ABSTRACT
The effect of osmotic solutions consisting of sucrose and humectants (sorbitol and glucose syrup) on the quality of osmo-dried cantaloupe was investigated. The ratios between sucrose and each humectant were varied (90:10, 80:20, and 70:30). The increments in water loss and solid gain were observed with increasing the sorbitol ratio in the mixtures, while increasing the glucose syrup ratio in the mixture decreased water loss and solid gain during osmotic dehydration. All samples immersed in the osmotic solution containing sucrose and sorbitol had higher L* and lower a* values than those of samples immersed in the osmotic solution containing sucrose and glucose syrup. The hardness tended to decrease with increasing sorbitol and glucose syrup ratio in the osmotic solution. In addition, the increment of all humectants ratios caused a decrease in water activity (aw). The reduction in vitamin C, phenolic content, and 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity was observed with increasing sorbitol concentration in the osmotic solution. Moreover, an increase in all humectant ratios could reduce 5-hydroxymethylfurfural content in the final product. The sensory attributes, including color, texture, and overall acceptability, were found to be better in the case of sucrose-sorbitol treated samples when compared to those of the sucrose-glucose syrup treated samples.
Introduction
The cantaloupe has high amounts of functional substances, especially carotenoids and vitamin C, which act as an antioxidant, minerals, and some amino acids. However, cantaloupe melon is a highly perishable fruit with short periods of storage that are insufficient to allow, without the use of some technology, its commercialization in distant markets (Ismail et al., Citation2010; Solval et al., Citation2012). Thus, preservation techniques should be applied to extend the shelf life of cantaloupe. Osmotically dehydrated air-dried cantaloupe is of increasing interest as it becomes more commercially important as a dried fruit in many countries, including Thailand. Humectants can be used to reduce water activity (aw) and act as texture modifiers in fruits like cantaloupes. In addition, humectants, including polyols, sugars, and salts, are incorporated into food materials in order to reduce aw to levels prevailing in the intermediate moisture range (usually between 0.5 and 0.9) even in the presence of a considerable amount of water (Sritongtae et al., Citation2011). The use of polyhydric alcohols during osmosis has the potential to maintain the moisture in the product and improve the quality of the texture because polyhydric alcohols can bind and retain water. A common humectant used in food products is sorbitol. This humectant is a sugar alcohol derived from glucose. It is used in dietetic or sugarless foods and is lower in calories than sucrose, so it is a popular choice among those who are dieting.
Factors affecting the quality of osmosed cantaloupe were investigated by several researches, including osmotic dehydration process (Phisut et al., Citation2013), concentration of osmotic agent and processing temperature (Pereira et al., Citation2006), and type of osmotic agent (Naknean et al., Citation2013). Sritongtae et al. (Citation2011) studied the effect of polyols (sorbitol and glycerol) on drying of osmosed cantaloupe. Partial replacement of sucrose solution (traditional osmotic agent) with each polyol at 10% and 15% (w/v) was performed. The result showed that the use of polyols could reduce aw in the final product. Moreover, glucose syrup is another humectant food additive used to replace sugar. It is not only found in sweet foods, but frequently in other foods like salad dressings. Sorbitol and glucose syrup have been widely used in foods, syrups, medicines, and cosmetics. It has been added to ice cream to improve the texture. In addition, sorbitol and glucose syrup can be used in bakery products and candies as a softening agent. Naknean et al. (Citation2013) studied the effect of type of osmotic agents (sucrose, invert sugar, sorbitol, and maltitol) on the quality of osmo-dried cantaloupe. It was found that the use of sugar alcohol could also improve the texture, color, and sensory qualities of the osmo-dried cantaloupe. However, the partial replacement of sucrose with humectant in the osmotic solution has rarely been reported in osmo-dried cantaloupe. Based on such reports of the high potential of sorbitol and glucose syrup in various food products, the report is further researched to investigate the effect of sucrose and humectant mixtures as the osmotic solution on the qualities and nutritional compositions of osmo-dried cantaloupe.
