ABSTRACT
Despite their economic importance, there is paucity of knowledge on fruit fly host status in Uganda. Therefore, this study set out to profile the host range of the main fruit fly pests and determine the susceptibility of selected fruits and mango cultivars across three main mango agro ecological zones, which included: Western Medium High Altitude Farmlands (WMHF), Lake Victoria Crescent (LVC), and the Northern Moist Farmlands (NMF) in Uganda. A wide range of fruits was sampled across the three zones. These were incubated at the National Agricultural Research Laboratories following standard protocols. Emerging fruit fly species were identified using standard keys and counted. Among the sampled fruits, 633 (35.0%) individual fruits from 15 plant families were positive for fruit fly infestation. Bactrocera invadens dominated (76.3%) of the positive samples, while infestation by native species, such as Ceratitis capitata and Ceratitis cosyra, was negligible. Annonaceae, Solanaceae, Rutaceae, and Anacardiaceae plant families recorded significantly more host species, while the number of pest fruit flies (species richness) per plant species followed a similar trend: Solanaceae > Rutaceae > Anacardiaceae. There was significant (P < 0.0001) variability in infestation among mango fruit cultivars, both within and across zones. When all zones were pooled together, Tommy Atkins and Kent, and Keitt, Kate, and Biire were the least and most infested, respectively. In conclusion, fruit flies have a diverse range of commercial and non-commercial hosts in Uganda. Strategies for fruit fly pest eradication in the country should ensure elimination or management of alternative fruit hosts and integration of tolerant mango cultivars in fruit development programs.
Introduction
Driven by rising incomes and the expansion of the middle class, global trade in fruit and vegetables has experienced tremendous growth in the last three decades (UNCTAD, Citation2014). The industry accounted for US$56.1 billion at the onset of the 21st century, but, by 2008, this had risen to US$139.6 billion (UNComtrade, Citation2011). Among the traded fruits, mango (Mangifera indica L., Anacardiaceae) experienced one of the highest increases in production and trade. Global production of mango has doubled in 30 years to around 35 million tonnes (Mt) (Evans, Citation2008) with Asia leading with 77% of the global production, followed by the Americas (13%) and Africa (10%) (FAO, Citation2012). International mango trade has also grown tremendously, with the United States (330,000 tonnes), Europe (225,000 tonnes), and Japan (10,000 tonnes) being the leading importers (UNCTAD, Citation2014). Consequently, there has been a rising interest by developing countries to actively participate in this trade to exploit the existing potential in the horticultural industry (Ekesi et al., Citation2006; Lux et al., Citation2003).
Unfortunately, fruit flies cause heavy losses on mango and other fruits and thus threaten to erase the benefits of the industry especially among developing countries (Ekesi et al., Citation2006; Mwatawala et al., Citation2009a; Rwomushana et al., Citation2008; UNCTAD, Citation2014). Consequently, the losses are accorded different economic status in different farming systems in the world.
These losses caused by fruit flies vary according to region, fruit species, fruit cultivars, and pest species. For instance, Lux et al. (Citation2003) reports losses of up to 40% in mango in East Africa, but losses have also been reported to be as high as 50% in Benin depending on season (Vayssières et al., Citation2009). Previous studies have, however, reported significant variation in levels of susceptibility among mango cultivars in Ghana (Ambele et al., Citation2012). It is possible that this may be the case among cultivars grown in Uganda. Due to differences in severity of damage, some studies have accorded fruit flies different economic status in different farming systems in the world (Mwatawala et al., Citation2009a). Thus, knowledge of their abundance in different systems could influence the choice of management strategies for their control.
Current fruit exports in Uganda declined from 3061 tonnes (1,158,000 US$) in 2005, to a mere 2166 tonnes (722,000 US$) in 2010 (http://www.uganda exportsonline.com). This has been attributed to damage by the fruit fly Bactrocera invadens. All this notwithstanding, there is paucity of country specific knowledge on fruit fly host status in Uganda. Previous efforts have been mainly at a regional or continent level (Copeland et al., Citation2002; De Meyer et al., Citation2002), or country specific (Mwatawala et al., Citation2009a; Rwomushana et al., Citation2008). As a result, these studies mostly fall short of giving any information on host range of the fruit flies particular to Uganda. Previous studies in Uganda, such as those by Nakasinga (Citation2002) were done before the invasion of B. invadens in the country, while others have concentrated on detection of specific species across the country (Nemeye, Citation2005; Okullokwany, Citation2006).
National economic programs, such as the National Agricultural Advisory Services (NAADS) and National Agricultural Research Organization (NARO) have introduced improved mango cultivars to diversify the country’s export base and promote non-traditional export crops in Uganda. However, the relative susceptibility of the different mango cultivars to fruit flies has not been studied. Knowledge about the different potential hosts and their relative utilization in a given area and the host partitioning between the different competitive fruit fly pests is crucial in development of a sustainable IPM program (Mwatawala et al., Citation2009a). For instance, Rwomushana et al. (Citation2008) and Mwatawala et al., (Citation2009a) showed mango and tropical almonds to be the most preferred hosts of B. invadens in Kenya and Tanzania, respectively. However, for Uganda, this information is scanty. Therefore, the specific objectives of this study were to profile the host range of the main fruit fly pests in the three main mango agro ecological zones and determine the susceptibility of selected fruits and mango cultivars grown to the various fruit fly pests in the country.
