 g. Inhibitory concentration (IC 50), which is the concentration of different papaya extracts required to scavenge the initial absorbance of DPPH by 50% was determined graphically. The IC 50 values for chemically extracted and the digestion control papaya were 9 μg, whereas the enzymatically digested papaya required only half the concentration of chemically extracted papaya, i.e., 4.5 μg and Trolox required 2.5 μg to scavenge the same amount of DPPH free radicals.
Antioxidant capacity as measured by ABTS radical scavenging ability (ABTSRSA) showed significant differences among the three extracts of papaya. The enzymatically digested papaya had 66.82 mg TE/100 g whereas the chemical extract had 43.82 mg TE/100 g of ABTSRSA. The lowest antioxidant capacity was found in the digestion control, which had 21.31 mg TE/100 g ABTSRSA. Reddy et al. (Citation2010) found that the papaya extracted in 60% methanol containing 0.1% HCl had 50 mg TE/100 g of ABTSRSA. Our chemical extract had a closer value, i.e., 43.82 mg TE/100 g. shows the ABTSRSA and DPPHRSA of different papaya extracts.
Correlation among total phenol and different antioxidant capacity parameters as well as flavonoid and flavonol is depicted in . A strong and positive relationship between total phenol and FRAP can be observed where R2 = 0.896 and p = 0.001 and that of total phenol and RPA is R2 = 0.935, p = 0.00. A strong and positive relationship of total phenol and ABTSRSA is also observed, i.e., R2 = 0.944, p = 0.00. shows a strong and positive relationship of total phenol with flavonoid and flavonol respectively.
FIGURE 1 Correlation between total phenol content and other biochemical characteristics of papaya extracts. ABTSRSA: ABTS radical scavenging ability; DPPHRSA: DPPH radical scavenging ability; FRAP: ferric reducing antioxidant power assay; RPA: reducing power assay; GAE: gallic acid equivalent; TE: trolox equivalent.
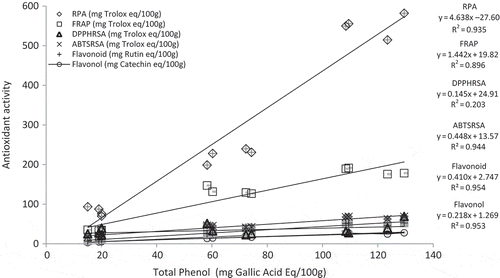
Enzymatically digested papaya samples had higher antioxidant capacity in all the antioxidant parameters as well as had higher carotenoid content, phenolic content and phenolic compounds than digestion control and the chemical extract. This clearly indicates the release of phenolic compounds and carotenoids during enzymatic digestion. Phenolic compounds are gradually released during the hydrolysis process in the digestive system (Hsu et al., Citation2004). According to Ryan and Prescott (Citation2010) when phenolic compounds are exposed to in vitro digestion, they are transformed into different structural forms and possess different chemical properties and functions. Similar results using simulated digestion for extraction of carotenoids and phenolic compounds were previously shown by Epriliati et al. (Citation2009) as well as Perez-Jimenez and Saura-Calixto (Citation2005). Other studies that have reported similar results after measurement of antioxidant potential of different food stuffs after gastrointestinal digestion are Akillioglu and Karakaya (Citation2010) in beans and Goni et al. (Citation2006) in fruits and vegetables.
Gawlik-Dziki et al. (Citation2009) evaluated the bioactivity of wheat and buckwheat after digestion with simulated intestinal fluid and reported an increase in the phenolic acids content as well as free radicals scavenging properties with the digestion time. Serrano et al. (Citation2007) have evaluated and shown a wide difference in the antioxidant capacity of plant foods in the Spanish diet measured by both chemical and physiological approach. Similarly, this experiment clearly shows the effect of enzymatic digestion on the release of phenolic compounds and its effect on antioxidant activity of papaya.
CONCLUSION
Results of this study show wide differences in chemical and physiological extracts of papaya thus proving that mere extraction by organic solvents may not be sufficient for the determination of antioxidant capacity. The reason behind the differences is that a significant part of the antioxidants contained in plant foods is not analyzed in most antioxidant capacity assays, where the antioxidant extraction is incomplete. Also, the quantity and quality of antioxidant compounds extracted by organic solvents may not relate to their physiological bioavailability. Such conventional extraction procedures may prove misleading for assessment of the antioxidant potential of foods. A physiological perspective on antioxidant capacity bioavailability yields more useful information about possible health effects of antioxidants of foods.
LITERATURE CITED
- Akillioglu, H.G. and S. Karakaya. 2010. Changes in total phenols, total flavonoids, and antioxidant activities of common beans and pinto beans after soaking, cooking, and in vitro digestion process. Food Sci. Biotechnol. 19(3):633–639.
- Ben-Amotz, A. and R. Fishler. 1998. Analysis of carotenoids with emphasis on 9-cis-carotene in vegetables and fruits commonly consumed in Israel. Food Chem. 62:515–520.