Materials and methods
Materials
Cantaloupe (Cucumis melo L. cv. Sun Lady), at commercial maturity, with 10–11% total soluble solid as measured by refractive index, was purchased from a local wholesale market. Sucrose was purchased from Mitr Phol Sugar Corp., Supanburi, Thailand. Glucose syrup (DE 40) was purchased from Taweekit, LTD, Bangkok, Thailand. Sorbitol was obtained from Thai Food and Chemical LTD, Bangkok, Thailand.
Sample preparation
The fruits were washed, hand peeled, and cut into slices of approximately 3 × 3.5 × 1.5 cm. Cantaloupe slices were pretreated by soaking in a solution of 2% calcium lactate (firming agent) for 3 h. After pretreatment, cantaloupe slices were washed in water to remove any excess of calcium solution at ambient temperature and then used for the osmotic dehydration process.
Osmotic dehydration process
The cantaloupe slices were transferred to a 50 °Brix solution for 24 h. In this 50 °Brix solution, the ratios of sucrose:sorbitol and sucrose:glucose syrup were varied from 100:0, 90:10, 80:20, and 70:30, which are named as 0, 10, 20, and 30% humectants, respectively. The sample immersed in the osmotic solution containing 0% of humectant was used as a control treatment. When the osmotic dehydration time reached 24 h, the excess solution on the surface was quickly removed by washing with water (50 °C). Thereafter, the slices were dried by using a hot air oven at 60 °C until their moisture content was below 18%.
To investigate the mass transfer of solutes and water between samples and osmotic solutions, water loss and solid gain were determined at 3-h intervals for 24 h and calculated using Eqs. (1) and (2), respectively (Shi et al., Citation1995):
where Mo is the initial mass of sample (g), Mt is the mass of osmosed sample at time t (g), Xso is the initial soluble solid content of sample (°Brix), Xst is the total soluble solid content of osmosed sample at time t (°Brix), Xwo is the initial water content of fruit sample (%, wet basis), and Xwt is the water content of osmosed sample at time t (%, wet basis).
Physical qualities measurement
Color measurement
The surface color on two sides of an individual piece (five pieces for each treatment) was measured by using a Hunter Lab colorimeter. A colorimeter (Colorflex, Hunterlab, Reston, VA, USA) with a port size of 1.0 inch was adjusted for reflectance, illuminant D 65, and angle of 10°. Instrumental color data was provided in accordance with the CIE system in terms of L* (lightness), a* (redness and greenness), and b* (yellowness and blueness).
Texture measurement
Texture measurement was carried out with a TAXT2i Texture Analyzer (Stable Micro Systems Ltd, Surrey, UK). The analyzer was linked to a computer that recorded data via a software program (Texture Expert, version 1.22; Stable Micro Systems, Surrey, UK). The hardness (N) of the products was evaluated using a knife blade probe. Test speed was set at 2 mm/s. The probe was programmed to cut through the samples at a distance of 17 mm. Ten measurements were performed on each sample to obtain the mean measurement for that sample (Phisut et al., Citation2013).
Chemical qualities analysis
Determination of pH and total acidity
The pH value was measured at ambient temperature with a pH meter (Satorious, Goettingen, Germany), which calibrated at pH 4.0 and 7.0. Total acidity was measured according to the procedure of Rangana (Citation1986) with a slight modification. Ten grams of sample were homogenized in 30 ml of distilled water and then made up to 50 ml. The homogenate was filtered and centrifuged at 5000 rpm for 10 min. After that, the supernatant was titrated with 0.01 N NaOH using 1% phenolphthalein solution as an indicator. The result was calculated as a percentage of citric acid and expressed in terms of dry basis (db).
Determination of moisture content and water activity
The moisture content was measured using a hot air oven. The sample (2–5 g) was placed in a pre-dried aluminum dish and dried in an oven at 110 °C for 6 h. The dried sample was placed in desiccators and cooled for 0.5 h to room temperature. The weight was recorded, and the percentage moisture based on the initial wet weight was calculated. Water activity was measured at room temperature using a water activity meter (Novasina, Thermostanter, Lachen, Switzerland). The sample was cut into tiny pieces and inserted into a sample cup and another water activity measurement was made immediately to restrict moisture transfer from the air to the samples.
Determination of total sugar and reducing sugar contents
The total sugar and reducing sugar contents were quantified by titration with Fehling’s reagents. The results were expressed as grams of glucose per 100 g of sample (db) (Rangana, Citation1986).
