Abstract
Longkong is one of the nutritious and economical fruits in Thailand. It has a very short shelf life due to its severe pericarp browning at ambient temperature storage. In the present study, the different concentrations (1, 2, and 3%) of citric acid were used to control longkong pericarp browning during storage at 18°C and an 85% relative humidity. Longkong treated with distilled water served as a control. An increased pericarp water loss and decreased fruit respiration rate was observed throughout the storage. Fruit pericarp ultrastructural observation showed the severe damage on the pericarp parenchymal tissues during at the end of storage. The different concentrations of citric acid effectively limited longkong pericarp browning throughout storage. Longkong treated with 3% citric acid significantly suppressed the increase of pericarp pH, phenylalanine ammonia lyase (PAL), polyphenol oxidase (PPO), peroxidase (POD) activities, and loss of pericarp total phenolics as compared to a low concentration and control fruit (P < 0.05). These findings have suggested citric acid is a promising agent that controls longkong pericarp browning and oxidoreductase activities during prolonged storage.
INTRODUCTION
Longkong (Aglaia dookkoo Griff.) is a non-climacteric tropical fruit. It is cultivated widely in the southern part of Thailand. At the fully ripened stage, the green pericarp of longkong is transformed into a bright yellow color (Nakasone and Paull, Citation1998; Paull et al., Citation1987). It contains a variety of nutrients, such as polyphenols, vitamins, minerals, carbohydrates, proteins, and low fat (Lim et al., Citation2007; Venkatachalam and Meenune, Citation2012). The pericarp of longkong has an antimicrobial property, and it is used to cure diarrhea and vomiting. It has a pleasant aroma with the combination of sweet and sour taste (Taesakul et al., Citation2012). However, after harvest, the pericarp’s yellow color turns into brown within 3 days at ambient temperature. Although the sensorial quality of the flesh is not affected, this alteration could reduce the commercial value of this fruit (Ketsa and Paull, Citation2008). Normally, the pericarp browning of fruits and vegetables is due to accelerating oxidation of phenolics by oxidoreductase enzymes. Enzymes, such as phenylalanine ammonia lyase (PAL), polyphenol oxidase (PPO), and peroxidase (POD), have been widely reported on accelerating the browning of fruits and vegetables by generating various phenolics and their oxidations (Kumar et al., Citation2012; Mishra et al., Citation2012; Ortega-Garcia et al., Citation2008). Longkong fruit browning during maturation and storage was contributed by PAL, PPO, and POD enzymes (Lichanporn et al., Citation2009; Venkatachalam and Meenune, Citation2012). Numerous approaches, such as coating, heat treatment, acid dipping, fungicide, fumigation, irradiation, storage temperature, and modified atmospheric package, have been widely done to prevent pericarp browning in many plant produce (Hetong et al., Citation2003).
On the other hand, longkong fruit is an economical fruit, and it needs an inexpensive and effectual treatment that can control its pericarp browning during storage. Low temperature storage, along with the acidulant treatment, controlled longkong pericarp browning and quality loss (Sangchote et al., Citation2012; Sangkasanya and Meenune, Citation2010). Ketsa and Paull (Citation2008) reported that longkong fruit stored at 18°C and 85% relative humidity increased the shelf life up to 21 days. Additionally, organic acids are an interesting alternative that effectively controlled pericarp browning and stabilized the color in many fruits and vegetables (Ducamp-Collin et al., Citation2008; Joas et al., Citation2005). Citric acid is an inexpensive organic acid with a wide range of benefits, such as an antioxidant, antibrowning agent, antimicrobial, and medicinal properties (Apai et al., Citation2009; Davey et al., Citation2000; Sangchote et al., Citation2012). It is a prominent inhibitor of oxidoreductase enzymes because of its chelating power, pH reducing, and acidulant abilities (Abbasi et al., Citation2013; Uylaser et al., Citation2014). However, there is no information available on effect of citric acid on longkong pericarp oxidoreductase enzymes and browning. Therefore, the present research has explored the effect of different concentrations of citric acid on longkong pericarp browning and its oxidoreductase enzyme activities during low temperature storage.