Materials and methods
Study area
The study was conducted in three agro ecological zones: Western Medium High Altitude Farmlands (WMHF), Lake Victoria Crescent (LVC), and Northern Moist Farmlands (NMF) (Wortman and Eledu, Citation1999). The three zones represent the major fruit growing regions in Uganda (). LVC encompasses areas along Lake Victoria that range in altitude from 1100 to 1400 meters above sea-level (masl). Temperatures are relatively stable and range between 18°C to 28°C. Rainfall is bimodal (rainy seasons in March–May and October–December), is evenly distributed and ranges between 700–2100 mm annually. Banana and coffee are the main crops that are often intercropped with annual and biennial root crops (sweet potato, cassava), vegetables, and fruits. Crops are commonly grown as polycultures on plots less than one hectare.
Figure 1. The location of the three Agro ecological zones in Uganda and the sampling sites. Major sites are areas where intensive systematic random collection of fruit hosts was undertaken, while minor sites are areas where selected important fruits were sampled randomly over the period of 2 years.
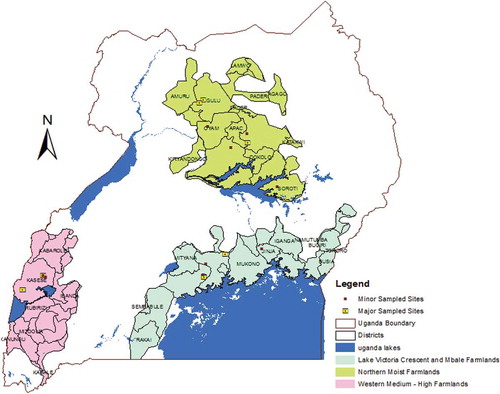
NMF are located in northern Uganda between latitudes 32.082 and 33.353. The zone ranges in altitude between 1000–1524 masl, with a temperature range of 15–32.5°C. Rainfall is largely unimodal (rainy seasons in April to September) and ranges between 700–1700 mm annually. Annual crops, mainly cotton and cereals, dominate the area and cassava is usually grown as monocultures. Many of the domesticated fruit fly host species present in the other two zones are not cultivated or are less frequent in the NMF. Where orchards and areas of substantial fruit growing is done, the areas are not surrounded by reasonable fruit habitats that would serve as important refuges and source areas for alternative hosts for fruit flies during fruit off season.
Finally, the WMHF are located in western Uganda along the Congo border, with an average altitude of 1235 masl, and an altitude range of 600–4500 masl. Rainfall in the zone is bimodal (rainy seasons in March–May and August–November) with some parts of the area, especially in the mountain areas, receiving more than 2250 mm as mean annual rainfall while the low lands receive about 1200 mm as mean annual rainfall. The zonal level mean temperature is 22.5°C. The main crops grown are bananas, coffee, beans, maize, and a variety of vegetables, fruit, and root crops. Bananas, coffee, and cotton are the main commercial crops.
Sampling procedure
As indicated above, the three zones do not harbor similar proportions and types of fruits and, hence, it was not possible to compare all fruit types across the three zones. Further, the fruits did not mature at the same time of the year across three zones; hence, it was not possible to synchronize sample size and seasonality. Therefore, as recommended by Mwatawala et al. (Citation2009a), sampling consisted of two protocols over a period of 24 months (Sept. 2010–Sept. 2012). The first (major) focused on intensive random collection of commercial and non-commercial fruit hosts from the three zones at specified orchards (). The second (minor) sampling strategy, which also lasted 24 months, involved sampling of selected important fruits and mango cultivars in each zone at random sites. The number of fruits per sample and the number of samples collected and incubated in each of the protocols depended mainly on fruit availability and abundance during the season. All samples comprised either tender skinned mature fruits or tender skinned immature fruits (Mwatawala et al., Citation2009a).
The selected important fruits included sweet orange (Citrus sinensis (L.) Osbeck), tropical almonds (Terminalia catappa L.), avocado (Persea americana Miller, cultivar ‘local’), guava (Psidium guajava L., cultivar ‘common guava’), and mango (Mangifera indica L., cultivar ‘Mixed’). The mango cultivars included Apple Mango, Biire, Boribo, Dodo, Glen, Kagogwa, Kate, Keitt, Kent, Tommy Akins, Palvin, and Zillatte. The 12 cultivars were classified according to their maturity seasonality into early, mid, and late maturing cultivars (Ambele et al., Citation2012).
In each case, collected fruits were transported to the rearing unit at the National Agricultural Research Laboratories (NARL), where they were labeled, kept in individual rearing buckets, and provided with appropriate medium for pupation as recommended by Copeland et al. (Citation2002). Conditions at the rearing unit were kept at 28 ± 2°C and 58 ± 5% relative humidity. The teneral adults that emerged were carefully removed and handled following methods described by White and Elson-Harris (Citation1992).