- Benzie, I. and J. Strain. 1999. Ferric radical antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 299:15–27.
- Benzie, I.F.F. 2003. Review: Evolution of dietary antioxidants. Compar. Biochem. Physiol. 126:113–126.
- Brand-Williams, M., M. Cuvelier, and C. Berset. 1995. Use of free radical method to evaluate antioxidant activity. LWT–Food Sci. Technol. 28:25–30.
- Cutler, R.G. 1984. Carotenoids and retinal: Their possible importance in determining longevity of prunate species. Proc. Natl. Acad. Sci. 81(76):27–31.
- Epriliati, I., B. D’arcy, and M. Gidley. 2009. Nutriomic analysis of fresh and processed fruit products. 1. During in vitro digestions. J. Agri. Food Chem. 57:3363–3376.
- FAOSTAT. 2010. Papayas: U.S. import-eligible countries; world production and exports. <http://faostat.fao.or/default.aspx>.
- Gawlik-Dziki, U., D. Dziki, B. Baraniak, and R. Lin. 2009. The effect of simulated digestion in vitro on bioactivity of wheat bread with Tartary buckwheat flavones addition. LWT–Food Sci. Technol. 42:137–143.
- Goni, I., J. Serrano, and F. Saura-Calixto. 2006. Bioaccessibility of β-carotene, lutein and lycopene from fruits and vegetables. J. Agri. Food Chem. 54:5382–5387.
- Gupta, S. and J. Prakash. 2009. Studies on Indian green leafy vegetables for their antioxidant activity. Plant Foods Hum. Nutr. 64:39–45.
- Hsu, C.L., S.L. Hurang, W. Chen, Y.M. Wenig, and C.Y. Tseng. 2004. Qualities and antioxidant properties of bread as affected by the incorporation of yam flour in the formulation. Int. J. Food Sci. Technol. 39(2):231–238.
- Isabelle, M., B.L. Lee, M.T. Lim, W.-P. Koh, D. Huang, and C.N. Ong. 2010. Antioxidant activity and profiles of common fruits in Singapore. Food Chem. 123(1):77–84.
- Kris Etherton, P.M., K.D. Heckar, A. Bonanome, S.M. Coval, A.E. Binkoski, K.F. Hilper, A.E. Griel, and T.D. Etherton. 2002. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am. J. Food Sci. Technol. 38(3):272–273.
- Luximon-Ramma, A., T. Bahorun, and A. Crozier. 2003. Antioxidant actions and phenolic and vitamin C contents of common Mauritian exotic fruits. J. Sci. Food Agric. 83:496–502.
- Moszik, G., B. Monika, J. Tibor, H. Fancisco, S. Joszef, and G. Toth. 1983. Tapalakozatud. Helyzete. Feldatia Magyarorszagon 8:781.
- Ong, D.E. and F. Chytil. 1983. Vitamin A and cancer, p. 105–144. In: G.D. Aurbach and D.B. McCormick ( eds.). Vitamins and hormones. Academic Press, New York.
- Oyaizu, M. 1986. Studies on product of browning reaction produced from glucose amine. Jap. J. Nutr. 44:307–315.
- Perez-Jimenez, J. and F. Saura-Calixto. 2005. Literature data may underestimate the actual antioxidant capacity of cereals. J. Agri. Food Chem. 53:5036–5040.
- Prior, R.L., J.A. Joseph, G. Cao, and B. Sukitt-Hale. 1999. Can foods forestall aging? Agric. Res. 47:15–17.
- Re, N., A. Pellegrini, A. Proteggente, M. Pannala, M. Yang, and C. Rice-Evans. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. 26:1231–1237.
- Reddy, C.V.K., D. Sreeramulu, and M. Raghunath. 2010. Antioxidant activity of fresh and dry fruits commonly consumed in India. Food Res. Int. 43:285–288.
- Ryan, L. and S.L. Prescott. 2010. Stability of the antioxidant capacity of twenty-five commercially available fruit juices subjected to an in vitro digestion. Int. J. Food Sci. Technol. 45:1191–1197.
- Sankat, C.K. and R. Maharaj. 1997. Papaya. Postharvest physiology and storage of tropical and subtropical fruits. CAB International, Wallingford, UK.
- Serrano, J., I. Goni, and F. Saura-Calixto. 2007. Food antioxidant capacity determined by chemical methods may underestimate the physiological antioxidant capacity. Food Res. Int. 40:15–21.
- Singleton, V.L., R. Orthofer, and R.M. Lamuela-Raventos. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteau reagent. Methods Enzymol. 299:152–178.
- Yermakov, A.I., V.V. Arasimov, and N.P. Yarosh. 1987. Methods of biochemical analysis of plants. John Wiley and Sons, New York.
- Zhishen, J., T. Mengcheng, and W. Jianming. 1999. Determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 64:555–559.