MATERIAL AND METHODS
Chemicals
All of the solvents were purchased from Merck, glutaraldehyde (25%, Sigma), Folin-Ciocalteu’s reagent (2N, Fluka), gallic acid (98%, Fluka), l-phenylalanine (98%, Sigma), 4-methyl catechol (95%, Fluka), guaiacol (98%, Merck), bovine serum albumin (˜66,000 Da, Sigma), citric acid (99%, Fluka), sodium carbonate (99%, Fluka), ethylenediamine tetra acetic acid (Fluka), polyvinyl pyrrolydine (PVP40, Sigma), hydrogen peroxide (30%, Merck), sodium hydroxide (98%, Fluka), and sodium phosphate (96%, Fluka).
Plant Material
The fully matured longkong fruit bunches were obtained from a selected garden in southern Thailand, and they were transported into the laboratory on the same day within 2 hours at ambient temperature. Fruits were cut into individuals from the bunch, undergone for screening to separate from any apparent pericarp damage and then fruits were sorted out according to uniform maturity, size (˜4 cm in diameter), and color of the pericarp. The obtained fruits were washed thoroughly in distilled water and used for physio-morphological study and treatment.
Physio-Morphological Analysis of Non-Treated Fruits
Non-treated longkong fruits were used to study the physio-morphological analysis, such as fruit pericarp water loss, respiration rate, and pericarp ultrastructural changes. The physio-morphological analysis was performed as follows.
Pericarp water loss
Measurement of pericarp water loss was based on a comparison of pericarp water content in the beginning and every storage interval until the end of storage. The following formula was used to calculate the water loss and results were expressed in percentage:
Pericarp ultrastructural changes
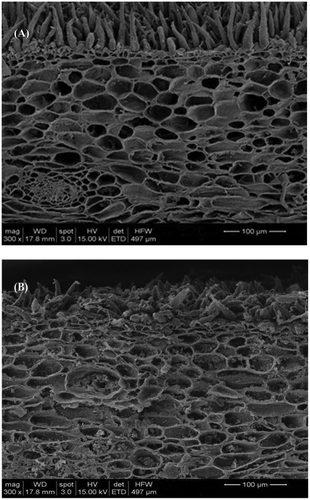
Ultrastructural changes in the fruit pericarp parenchymal tissue were observed in the beginning and at the end of storage. Fruits pericarp was fixed in 2.5% glutaraldehyde in a 0.1 M sodium phosphate buffer (pH 7.2) for 2 h. Then, specimens were rinsed twice in a buffer solution and rinsed once in distilled water every 20 min. After that the pericarp tissues were dehydrated in a graded alcohol series, sputtered with platinum/palladium, and then dried by a critical point dryer. The dried peels were mounted on the stubs, gold coated twice, and then they were observed under a scanning electron microscope (JOEL, JSM 5800 LV, SEC-PSU, Hatyai, Thailand).
Fruit respiration rate
Fruit respiration rate was measured as the amount of CO2 evolved by fruit during storage as described by Caleb et al. (Citation2012) with slight modifications. Twenty fruits per replication were placed in a 3-L sealed glass chamber for 4 h. After 4 h incubation, 1 ml of a gas sample was taken with a syringe and injected in a gas chromatography (Perkin Elmer (Auto systems XL), PSU, SuratThani, Thailand) using an 80/100-mesh Pora pack-Q column and a thermal conductivity detector. The results were expressed in ml CO2kg−1 h−1.
Citric Acid Treatment and Storage
Longkong fruits were separated into four groups. The first group was dipped in distilled water, and it was used as a control. The 2nd, 3rd, and 4th group were dipped in 1%, 2%, and 3% citric acid solutions, respectively. All of the groups were dipped for 10 min and after that, fruits were air dried thoroughly by an electric fan and each group was packed in a perforated transparent PVC punnet (20 fruits per punnet) and stored at 18°C, 85% RH. For each treatment, five replicates were used. The samples for analysis were taken directly after treatment and at every 3 days of interval until the end of storage. The storage life of longkong was terminated when the visible mold growth appeared on the pericarp.