Determination of vitamin C content
Vitamin C was analyzed according to the method of Guimares et al. (Citation2010). The sample (450 mg) was extracted with metaphosphoric acid (1%, 30 ml) for 45 min at room temperature and then filtered through Whatman No. 4 filter paper. The filtrate (1 ml) was mixed with 2,6-dichloroindophenol (9 ml) and the absorbance was measured within 30 min at 515 nm against a blank. Quantification of vitamin C was carried out using the external standard method and expressed as mg of vitamin C per g of sample (db).
Determination of phenolic content
The phenolic content in each sample was evaluated according to the method described by Naknean et al. (Citation2013). The sample (10 g) was homogenized in 30 ml of distilled water and then made up to 50 ml. The homogenate was filtered and centrifuged at 5000 rpm for 10 min. The supernatant (0.5 ml) was mixed with 0.5 ml of distilled water. After that, 0.5 ml of Folin-Ciocalteu reagent (1:1 with water) and 2.5 ml of sodium carbonate solution (2%) were added. The mixture was mixed thoroughly and placed in the dark for 40 min and the absorbance was read at 725 nm. The total phenolic content was calculated from the standard curve of gallic acid and expressed as µg gallic acid per g dry sample after blank subtraction.
Determination of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical-scavenging activity
DPPH radical-scavenging activity was analyzed by DPPH assay, as described by Binsan et al. (Citation2008) with a slight modification. The sample (10 g) was homogenized in 30 ml of distilled water and then made up to 50 ml. The homogenate was filtered and centrifuged at 5000 rpm for 10 min. The supernatant was used to measure DPPH radical-scavenging activity. The sample (1.5 ml) was mixed with 1.5 ml of 0.15 mM DPPH in methanol. The mixture was allowed to stand at room temperature in the dark for 60 min. Thereafter, the absorbance was recorded at 517 nm using a spectrophotometer. The blank was prepared in the same manner, except that distilled water was used instead of the sample. L-ascorbic acid was used as the external standard. The activity was expressed as μg ascorbic acid equivalents per gram dry sample.
Determination of 5-hydroxymethylfurfural (HMF) content
HMF content was carried out according to the procedure of Rattanathanlerk et al. (Citation2005) with some modifications. Briefly, the sample (10 g) was homogenized in 30 ml of distilled water and then made up to 50 ml. The homogenate was filtered and centrifuged at 5000 rpm for 10 min. The supernatant was used to measure HMF content. Next, 2 ml of supernatant was added into the tube; then 2 ml of 12% trichloroacetic acid and 2 ml of 0.025 M thiobabituric acid were subsequently mixed thoroughly and then placed in a water bath at 40 °C for 50 min. After that, the tube was cooled immediately, using water. Readings were recorded at 443 nm by using a spectrophotometer. HMF (Sigma-Aldrich, St. Louis, MO, USA) was used to perform the calibration curves and the content of HMF was reported in terms of dry sample.
Sensory evaluation
Acceptance testing was used to measure magnitude of like/dislike for the final product. Each osmo-dried cantaloupe sample was evaluated by 50 non-trained panelists. It was based on a 9-point hedonic scale for overall liking, where 9 = like extremely and 1 = dislike extremely. Sensory attributes, including color, appearance, flavor, texture, and overall acceptability, were determined. Samples were randomly coded with three-digit numbers and their order of presentation was completely randomized for each panelist.
Statistical analysis
The experiment was done in two batches and the measurement was carried out in at least duplicates per each batch. The mean ± SD values were reported based on at least four measurements obtained from two batches (at least two measurements from each batch). The experimental design was a completely randomized design (CRD). The experimental design for sensory evaluation was a randomized complete block design (RCBD). Data was subjected to analysis of variance (ANOVA). Comparison of means was carried out by Duncan’s multiple-range test.