Fruit fly species identification
To identify the emerging fruit fly species, different types of keys were used as recommended by Mwatawala et al., (Citation2009a). When in doubt, confirmation of the identification was done at Royal Museum of Central Africa under the guidance of Dr. De Meyer. Voucher specimens are kept in collections at the National Agricultural Research Laboratories, Uganda and Makerere University Zoology Museum, and the Royal Museum for Central Africa, Belgium.
Data analysis
To compare infestation rate among the different fruit species and cultivars, two parameters were determined: incidence and infestation rate. Incidence is the number of infested or ‘positive’ samples (= samples from which fruit flies emerged) in comparison to total number of samples per fruit species or variety (Mwatawala et al., Citation2009a). Infestation rate was calculated as the number of larvae or adult flies that emerged per Kg of fruit (Copeland et al., Citation2002; Cowley et al., Citation1992). All variables were tested for normality using Shapiro-Wilk and Anderson test, and the strongly skewed variables were transformed prior to analyses if necessary to meet the assumption of parametric tests. Percentage incidence (%) were arcsine-square-root (+0.5) transformed; while the number of larvae per Kg were log(log(x + 1)) transformed. Where log transformation of the raw data was not sufficient to improve data shape, an appropriate nonparametric test, such as Kruskal-Wallis, was applied. Differences in mango variety infestation across zones were tested with General Linear Model (GLM) analysis of variance (ANOVA). Where the GLM test indicated significant differences, post-hoc Tukey (HSD) test was used. Chi-square was used to test for differences in fruit fly incidence among fruits, mango cultivars, and zones. All of these analyses were done using PAST computer program (Hammer et al., Citation2001).
Results
Fruit fly host range and susceptibility
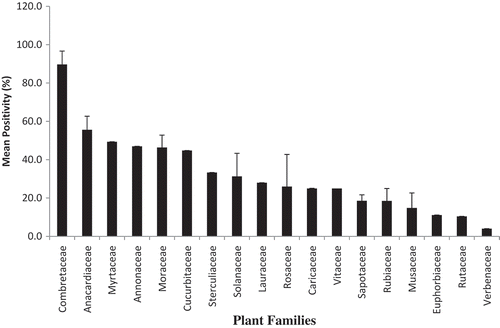
Over the sampling period, 1812 fruit samples, belonging to 38 fruit species, from 30 plant genera in 18 plant families were sampled (). Among these, 633 (35.0%) samples were positive for fruit fly infestation. The highest positivity (% infested fruits) were recorded in Combretaceae (89.7 ± 7%), followed by Anacardiaceae (55.6 ± 7%) and Myrtaceae (49.3 ± 0.01%). The least preference was recorded in Verbenceae, Rutaceae, and Euphorbiaceae at 4.0%, 10.5%, and 11.1%, respectively (). Anacardiaceae, Myrtaceae, Annonaceae, Caricaceae, Cucurbitaceae, Lauraceae, Moraceae, Rosaceae, Rubiaceae, Sapotaceae, Solanceae, and
Table 1. The range and abundance of fruits from the various host plant families, and the relative positivity (positive samples) of the various fruit hosts across the three agro ecological zones in Uganda. fruit fly richness indicates the number of fruit fly species that infested the plant family or species.
Figure 2. Diversity of plant species (plant species richness) per plant family, and the species richness (number of species) of fruit flies infesting/utilizing plant species from each plant family across the three agro ecological zones in Uganda.
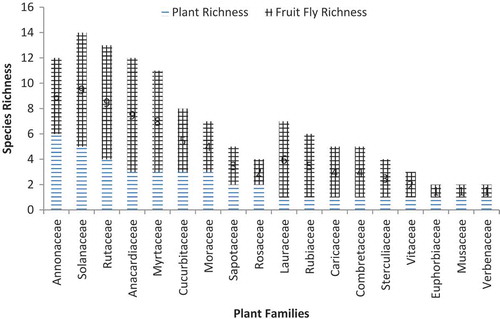
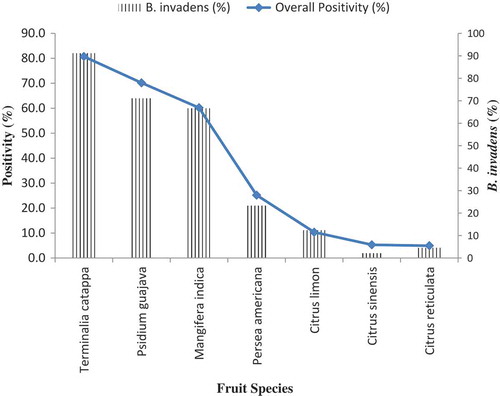
Figure 3. Proportion of fruit samples infested/utilized (% positivity) by fruit flies per plant family across the three major mango agro ecological zones in Uganda.