Analysis of Citric Acid-Treated and Control Fruits
Pericarp color (L*, a*, and b*)
The surface color of four sides of 20 individual fruits pericarp was measured by using a Hunter Lab Colorimeter in terms of CIE lightness (L*), redness (a*), and yellowness (b*) values.
Pericarp pH
Pericarp tissues (4 g) were homogenized in 40 ml of distilled water by using a blender and then the homogenate filtered through a muslin cloth. After that the pericarp filtrate was used to measure pH by using a Sartorius PB-20 digital pH meter.
Pericarp total phenolics
Pericarp total phenolic content was measured in accordance with the method of Singleton and Rossi (Citation1965). Pericarp tissues (2 g) were homogenized with 20 ml of 80% ethanol (1:1 w/v) at 4°C. The homogenized sample was centrifuged at 12000g for 20 min at 4°C. Next, 0.4 ml of supernatant was mixed with 0.4 ml of Folin-Ciocalteu’s reagent and then followed by 1 ml of 7% sodium carbonate solution. After that, the volume was increased to 10 ml with distilled water, vortexed, and then incubated for 1 h at room temperature. Absorbance was measured at 750 nm. Total phenolic content was calculated from a standard curve prepared with gallic acid.
Pericarp enzyme analysis
PAL activity measurement
Pericarp tissues (2 g) were homogenized at 4°C in 20 ml of a 0.1 M sodium borate buffer (pH 8.0) solution, which contained 0.2 g of insoluble PVP, 5 mM mercaptoethanols, and 2 mM ethylenediamine tetra acetic acid (EDTA). The homogenate was centrifuged at 19000g for 20 min at 4°C and then the supernatant was collected for the PAL enzyme assay. PAL activity was determined in accordance with the method of Jiang and Joyce (Citation2003). A mixture of 0.1 ml of enzyme extract and 2.9 ml of 0.1 M sodium borate buffer (pH 8.0) solution that contained 3 mM l-phenylalanine was incubated for 1 h at 37°C. An increase of PAL activity was measured at 290 nm. One unit of enzyme activity was defined as the amount that caused an increase of 0.01 in the absorbance per hour. The specific activity was expressed as unit/mg protein.
PPO activity measurement
Pericarp tissues (2 g) were homogenized at 4°C in 20 ml of 0.2 M sodium phosphate buffer (pH 6.4). After that, the homogenized sample was filtered through the cheesecloth and then filtrate was centrifuged at 12000g for 30 min at 4°C. The supernatant was collected for measuring PPO activity (Tian et al., Citation2002). The reaction mixture contained 3 ml of 0.5 M 4-methylcatechol in 0.2 M sodium phosphate buffer (pH 6.4) and 0.1 ml of enzyme extract. The absorbance was measured at 398 nm for 1 min. One unit of enzyme activity was defined as an increase in one-absorbance unit per min. The specific activity was expressed as unit/mg protein.
POD activity measurement
Pericarp tissues (2 g) were homogenized at 4°C in 20 ml of 0.05 M phosphate buffer (pH 7) solution and 0.2 g of insoluble polyvinylpyrrolidone (PVP). The homogenate was filtered through the cheesecloth and then the filtrate was centrifuged at 19000g for 20 min at 4°C. The collected supernatant was used to measure POD activity (Zhang et al., Citation2005). A 3-ml reaction mixture contained 25 μl of crude enzyme extract, 2.78 ml of 0.05 M phosphate buffer (pH 7.0), 0.1 ml of 20 mM hydrogen peroxide (H2O2), and 0.1 ml of 20 mM guaiacol. An increased POD activity in pericarp was recorded at 470 nm for 2 min. One unit of enzyme activity was defined as the amount that caused a change of 0.01 in the absorbance per min. The specific activity was expressed as unit/mg protein.
Protein analysis
Protein content in the pericarp crude enzyme extract was determined in accord with the method of Bradford (Citation1976). Bovine serum albumin (BSA) is used as a standard.