Results and discussion
Effect of osmotic solutions on water loss and solid gain during osmotic dehydration
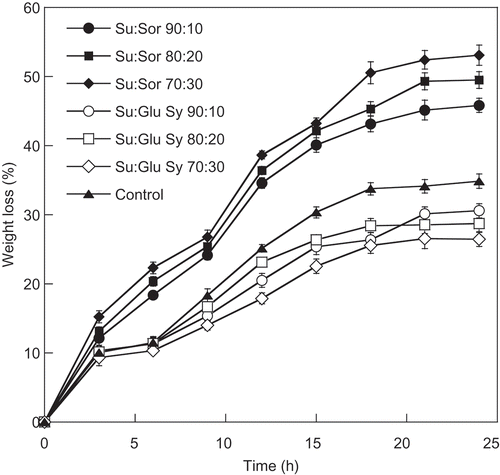
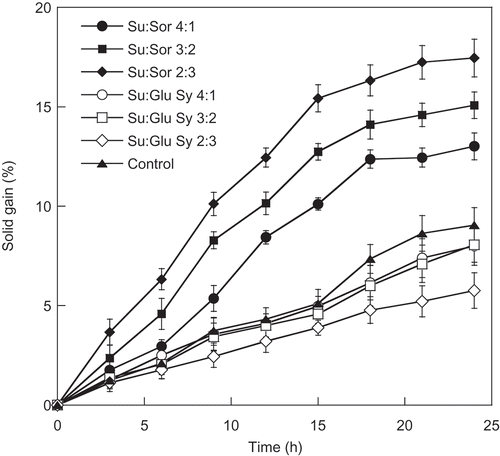
Initially, the moisture content and total soluble solid of fresh cantaloupe were 90.11% and 10.50 °Brix, respectively. During the osmotic dehydration of cantaloupe slices using the various osmotic solutions, water loss and solid gain were monitored, as presented in and . It was found that the immersion of cantaloupe slices in different osmotic solutions (50 °Brix) for 24 h had a significant (P ≤ 0.05) effect on solid gain and water loss during osmotic treatment. Water loss and solid gain of all treatments increased gradually as the osmosis dehydration time increased up to 21 h (P ≤ 0.05). Thereafter, no change in water loss and solid gain of all treatments was observed (P > 0.05). The highest solid gain and water loss were observed in the sample immersed in the osmotic solution containing 70:30 ratio of sucrose to sorbitol. The result indicated that the use of sorbitol could enhance an increase in solid gain and water loss, suggesting that sorbitol has a lower molecular weight than sucrose and glucose syrup. Increasing the amount of low molecular weight osmotic agent, such as sorbitol, increased the osmotic pressure gradient and thereby increased the water loss. Sritongtae et al. (Citation2011) reported that increasing concentration of sorbitol led to an increase in solid gain during osmotic dehydration of cantaloupe. Naknean et al. (Citation2013) revealed that higher solid gain and water loss were observed in the sorbitol-treated sample compared to those of the sucrose-treated sample. In addition, a similar result observed the osmotic dehydration of mandarin that used a mixture of glycerol and sucrose as the osmotic agent (Pattanapa et al., Citation2010). Sorbitol is the result of hydrogenation of dextrose changing the aldehyde group to an additional hydroxyl group; hence, it can be named as sugar alcohol. Molecular weight of sorbitol (C6H14O6) is smaller than sucrose (C12H22O11). Sucrose has α-glucose and fructose, joined by a glycosidic bond. The utilization of a low molecular weight osmotic agent could promote an increase in osmotic pressure, leading to a higher penetration rate of solid into fruit cell as mentioned previously (Chauhan et al., Citation2011; Naknean, Citation2012; Ozen et al., Citation2002; Pattanapa et al., Citation2010). However, the lowest solid gain and water loss was observed in the sample immersed in the osmotic solution containing sucrose and glucose syrup. An increase in the amount of glucose syrup in the sucrose-glucose syrup mixture from 90:10 to 70:30 (sucrose/glucose syrup) affected water loss and solid gain (P ≤ 0.05). The increment of glucose syrup ratio in the mixtures caused a decrease in water loss and solid gain. The fact is that the glucose syrup solution had higher viscosity and molecular weight than sucrose and sorbitol. Sritongtae et al. (Citation2011) reported that a high viscosity of osmotic solution might retard the penetration of solute into the cell, limiting the solute uptake. El-Aouar et al. (Citation2006) studied the influence of the osmotic agents (sucrose and corn syrup solid) on mass transfer during osmotic dehydration of papaya. It was found that the sample dehydrated in sucrose solution had values of solid gain and water loss higher than that obtained from the samples processed in corn syrup solution. In addition, the effects of osmotic agents (fructo-oligosaccharide and sucrose) in osmotic dehydration of apple cubes were reported by Matusek et al. (Citation2008). It was found that the solid gain in the case of fructo-oligosaccharide was less than half of the solid gain in the case of sucrose. This was probably due to fructo-oligosaccharide having a higher molecular weight and viscosity than sucrose, resulting in a lower rate of diffusion.