Of the seven selected fruit types, tropical almond showed significantly (P < 0.004) higher incidence (87.9%; ). Other major hosts that recorded high incidences are guava and mango, while citrus was the least infested. Persea americana (avocado) registered intermediate infestation, but was significantly different from the citrus cultivars. Among the emerging fruit flies, B. invadens was the dominant species recorded in 29 out of the 38 plant species, while out of the 633 positive samples, 483 (76.3%) were due to B. invadens (). The latter infested tropical almonds significantly (82.1%). Host fruit fly incidence for the rest of the fruit fly species ranged between 7.9% in T. coffeae to 65.8% in C. rosa, with relative positivity by the rest of the species ranking very low. The lowest and highest proportion was seven samples out of 633 (1.11%) for T. coffeae and 31 (4.9%) for C. capitata, respectively. All of the 29 plant species that B. invadens utilized were recorded in 15 plant families, ranking second behind C. rosa (16 families) in plant family resource diversity. Other species, with relatively more plant family resource diversity, included C. capitata (14) and C. anonae (10), while the least host spectrum species was T. coffeae (3).
Mango fruit host utilization
Mango fruit fly incidence and infestation intensity across the selected cultivars differed significantly across and within zones (). In the LVC, the highest incidence was recorded in Biire (44.7%) and Palvin (44.4%), while the least was in Tommy Atkins (13.5%), Zillate (22.6%), and Kent (19.5%). In terms of infestation intensity, the difference was significant too (F11, 1808 = 7.584, P = 0.0001), with Glen (92.4 larvae/ Kg) and Boribo (86.7 larvae/Kg) being the highest, and Tommy Atkins, Keitt, Zillate, and Kent the least infested cultivars. Post-hoc analysis showed that Biire was significantly (P < 0.000) more infested than all cultivars, with the exception of Glen, Boribo, and Palvin. Likewise, Glen was significantly different from Tommy Atkins (P < 0001), Kent (P < 0.003), Zillate (P < 0.018), and Kagogwa (P < 0.03). Boribo differed with Tommy Atkins (P < 0.006) and Kent (0.016), while Dodo was significantly more infested than Tommy Atkins (0.000) and Kent (P < 0.011). Similarly, Kate was significantly different from Tommy Atkins (P < 0.000) and Kent (P < 0.008), while Kagogwa was more infested than Tommy Atkins (P < 0.039).
Figure 5. Mean (±SD) proportion of mango cultivar samples infested by fruit flies (%) and number of larvae per Kg (infestation intensity) for the different cultivars in the three zones in Uganda: Northern Moist Farmland (NMF), Lake Victoria Crescent (LVC), and Western Moist High Farmlands (WMHF), and all three (All Zones) pooled. “Tommy” = ‘Tommy Atkins’.
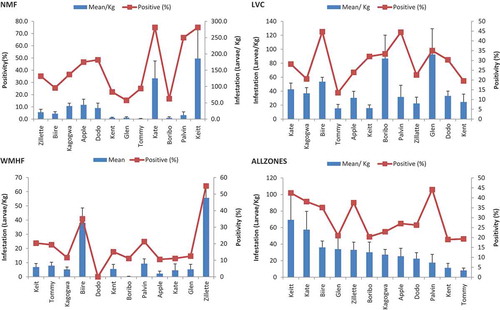
In the NMF, significant difference in mango cultivar fruit fly incidence was recorded too (). The highest incidence was recorded in Keitt and Kate (75% each), while the least was in Glen, Boribo, and Kent, with 15.4%, 16.7%, and 22.2%, respectively. There were significant differences in intensity (F11, 243 = 2.807, P < 0.0002) with Keitt (185.8 larvae/Kg) and Kate (125 larvae/Kg) being the highest, while the least infested cultivars were Tommy Atkins, Boribo, Kent, and Palvin. Post-hoc analysis showed that Keitt and Kate were significantly (P < 0.001) more infested than all cultivars, with the exception of Apple mango. The latter was significantly (P < 0.03) more infested than Tommy Atkins, Glen, and Kent, while Dodo was marginally (P < 0.046) more infested than Tommy Atkins and Glen. Similar to the LVC, Kagogwa was more infested than Tommy Atkins (P < 0.047), but in this case was also different from Glen (P < 0.048).
The highest incidence in WMHF was recorded on Zillate and Biire (57% and 44%, respectively), while the least was on Dodo (). In terms of infestation intensity, the difference was significant too (P < 0.0001), and similarly Zillate and Biire (48 and 38 larvae/Kg, respectively) being the highest, while the least infested cultivars were Dodo, Boribo, Apple, and Kagogwa. Post-hoc analysis showed that Zillate and Biire were not significantly different, but differed significantly (P < 0.05) with the rest of the cultivars. Mango fruit infestation was significantly higher in NMF and least in WMHF (P < 0.000).