Statistical Analysis
All of the experiments were done in five replications. Significant differences between means were estimated by Duncan’s new multiple range tests (DMRT), with a level of significance of 0.05. The Statistical Package for Social Science (SPSS version 6.0 for windows, SPSS Inc., Chicago, IL, USA) was used for data analysis.
RESULTS AND DISCUSSION
Physio-Morphological Changes of Non-Treated Fruits
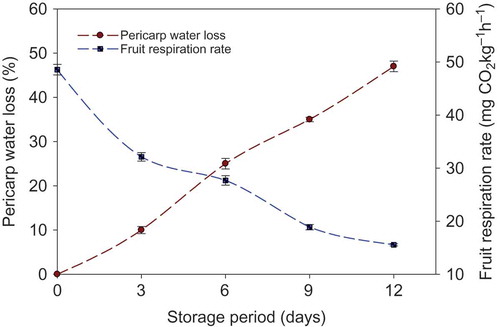
Longkong pericarp water loss was shown in . The results showed that an increase of water loss found throughout the storage. The percentage of water loss was steadily increased with the increase of storage time. The higher percentage of water loss was recorded from the 6th day of storage (25.2%) until the end of storage (47.4%). Plants’ cell membranes are one of the prime targets of plant stress and the alteration in cell membrane structure may induce a modification in the cellular compartment (Gonzalez and Barrette, Citation2010). Normally, the phenolic compound and oxidoreductase enzymes are confined in separate cellular compartments, and the browning reaction does not usually take place until the disruption of the cellular compartments (Olivos et al., Citation2012). Water loss of freshly harvested plant produce has been attributed to a loss and/or damage of cellular compartments of the plants, thus increasing the browning reaction (Jiang et al., Citation1999; Jongen, Citation2002; Maalekuu et al., Citation2006). On the other hand, fruit respiration rate was gradually decreased with the increased storage time (). This was in agreement with the results published on longkong by Lichanporn et al. (Citation2009). Jiang et al. (Citation2011) reported that a decrease of respiration rate is seen usually in non-climacteric fruit during storage.
FIGURE 1 Pericarp water loss and respiration rate of non-treated longkong fruit during storage at 18°C (85% RH). Vertical bars represent the standard deviations.
Longkong pericarp ultrastructural changes were examined in the beginning and at the end of storage (, ). The observation showed that the increased storage time gradually damaged the pericarp parenchymal cells in the hyper-dermal tissues and, consequently, an increase of pericarp browning was steadily observed all over the storage. The oxidoreductase enzymes are present in the parenchymal cells and these enzymes could possibly interact with substrates to produce browning during the parenchymal damage (Ortega-Garcia et al., Citation2008). Lichanporn et al. (Citation2009) reported that longkong fruit during storage had severe ultrastructural changes in the fruit pericarp and thus, increased the pericarp browning. Jiang et al. (Citation2011) reported that the stored longan fruit had a gradual breakdown of cellular ultrastructure and this breakdown commenced in the mesocarp tissue at the beginning of storage and then followed by sclerenchyma cells during the prolonged storage.
Effect of Citric Acid on Longkong Pericarp Color
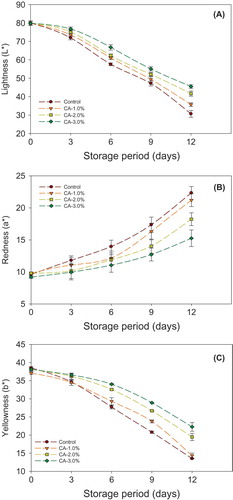
Longkong pericarp colors, such as lightness (L*), redness (a*), and yellowness (b*), were changed throughout the storage time (–C). Pericarp L* and b* levels were depleted ( and C), whereas a* levels were raised throughout the storage in both citric acid treated and the control fruit pericarp (). Although the citric acid treated fruits pericarp held a better color value than the control. On the other hand, pericarp L* and b* values were severely decreased, and a* values continuously increased in the control. The different concentration of citric acid treatment had a significant effect on holding the pericarp fruit colors (L*, a*, and b*) (P < 0.05). Normally, a decrease of L* and b* values and an increase of a* values referred as an indicator for the raising of pericarp browning in longkong (Ketsa and Paull, Citation2008; Paull et al., Citation1987; Venkatachalam and Meenune, Citation2012). The higher concentrations (2% and 3%) of citric acid treatment were given the better control on increasing in pericarp browning than a low concentration (1%) and the control. Longkong fruit pericarp browning was due to the consequence of changes of the oxidoreductase (PPO and POD) enzymes in pericarp that converts phenols into brown pigments (Lichanporn and Techavuthiporn, Citation2013; Venkatachalam and Meenune, Citation2012). Previous studies found that using the aqueous acid dipping treatment between a low to medium concentration on fruits and vegetables would limit the activities of PPO and POD enzymes and reduced the color changes (Apai et al., Citation2009; Lichanporn et al., Citation2002; Suttirak and Manurakchinakorn, Citation2010).