Effect of osmotic solutions on the physical qualities of osmo-dried cantaloupe
The color of osmo-dried cantaloupe was measured as expressed in . The result showed that the immersion of cantaloupe slices in different sucrose-humectant solutions had a significant effect on L* and a* values (P ≤ 0.05). However, no significant difference in b* value was found among the samples (P > 0.05). The color characteristics of fresh cantaloupe using L*, a*, and b* values were 61.47, 8.63, and 17.52, respectively. After the drying process, the decrease in L* value and increase in a* value were observed in all treatments. This result might be due to the increment in concentration of carotenoids that resulted from water loss. Heredia et al. (Citation2009) reported that water loss could increase the concentration of β-carotene and lycopene in cherry tomato. Additionally, Pereira et al. (Citation2006) reported that pigment was concentrated during osmotic dehydration of melon. In addition, a decrease in L* values and an increase in a* values could be a result of browning reactions occurring during hot-air drying. After the drying process, L* values were observed to be the highest in the case of sucrose-sorbitol-treated samples, followed by the case of sucrose-glucose syrup-treated samples and the lowest in the case of the control treatment. The lowest a* value was observed in the samples immersed in sucrose-sorbitol mixtures, while the highest a* value was found in the control treatment. Decreasing the sucrose/glucose syrup ratio from 90:10 to 70:30 and sucrose/sorbitol ratio from 90:10 to 80:20 did not result in a significant difference in the L* and a* value, while an increase in the amount of sorbitol in the sucrose-sorbitol mixture from 80:20 to 70:30 (sucrose/sorbitol) affected L* and a* values. The highest L* value and lowest a* value was found when the osmotic solution of 70:30 (sucrose/sorbitol) was used. Generally, L* value decreases while a* value increases during browning reactions. Thus, the development of brown color may contribute to the non-enzymatic browning reactions as mentioned previously. The Maillard reaction is mainly responsible for browning development in osmo-dried cantaloupe during the drying process as discussed earlier. The Maillard reaction that occurs between reducing sugars and compounds containing a free amino group is important for food processing because it leads to the development of brown color, which affects flavor, color, and nutritional quality. For the control treatment, only sucrose, non-reducing sugar, was used as an osmotic solution for dewatering cantaloupe. However, reducing sugars (fructose and glucose) formed by sucrose inversion during the drying process could participate in Maillard reaction (Pattanapa et al., Citation2010). Thus, the highest browning development (highest a* value and lowest L* value) was observed in the control treatment. The use of sorbitol and glucose syrup in the osmotic solution could reduce brown color in osmo-dried cantaloupe as evidenced by higher L* value and lower a* value when compared to the control treatment. Naknean et al. (Citation2013) reported that lower browning development was found in the sorbitol-treated sample compared to the sucrose-treated sample. However, the samples immersed in the osmotic solution containing sucrose and glucose syrup became darker than those immersed in the osmotic solution containing sucrose and sorbitol. This can be related to the fact that sugar alcohols do not undergo the Maillard reaction (Lin et al., Citation2003), while polydextrose, such as glucose syrup, is able to promote browning reactions as a result of thermal degradation, resulting in higher browning development being observed in the sample immersed in the osmotic solution containing sucrose and glucose syrup.
Table 1. Physical quality of osmo-dried cantaloupe.