The results further showed significant (F22 = 4.852, P < 0.0001) interaction between zones and variety infestation levels, with Glen and Boribo showing significantly higher infestation in LVC. Similarly, Kate (P < 0.048) and Keitt (P < 0.027) recorded significantly higher infestation in the NMF. No particular cultivar registered relatively higher infestation for the WMHF, as infestations in this area were generally low. Across all of the zones, the 12 mango cultivars all showed a relatively high (63%) vulnerability to infestation, as depicted by incidence and positivity. There were significant differences in mango variety susceptibility to infestation when data from all zones was pooled (F11, 720 = 11.215, P < 0.0001; ). The highest incidence was recorded in Zillate and Biire (55% and 35%, respectively), while the least was in Dodo, Apple, Boribo, Kate, and Kagogwa. A similar trend was followed for infestation intensity: Zillate and Biire (56 and 38 larvae/Kg, respectively), while the least was in Dodo, Apple, Boribo, Kate, and Kagogwa. Post-hoc analysis showed that Zillate was significantly more infested than Biire (P < 0.0001), while the latter two are significantly different in infestation from all the other cultivars (P < 0.001). The rest of the cultivars did not differ significantly.
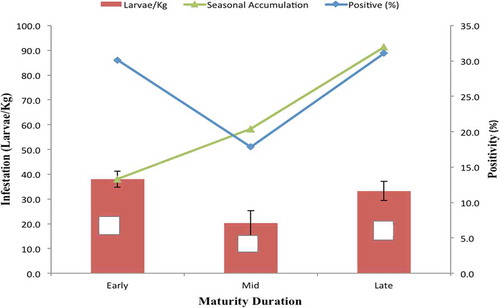
Mangoes that mature at different stages (early, mid-, and late) of the fruiting season showed significant difference in incidence (%) and total number of puparia recovered (infestation) (Kruskal-Wallis H′ = 15.72, P < 0.0003; ). Early maturing cultivars recorded the highest infestation (38.0 Larvae/Kg), while the mid season cultivars were the least infested. Late and Early season cultivars did not differ significantly (P < 0.5246), but the two were different from mid-season cultivars (P < 0.000) in incidence and infestation intensity. Further, as the season progressed, there was orchard level accumulation of incidence and intensity of fruit damage of mango fruits as the population of the fruit flies increased from the onset of the season ().
Discussion
Fruit fly host spectrum
This study has revealed a high fruit fly plant host range in Uganda. Among the fruit fly species recovered from the fruit samples, B. invadens was the most polyphagous species, infesting 29 hosts from 15 plant families. Mwatawala et al. (Citation2009a) reported B. invadens in 81.7% of all the positive samples, comparable to the 76.3% in this study. The results also agree entirely with previous studies about the increasingly negligible ecological role of the rest of the species in the ecosystem (Mwatawala et al., Citation2006, Citation2009a, Citation2009b; Rwomushana et al., Citation2008; Vayssières et al., Citation2009).
With a few exceptions, all of the major and minor fruit fly hosts encountered in this study (), have been documented elsewhere (Mwatawala et al., Citation2006a, Citation2009a, Citation2009b; Rwomushana et al., Citation2008; Vayssières et al., Citation2009). The study reports for the first time the infestation of B. invadens on T. catappa, A. toxicaria, E. uniflora, A. sellowiana, T. cacao, and C. oblonga in Uganda. Apart from T. catappa, whose association with B. invadens is widely appreciated, the rest of these new records had low frequency, ranging between one and five fruits. The study therefore recommends more confirmatory assessments, as presence of low numbers of larvae in fruit are not indicative of host acceptability (Aluja and Mangan, Citation2008).
Notable too, in this case, was the positive rearing from Musa spp. and C. arabica. These two records are unusual (but not unexpected), and will require further confirmation studies, as they are Uganda’s important staple and export crops, respectively. For coffee, similar findings by Mwatawala et al. (Citation2009a) have been reported, with up to 370 flies/ Kg of fruit infestation. The bananas (Musa spp.) from which the flies emerged in the study were mainly ripe, sometimes ruptured and close to rotting; factors that could have enhanced chances of fruit fly oviposition. It is common for fruit flies to oviposit in hosts that are inadequate for larval development, such as ruptured, tender, but not necessarily appropriate hosts. A high level of acceptance for oviposition for such punctured or intact poor hosts does not necessarily imply suitability of the infested fruit for further larval development or adult emergency (Rengifo et al., Citation2011). Polyphagous species, such as B. invadens circumvent physical resistance to oviposition by utilizing punctures, holes, pecks and crevices (Aluja and Mangan, Citation2008), which could ultimately explain the Musa spp. records here.
The highly polyphagous nature of C. capitata (22 hosts) and B. invadens (29 hosts) recorded in this study could be explained by their ability to oviposit in a wide range of fruit crops (Aluja and Mangan, Citation2008). Globally, in the genus Ceratitis, C. capitata is regarded as the most polyphagous species (Copeland et al., Citation2002; White and Elson-Harris, Citation1992). Earlier on in Uganda, Nakasinga (Citation2002) showed C. capitata to be the most polyphagous species among many native Ceratitis members, while Copeland et al. (Citation2002) demonstrated its wide host spectrum in Kenya (51 indigenous hosts).