Effect of Citric Acid Treatment on Longkong Pericarp pH
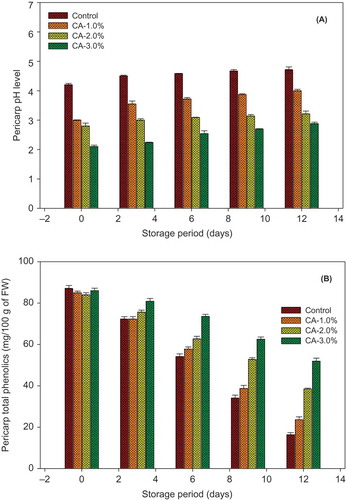
Longkong pericarp pH changes during storage were shown in . The citric acid treated and the control fruits were gradually increased in pericarp pH level during storage. Hetong et al. (Citation2003) reported that the increase of pH level in fruit pericarp could be attributed by the loss of cellular compartmentalization and pericarp water loss during storage. This is in agreement with our results that are shown in and . The different concentration of citric acid treatment was significantly maintained a low pH profile in fruit pericarp as compared to the control (P < 0.05). The increased concentration of citric acid helped to reduce the pH level of pericarp during storage. This experiment demonstrated that 3% of citric acid treatment was given the lower pH level (2.88) as compared to the higher level of pH in the control fruit pericarp (4.71) and 1.0% (4.00) and 2.0% (3.21) citric acid treatment during at the end of storage time. However, the increased acidification level of fruit pericarp could cause its surface to become more fragile and then, in an appropriate condition, can accelerate the browning reaction (Joas et al., Citation2005). Lichanporn et al. (Citation2002) observed that the longkong fruit treated with the higher concentration of citric acid (4% and 6%) had increased pericarp browning during storage.
Effect of Citric Acid Treatment on Pericarp Phenolics
Changes in longkong pericarp total phenolics level during storage were shown in . Longkong pericarp total phenolics were continuously declined all over the storage period. Citric acid treatment minimized the reduction of longkong pericarp total phenolics compared to the control (P < 0.05). Longkong fruit pericarp treated with 3% citric acid retained a higher level of phenolics (51.89 mg/100 g FW) than the control (16.37 mg/100 g FW) and 1% (23.62 mg/100 g FW) and 2% (38.47 mg/100 g FW) of citric acid treatments. However, the different concentrations of citric acid treatment non-significantly maintained the longkong pericarp total phenolics. The higher concentration of citric acid treatment was restrained reduction of total phenolics in loquat and longan fruit during storage as compared to the control (Abbasi et al., Citation2013; Apai et al., Citation2009). Polyphenolic compounds have various unique characteristics, such as antimicrobial, antioxidant, and medicinal properties, and additionally, it controls the increase of senescence rate in many fresh produce (Pandey and Rizvi, Citation2009; Rekika et al., Citation2005). Meanwhile, polyphenols are important substrates for the oxidoreductase enzymes (PPO and POD). It is reported that PPO and POD are the key enzymes that are responsible for pericarp browning of longkong fruit, by catalyzing the oxidation of phenolic compounds (Venkatachalam and Meenune, Citation2012).