The effect of the sucrose-humectants ratios on the hardness of the osmo-dried cantaloupe was investigated as presented in . An increase in the amount of glucose syrup in the sucrose-glucose syrup mixture from 90:10 to 70:30 (sucrose to glucose syrup) caused a decrease in the hardness of osmo-dried cantaloupe. The lowest hardness was observed when the osmotic solution of 80:20 and 70:30 (sucrose to glucose syrup) was used. It indicated that glucose syrup could help to keep the product moister but with a lower water activity. Additionally, the sample immersed in the osmotic solution containing sucrose and sorbitol presented lower hardness than the control treatment. An increase in the amount of sorbitol in the sucrose-sorbitol mixture from 90:10 to 70:30 (sucrose/sorbitol) caused a decrease in the hardness of osmo-dried cantaloupe. Sorbitol also acts as a humectant that absorbed water to its molecule in terms of bound form, thus aw was reduced while a sample was still kept moist (Naknean et al., Citation2013). Therefore, the increased addition of humectant in the osmotic solution reduced the hardness of the osmo-dried cantaloupe. Naknean et al. (Citation2013) have reported that lower hardness was observed in the sorbitol-treated sample compared to those of the sucrose-treated sample. Ronda et al. (Citation2005) found that the use of polyols, such as sorbitol, could reduce the hardness of sponge cake. Moreover, the reduction in hardness was found in osmotically dehydrated mandarin that immersed in a mixture of glycerol and sucrose as the osmotic agent (Pattanapa et al., Citation2010). In addition, the moisture content in the final product might affect the hardness. The lowest moisture content detected in the control sample was another factor that was responsible for the highest hardness found in the control sample.
Effect of osmotic solutions on the chemical qualities of osmo-dried cantaloupe
The chemical properties and nutritional compositions of osmo-dried cantaloupe were shown in and . The pH and total acidity of fresh cantaloupe were 6.33 and 0.13%, respectively. An increase in total acidity and a decrease in pH were found in all samples after the drying process. This was due to an increased concentration of organic acid that resulted from water loss. In addition, no difference in pH and total acidity was observed in all treatments.
Table 2. Chemical quality of osmo-dried cantaloupe.
Table 3. Nutritional compositions of osmo-dried cantaloupe.
Water activity (aw) is a measure of the water available for microbial growth. Water activity is not necessarily linked to water content. Two products with similar water content can have different aw and, therefore, may have different spoilage rates. Both moisture content and aw are highly important for the shelf life of osmo-dried cantaloupe during storage. No difference in moisture content was found in all treatments. Lower aw was observed in all samples immersed in the osmotic solution containing sucrose and humectants when compared to the control treatment. Wangcharoen (Citation2013) also revealed that the reduction in aw was also observed in longan aril that was immersed in the concentrated sucrose solution. According to the results, increasing the amount of humectants (sorbitol or glucose syrup) in the mixtures could reduce aw of the osmo-dried cantaloupe. The samples immersed in either 70:30 (sucrose/sorbitol) or 70:30 (sucrose/glucose syrup) solutions had the lowest aw. Glucose syrup is a polydextrose obtained from starch hydrolysis. There are many –OH groups in a structure of glucose syrup that can interact with water molecules via hydrogen bond, resulting in the increment of bound water in osmo-dried cantaloupe. Moreover, glucose syrup acts as a humectant that has a property of absorbing water as mentioned previously. Sorbitol has six hydroxyl groups that can form hydrogen bonds with water in osmo-dried cantaloupe. Thus, the use of humectant in the osmotic dehydration process could enhance the shelf life of osmo-dried cantaloupe slices by reducing aw. This result was in agreement with the work of Korsrilabut et al. (Citation2010) and Sritongtae et al. (Citation2011).