Terminalia catappa, the most infested and suitable resource for B. invadens in this study, has been reported in many other cases (Mwatawala et al., Citation2006, Citation2009a; Rwomushana et al., Citation2008). This potentially weedy plant is largely planted as ornamental, shade tree, for soil conservation, or as a fruit. The higher affinity for T. catappa by B. invadens has been related to the higher plant concentration of methyl eugenol, a phenylpropanoid compound found in over 200 plant species representing 32 families (Raghu, Citation2002). After ingesting the compound, males sequester breakdown products of methyl eugenol in the rectal gland and use these metabolites to synthesize a sex pheromone attractive to females (Raghu, Citation2002). Hence, the overwhelmingly high incidence (87.9%) and infestation by B. invadens (82.1%) in this study should not be surprising. However, the fruit fly management implication is that the plant should be of concern as a reservoir host for B. invadens.
Major commercial crops, such as mango (Anacardiaceae), avocado, and guava (Myrtaceae), showed a high infestation incidence for B. invadens, as was the case elsewhere (Mboyine et al., 2012; Mwatawala et al., Citation2006, Citation2009a, Citation2009b; Rwomushana et al., Citation2008; Vayssières et al., Citation2009). Among the commercial fruits recorded, oranges do not comprise a favorable fruit fly host in this study and elsewhere (Mwatawala et al., Citation2009a; Rwomushana et al., Citation2008).
The poor diversity of cucurbits reared in this study could have affected the study’s infestation records for this family and, hence, further efforts are recommended. C. rosa was the major infester for high altitude fruits, such as coffee and annona fruits (albeit based on few samples), as was the case by Mwatawala et al. (Citation2006). Despite the polyphagous nature of C. cosyra, constant displacement by B. invadens has mainly restricted its host range to the most favorable and abundant Anacardiaceae hosts, with single samples in five families (). The only two families with two hosts, Anacardiaceae and Annonaceae, are recognized major host plant families for C. cosyra, while mango and marula, are known to be important hosts for C. cosyra (Mwatawala et al., Citation2009a).
Mango cultivars
Mango cultivar infestation varied significantly within and across zones, corroborating earlier reports (Ambele et al., Citation2012; Vayssières et al., Citation2009). Fruit flies are resilient pests with typical ecological adaptiveness. They may differ in distribution patterns and host utilization preferences but most species can survive, with some degree of flexibility, in a wide range of habitats of varying environmental conditions. Fruit flies can travel over short distances to feed and breed or they can disperse passively over long distances with the aid of wind currents. Since different zones offer these resources in varying proportions, in addition to the obvious ecological and climatic differences and farmer practices (e.g., cultivar choices and mixes), it is not surprising that zones ultimately differ in pest infestation.
Across the study zones, the factors that are varying and could explain the observed differences in variety susceptibility include: water or nutrient availability, wind exposure and amount of shade, or the presence of other herbivores. If a mango cultivar’s suitability were phenotypically plastic in response to each of these environmental variables, fruit fly host suitability in a given zone would be increased potentially by orders of magnitude. Variations in fruit cultivar infestation among the zones could therefore indicate that cultivars have specific adaptations in their ecological requirements. The bulk of mangoes consumed in Uganda are either the local cultivars (Kagogwa and Dodo) or the local selections (Kate and Biire). The perception that the exotic cultivars introduced in 1990s are generally the most susceptible to fruit flies has been partly disproved. Across the three zones, Biire and Kate were consistently among the most infested, while Kagogwa and Dodo showed intermediate infestation levels. Indeed, with all data pooled, the most infested cultivars were Keitt, Kate, and Biire, while the least were Tommy Atkins and Kent, both ‘exotics’.
Two possible hypotheses may explain the differences in B. invadens preference for ‘local’ selection cultivars (Kate and Biire) and in some cases Kagogwa over the ‘exotics’. First, most polyphagous species (like B. invadens) adapt quickly to a new host, with larval survivorship increasing linearly over 10 generations in a new host (Hawthorne, Citation1999). The relatively higher preference of the flies for the local selection and Kagogwa cultivars may, therefore, be a result of an increase in performance on these hosts. Second, Egas and Sabelis (Citation2001) have shown that experience or learning may affect host choice of phytophagous insects. B. invadens probably already adapted for living on these cultivars because of their predominant availability in the country over the ‘exotics’. Therefore, the countrywide distribution of local selections and Kagogwa cultivars might have favored adaptation, which may provide an explanation for their high preference.
Several studies have revealed that behavioral and ecological factors (biotic and abiotic) play an important role in host use. Pre-alighting factors (fruit color, host plant structure, shape, and size) and post-alighting factors (e.g., pericap toughness), should explain the study’s observed variations in infestation among cultivars. A conjugation of plant physical and chemical factors influences the choice and balance between positive and negative stimuli, might ultimately determine the final host selection. For instance, peel thickness and firmness (PTF), percent total acids and total soluble solids and developmental times were reported to be the main cause of mango fruit variety variation in fruit fly tolerance in Ghana (Ambele et al., Citation2012). The latter studies reported peel firmness and thickness, as important determinants for oviposition preference among fruit flies, with female tephritids having oviposition preference for fruits with softer pericarps.