Effect of Citric Acid Treatment on Pericarp PAL, PPO, and POD Activities
Changes in longkong fruit pericarp PAL, PPO, and POD activities during storage were shown in –C. The PAL activity during the initial storage slightly obtained a low level and then it gradually increased all over the storage time (). PAL is the key regulatory enzyme that participates in the synthesis of polyphenols. The increased activity of PAL could be promoted by various biotic and abiotic stresses (Mishra et al., Citation2012). The citric acid treatment was significantly maintained a low PAL activity as compared to the control during storage. Longkong pericarp that was treated with 3% citric acid maintained fewer levels of PAL activity during storage as compared to the other concentrations and control. The ionizable groups of protein structure of PAL enzyme could be controlled by the environmental pH (He and Luo, Citation2007). The inhibition of PAL activity by citric acid treatment controlled the biosynthesis of the precursor for the formation of brown pigments (Loaiza-Velarde and Saltveit, Citation2001; Uylaser et al., Citation2014).
FIGURE 5 Effect of different concentration of citric acid on longkong pericarp PAL, PPO, and POD activities during storage at 18°C (85% RH). Vertical bars represent the standard deviations.
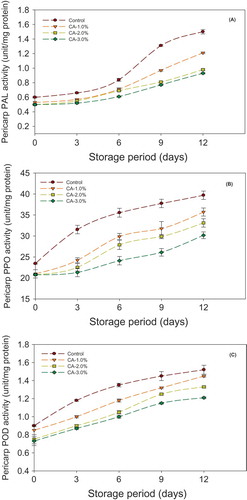
The effect of citric acid treatment on PPO and POD activities in longkong pericarp during storage is shown in and C. The results indicated that PPO and POD activity was increased rapidly in the control fruit pericarp, and a slower increase was seen in citric acid-treated fruit pericarp. The different concentrations of citric acid treatment significantly limited the pericarp browning during the storage by suppressing the activities of PPO and POD. The higher concentration of citric acid maintained fewer levels of PPO and POD activities in fruit pericarp during storage. This could be due to the citric acid treatment maintaining a low level of pH in pericarp, which produced the unsuitable conditions for PPO and POD activities (He and Luo, Citation2007). Usually, PPO and POD enzymes are highly pH dependent. Mizobutsi et al. (Citation2010) reported that PPO and POD activities were not noticed in litchi pericarp when the pH level was lowered to below 2.5. Moreover, citric acid could effectively control the PPO activity in different fruits and vegetables because of its potential as a chelating agent, which can chelate the copper from the active site of PPO (Abbasi et al., Citation2013; Jiang et al., Citation1999).
The increase of pericarp water loss and membrane destruction provided the suitable condition for oxidoreductase activities and, therefore, the rapid increase of pericarp browning observed in non-treated fruits. The combination of citric acid treatment and storage at 18°C and an 85% relative humidity effectively controlled longkong pericarp browning. The higher concentration of citric acid (3%) treatment significantly hindered the raising of browning parameters, such as pericarp pH, PAL, PPO, POD, and pericarp total phenolic loss, as compared to a low concentration and the control. Longkong fruit shelf life was discontinued at the 12th day of storage and it was due to the visible mold growth found on the longkong pericarp. Overall, citric acid treatment is an economical and effective treatment that keeps the longkong fruit away from severe pericarp browning and quality loss.
ACKNOWLEDGMENT
The author is very grateful to Prince of Songkla University for the given financial support (No. 5107010199) to successfully accomplish this research.
LITERATURE CITED
- Abbasi, A.N., A. Akhtar, A. Hussain, and I. Ali. 2013. Effect of anti browning agents on quality changes of loquat (Eriobotrya Japonica Thunb.) fruit after harvest. Pak. J. Bot. 45:1391–1396.
- Apai, W., V. Sardsud, P. Boonprasom, and U. Sardsud. 2009. Effect of chitosan and citric acid on pericarp browning and polyphenol oxidase activity of longkong fruit. Songklanakarin J. Sci. Tech. 31:621–628.
- Bradford, M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254.
- Caleb, O.J., P.V. Mahajan, U.L. Opara, and C.R. Witthuhn. 2012. Modelling the respiration rates of pomegranate fruit and arils. Postharv. Biol. Technol. 64:49–54.
- Davey, M.W., M.V. Montagu, D. Inzé, M. Sanmartin, A. Kanellis, N. Smirnoff, I.J.J. Benzie, J.J. Strain, D. Favell, and J. Fletcher. 2000. Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agr. 80:825–860.
- Ducamp-Collin, M.N., H. Ramarson, M. Lebrun, G. Self, and M. Reynes. 2008. Effect of citric acid and chitosan on maintaining red colouration of litchi fruit pericarp. Postharv. Biol. Technol. 49:241–246.
- Gonzalez, E.M., and M.D. Barrette. 2010. Thermal, high pressure and electric field processing effects on plant cell membrane integrity and relevance to fruits and vegetable quality. J. Food Sci. 75:121–130.
- He, Q., and Y. Luo. 2007. Enzymatic browning and its control in fresh-cut produce. Stewart Postharv. Rev. 6:1–7.
- Hetong, L., C. Shaojun, and X. Yufang. 2003. Commercial postharvest handling and storage technology of lichi fruit. Trans. CSAE 19:126–134.
- Jiang, Y., J. Fu, G. Zauberman, and Y. Fuchs. 1999. Purification of polyphenol oxidase and the browning control of litchi fruit by glutathione and citric acid. J. Sci. Food Agr. 79:950–954.
- Jiang, Y., and D. Joyce. 2003. ABA effects on ethylene production, PAL activity, anthocyanin and phenolic contents of strawberry fruit. Plant Grow. Regul. 39:171–174.
- Jiang, Y., D. Joyce, and H. Lin. 2011. Longan (Dimocarpus Longan Lour.), p. 408–422. In: E. Yahia (ed.). Postharvest biology and technology of tropical and subtropical fruits. Woodhead Publishing Limited, UK.
- Joas, J., Y. Caro, M.N. Ducamp, and M. Reynes. 2005. Postharvest control of pericarp browning of litchi fruit (Litchi chinensis Sonn cv Kwaï Mi) by treatment with chitosan and organic acids: I. Effect of pH and pericarp dehydration. Postharv. Biol. Technol. 38:128–136.
- Jongen, W.M.F. 2002. Fruit and vegetable processing: Improving quality. CRC Press, England.
- Ketsa, S., and E.R. Paull. 2008. Meliaceae, p. 468–472. In: J. Janick and R.E. Paull (eds.). The encyclopeadia of fruits and nuts. CAB International, UK.
- Kumar, S., B.B. Mishra, S. Saxena, N. Bandyopadhyay, V. More, S. Wadhawan, S.N. Hajare, S. Gautam, and A. Sharma, 2012. Inhibition of pericarp browning and shelf life extension of litchi by combination dip treatment and radiation processing. Food Chem. 131:1223–1232.
- Lichanporn, I., V. Srilaong, C. Wongs-Aree, and S. Kanlayanarat, 2009. Postharvest physiology and browning of longkong (Aglaia dookkoo Griff.) fruit under ambient conditions. Postharv. Biol. Technol. 52:294–299.
- Lichanporn, I., and C. Techavuthiporn. 2013. The effects of nitric oxide and nitrous oxide on enzymatic browning in longkong (Aglaia dookkoo Griff.). Postharv. Biol. Technol. 86:62–65.
- Lichanporn, I., C. Techavuthiporn, and S. Kanlayanarat. 2002. Effect of ascorbic acid and citric acid on browning of longkong (Aglaia dookoo Griff.). Agric. Sci. J. 22:119–121.
- Lim, Y.Y., T.T. Lim, and J.J. Tee. 2007. Antioxidant properties of several tropical fruits: A comparative study. Food Chem. 103:1003–1008.
- Loaiza-Velarde, G.J., and E.M. Saltveit. 2001. Heat shocks applied either before or after wounding reduce browning of lettuce leaf tissue. J. Amer. Soc. Hort. Sci. 126:227–234.