Therefore, it is possible that B. invadens is able to discriminate the mango cultivars based on pre-alighting factors, such as colors and host colors, which play an important role on oviposition site selection (Katsoyannos and Kouloussis, Citation2001). Saha et al. (Citation1996) noted a very low response of adult B. cucurbitae for oviposition to yellow-, orange-, or white-colored egging substrates. It may, therefore, stand to reason that the bright color of Tommy Atkins enables it to partly escape fruit fly oviposition. Tommy Atkins mangoes, a mid-season cultivar, is green to purplish red and turns a bright yellowish/orange when ripe.
Mango losses associated with fruit fly infestation are relatively high in relation to the total fruit yields. Thus, understanding the extent of distribution and damage of important species (B. invadens) would provide important information required for the design of suitable management strategy. Even though this species is important in terms of losses associated with its infestations, little effort has been made in the past to reduce its population on an area-wide scale. Information on the ecological requirement that is reflected in geographic distribution and population dynamics along the seasons is a prerequisite in priority setting for fruit fly management. Findings from this study have particular relevance to fruit production in Uganda. Results here have revealed that fruit fly infestation builds during fruit growing season, with the highest damage to early and late cultivars, as demonstrated by Vayssières et al. (Citation2009).
The results also suggest variability in zonal infestation. Orchards in the WMHF recorded low infestation, while the NMF were relatively more infested. It is not clear cut whether the higher infestation in NMF is due to low investment by farmers in pest control, or harsh environmental conditions capable of reducing plant defense mechanisms resulting in higher mango fruit damage and yield losses in this area. It is, therefore, recommended that studies on knowledge, attitudes, and practices in the management of fruit flies be conducted to understand the observed differences.
Conclusion
Fruit flies have a diverse range of commercial and noncommercial or wild hosts in Uganda. The diverse range is also highly variable across the different zones in the country. This wide and variable spectrum of host range offers management implications, as no single management package can suffice all of the zones, hence calling for site-specific approaches. Tropical almonds and B. invadens were the most suitable host and dominant fruit fly species, respectively. Mango cultivars varied in their susceptibility to fruit fly infestation within and across zones, hence making definite conclusions of the most and least susceptible cultivars difficult. However, early and late season maturing mango cultivars were more susceptible. New fruit fly-host associations in Uganda were also recorded probably due to the adaptative evolution of polyphagous species in highly disturbed ecosystems. Further studies should improve the knowledge of the biology of native and other invasive Tephritid fruit flies in the different edapho-climatic conditions of Uganda.
Acknowledgments
We would like to thank Mayanja Robert, Kirunga Martin, Nampeera Florence, Waswa William, and Agnes Wenene for their help with practical and fieldwork, and the Mango farmers for their kind cooperation. We also acknowledge the support from the National Agricultural Research Laboratories. Dr. De Meyer (Royal Museum of Central Africa) and Maulid Mwatawala (Sokoine University of Agriculture) guided in the confirmation of species during the Training course in Taxonomy and Systematic of African fruit flies at the Museum.
Funding
We thank the Uganda National Council for Science and Technology, through the Millennium Science Initiative that funded Brian E. Isabirye’s PhD studies, for which this paper was a part.
Additional information
Funding
Literature cited
- Aluja, M. and R.L. Mangan. 2008. Fruit fly (Diptera: Tephritidae) host status determination: Critical conceptual, methodological and regulatory consideration. Annu. Rev. Entomol. 53:473–502.
- Ambele, F.C., M.K. Billah, K. Afreh-Nuamah, and D. Obeng-Ofori. 2012. Susceptibility of four mango cultivars to the Africa Invader fly, Bactrocera invadens Drew, Tsuruta and White (Diptera: Tephritidae) in Ghana. J. Appl. Biosci. 49:3425–3434.
- Copeland, R.S., R.A. Wharton, Q. Luke, and M. De Meyer. 2002. Indigenous hosts of Ceratitis capitata (Diptera: Tephritidae) in Kenya. Ann. Entomol. Soc. Amer. 95:672–694.
- Cowley, J.M., R.T. Baker, and D.S. Harte. 1992. Definition and determination of host status for multivoltine fruit fly (Diptera: Tephritidae) species. J. Econ. Entomol. 85:312–317.
- De Meyer, M., R.S. Copeland, S.A. Lux, M. Mansell, S. Quilici, R.A. Wharton, I.M. White, and N. Zenz. 2002. Annotated checklist of host plants for Afrotropical fruit flies (Diptera: Tephritidae) of the genus Ceratitis. Doc. R. Zoological Museum Central Africa, Tervuren 27:1–91.
- Egas, M., and M.W. Sabelis. 2001. Adaptive learning of host preference in a herbivorous arthropod. Ecol. Lett. 4:190–195.
- Ekesi, S, P.W. Nderitu, and I. Rwomushana. 2006. Field infestation, life history and demographic parameters of Bactrocera invadens Drew, Tsuruta and White, a new invasive fruit fly species in Africa. Bull. Entomol. Res. 96:379–386.