- Maalekuu, K., Y. Elkind, A. Leikin-Frenkel, S. Lurie, and E. Fallik, 2006. The relationship between water loss, lipid content, membrane integrity and LOX activity in ripe pepper fruit after storage. Postharv. Biol. Technol. 42:248–255.
- Mishra, B.B., S. Kumar, S. Wadhawan, S.N. Hajare, S. Saxena, V. More, S. Gautam, and A. Sharma. 2012. Browning of light fruit pericarp: Role of polyphenol oxidase, peroxidase, phenylalanine ammonia lyase and effect of gamma radiation. J. Food Biochem. 36:604–612.
- Mizobutsi, P.G., L.F. Finger, A.R. Ribeiro, R. Puschmann, M.L.L. Neves, and F.W. Mota. 2010. Effect of pH and temperature on peroxidase and polyphenoloxidase activities of litchi pericarp. Sci. Agricol. 67:213–217.
- Nakasone, H.Y., and R.E. Paull. 1998. Tropical fruits. CAB International, England.
- Olivos, A., S. Johnson, Q. Xiaoqiong, and C.H. Crisosto. 2012. Fruit phosphorous and nitrogen deficiencies affect ‘Grand Pearl’ nectarine flesh browning. HortScience 47:391–394.
- Ortega-Garcia, F., S. Blanco, M.A. Peinado, and J. Peragon. 2008. Polyphenol oxidase and its relationship with oleuropein concentration in fruits and leaves of olive (Olea europaea) cv. ‘Picual’ trees during fruit ripening. Tree Physiol. 28:45–54.
- Pandey, B.K., and I.S. Rizvi. 2009. Plant polyphenols as dietary antioxidants in human health and disease. Oxidat. Med. Cell. Long. 2:270–278.
- Paull, E.R., T. Goo, and N.J. Chen. 1987. Growth and compositional changes during development of lanzone fruit. HortScience 22:1252–1253.
- Rekika, D., S. Khanizadeh, M. Deschenes, A. Levasseur, and T.M. Charles. 2005. Antioxidant capacity and phenolic content of selected strawberry genotypes. HortScience 40:1777–1781.
- Sangchote, S., N. Khewkhom, and T. Sungsiri. 2012. Fruit rot control in longkong (Aglaia dookkoo Griff.) with chemical and hot water treatment. Acta Hort. 943:219–222.
- Sangkasanya, S., and M. Meenune. 2010. Physical, chemical and sensory qualities of longkong (Aglaia dookkoo Griff.) as affected by storage at different atmospheres. Asian J. Food Agro. Indus. 2:64–74.
- Singleton, V.L., and A.J. Rossi. 1965. Colorimetry of total phenolics with phosphomlybdic-phophotungstic acid reagents. Amer. J. Econ. Vit. 16:144–158.
- Suttirak, W., and S. Manurakchinakorn. 2010. Potential application of ascorbic acid, citric acid and oxalic acid for browning inhibition in fresh cut fruits and vegetables. Walailak J. Sci. Tech. 7:5–14.
- Taesakul, P., N. Pradisthakarn, S. Chantaksinopas, and J. Siriphanich. 2012. Longkong fruit abscission and its control. Postharv. Biol. Technol. 64:91–93.
- Tian, S., Y. Xu, A. Jiang, and Q. Gong. 2002. Physiological and quality responses of longan fruit to high O2 or high CO2 atmospheres in storage. Postharv. Biol. Technol. 24:335–340.
- Uylaser, V., B. Incedayi, and G. Yildiz. 2014. Effect of citric acid and Na-metabisulphite on the shelf life of minimally processed Haciomer cv. Chestnut. Int. J. App. Sci. Tech. 4:127–135.
- Venkatachalam, K., and M. Meenune. 2012. Changes in physiochemical quality and browning related enzyme activity of longkong fruit during four different weeks of on-tree maturation. Food Chem. 131:1437–1442.
- Zhang, Z., X. Pang, D. Xuewu, Z. Ji, and Y. Jiang. 2005. Role of peroxidase in anthocyanin degradation in litchi fruit pericarp. Food Chem. 90:47–52.