- Evans, E.A, 2008. Recent trends in world and U.S. mango production, trade and consumption. EDIS document FE718, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida, Gainesville, FL.
- FAO. 2012. The Food and Agriculture Organization database (FAOSTAT). Published on the internet: http://apps.fao.org.
- Hammer, Ø., D.A.T. Harper, and P.D. Ryan. 2001. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 4(1):1–9.
- Hawthorne, D.J. 1999. Physiological not behavioral adaptations of leafminers to a resistant host plant: A natural selection experiment. Environ. Entomol. 28:696–702.
- Katsoyannos, B.I., and N.A. Kouloussis. 2001. Captures of the olive fruit fly Bactrocera oleae on spheres of different colours. Entomol. Expt. et Appl. 100:165–172.
- Lux, S.A., S. Ekesi, S. Dimbi, S. Mohamed, and M.K. Billah. 2003. Mango infesting fruit flies in Africa—Perspectives and limitation of biological approaches to their management, p. 277–293. In: P. Neuenschwander, C. Borgemeister, and J. Langewald (eds.). Biological control in integrated pest management systems in Africa. CABI, Wellingford, UK.
- Mboyine, J.A., M.K. Billah, and K. Afreh-Nuamah. 2002. Species range of fruit flies associated with mango from three agro-ecological zones in Ghana. J. Appl. Biosci. 52:3696–3703.
- Mwatawala, M.W., M. De Meyer, R.H. Makundi, and A.P. Maerere. 2006. Biodiversity of fruit flies (Diptera, Tephritidae) in orchards in different agro-ecological zones of the Morogoro region, Tanzania. Fruits 61:321–332.
- Mwatawala, M.W., M. De Meyer, R.H. Makundi, and A.P. Maerere. 2009a. An overview of Bactrocera (Diptera: Tephritidae) invasions and their speculated dominancy over native fruit fly species in Tanzania. J. Entomol. 6:18–27.
- Mwatawala, M.W., M. De Meyer, R.H. Makundi, and A.P. Maerere. 2009b. Host range and distribution of fruit-infesting pestiferous fruit flies (Diptera, Tephritidae) in selected areas of Central Tanzania. Bull. Entomol. Res. 99(6):629–641.
- Nakasinga, J.K. 2002. Studies on fruit fly species occurrence, distribution and composition on mango in Uganda. MSc Thesis, Makerere University, Kampala-Uganda.
- Nemeye, P. 2005. Surveillance and Management of Bactrocera invadens in East Africa. Progress Report No. 2. 20 pp. Ministry of Agriculture Animal Industry and Fisheries (MAAIF), Kampala-Uganda.
- Okullokwany, F.S. 2006. Report on surveillance and management of Bactrocera invadens in East Africa—Phase II (Uganda). Ministry of Agriculture Animal Industry and Fisheries (MAAIF), Kampala-Uganda.
- Raghu, S. 2002. The autecology of Bactrocera cacuminata (Hering). Functional significance of resources. PhD Thesis, Griffith Univ., Queensland, Australia.
- Rengifo, J.A, J.G. Garcia, J.F. Rodriguez, and K.A.G. Wyckhuys. 2011. Host status of purple passion fruit for the Mediterranean fruit fly (Diptera: Tephritidae). CIAT, Cali, Colombia.
- Rwomushana, I., S. Ekesi, C. Ogol, and I. Gordon. 2008. Effect of temperature on development and survival of immature stages of Bactrocera invadens (Diptera: Tephritidae). J. Appl. Entomol. 132(9–10):832–839.
- Saha, A.K., R.M. Shahjahan, A.D. Bhuiya, and M.A. Wadud. 1996. Studies on the laboratory rearing of melon fly, Bactrocera cucurbitae (Coq.) (Diptera: Tephritidae) in different host and artificial diet. Bangladesh J. Entomol. 6:23–30.
- UNComtrade. 2011. Exports fruits and vegetables. United Nations Commodity Trade Statistics Database. Retrieved April 7, 2011, from http://comtrade.un.org/db/default.aspx.
- United Nations Conference on Trade and Development (UNCTAD). 2014. INFOCOM COMMODITY PROFILE-MANGO. Retrieved October 3, 2014, from http://www.unctad.info/en/Infocomm/AACP-Products/COMMODITY-PROFILE—Mango/
- Vayssières, J.F., S. Korie, and D. Ayegnon. 2009. Correlation of fruit fly (Diptera: Tephritidae) infestation of major mango cultivars in Borgou (Benin) with abiotic and biotic factors and assessment of damage. Crop Protec. 28(6):477–488.
- White, I.M., and M. Elson-Harris. 1992. Fruit flies of economic significance: Their identification and bionomics. CAB International, Wallingford UK, 601 p.
- Wortman, C.S., and C.A. Eledu. 1999. Uganda’s agroecological zones: A guide to planners and policy makers. Centro Internationale de Agricultural Tropical (CIAT), Kawanda, Uganda.