Initial total sugar and reducing sugar content of fresh cantaloupe were 5.42% and 11.34%, respectively. An increase in reducing sugar and total sugar content was found in all samples after the drying process. This indicated that the osmotic agent penetrated into the cell of fruit during osmotic dehydration, resulting in the increment of total sugar content. Additionally, an increase in reducing sugar was due to the hydrolysis of sucrose. The highest reducing sugar content was found in the control treatment, followed by the sample immersed in the osmotic solution containing sucrose and glucose syrup. This could be attributed to the control treatment containing the highest sucrose content, resulting in the promotion of inversion reaction. Moreover, glucose syrup contains glucose, maltose, maltotriose, and higher molecular mass carbohydrates. During the drying process, the hydrolysis of maltose and maltotriose could take place. The lowest reducing sugar content was found in the sample immersed in the osmotic solution containing sucrose and sorbitol (70:30). An increase in the amount of sorbitol in the sucrose-sorbitol mixture from 90:10 to 70:30 (sucrose/sorbitol) could reduce the reducing sugar content in the product. This was due to the ratio of sucrose reduced by the addition of sorbitol, resulting in the reduction of substrate for participating in the inversion reaction. The highest total sugar content was found in the control treatment, while the sample immersed in the osmotic solution containing sucrose and sorbitol (70:30) presented the lowest total sugar content. Thus, an increase in the amount of sorbitol in the sucrose-sorbitol mixture could reduce the total sugar content in the product.
In the acid medium of this product, the dehydration of carbohydrates, especially hexose, causes the formation of HMF. Additionally, the Maillard reaction can also take place, giving rise to Amadori compounds during the first step of the reaction, and HMF as a consequence of further reaction. HMF could be used as an indicator of heat stress or overheating during the thermal process for sugar-based foods because of its toxicological status. The highest HMF content was detected in the control treatment. This could be explained by the highest Maillard reaction taking place in the control treatment. The lowest HMF content was observed in the sample immersed in the osmotic solution containing sucrose and sorbitol. An increase in the amount of sorbitol in the sucrose-sorbitol mixture from 90:10 to 70:30 (sucrose/sorbitol) was not affected the HMF content (P > 0.05). This is probably due to the fact that sorbitol cannot undergo the Maillard reaction as mentioned previously. Naknean et al. (Citation2013) also revealed that the use of polyol as the osmotic agent could reduce MHF content in osmo-dried cantaloupe.
Vitamin C, also known as ascorbic acid, is a water-soluble vitamin. The amount of vitamin C in the osmo-dried cantaloupe was lower than in fresh cantaloupes (50.38 mg per 100 g for fresh sample). This effect could be attributed to the leaching of vitamin C during osmotic dehydration (Brochier et al., Citation2015). Azoubel et al. (Citation2009) also reported that the loss of vitamin C was detected during osmotic dehydration of cashew apple. In addition, vitamin C is the least stable of all vitamins and is easily destroyed during processing. The lowest vitamin C content was found in the sample immersed in the osmotic solution containing 70:30 ratio of sucrose to sorbitol while an increase in the amount of sorbitol in the sucrose-sorbitol mixture from 90:10 to 80:20 (sucrose/sorbitol) did not affect the vitamin C content (). Results suggested that increasing the water loss caused an increased leaching of vitamin C during the osmotic dehydration process. However, an increase in the amount of glucose syrup in the sucrose-glucose syrup mixture from 90:10 to 70:30 (sucrose/glucose syrup) was not affected by the vitamin C content (P > 0.05).
The phenolic compounds of all osmo-dried cantaloupes were analyzed as shown in . The phenolic content of fresh cantaloupe was 1023 µg GAE/g sample as reported by Phisut et al. (Citation2013). Different osmotic solutions induced loss of phenolic compounds by migration into the osmotic solution. An increase in the amount of sorbitol in the sucrose-sorbitol mixture from 90:10 to 70:30 (sucrose/sorbitol) affected the phenolic content of osmo-dried cantaloupe. Phenolic content tended to decrease with increasing sorbitol concentration in the osmotic solution (P ≤ 0.05), while the increment of glucose syrup concentration in the osmotic solution was not affected the phenolic content (P > 0.05). This could be attributed to the fact that small phenolic molecules may diffuse into the osmotic solution. Increasing the water loss resulted in an increased leaching of solutes, such as acids, water soluble vitamin, and small phenolic molecules, into the osmotic solution (Devic et al., Citation2010).
DPPH• assay is a reliable method to determine the antioxidant capacity of biological substrates. DPPH radical scavenging activity of osmo-dried cantaloupes was also investigated as presented in . An increase in the amount of sorbitol in the sucrose-sorbitol mixture from 90:10 to 80:20 (sucrose/sorbitol) was affected by DPPH radical scavenging activity of osmo-dried cantaloupe. DPPH radical scavenging activity tended to decrease with increasing sorbitol concentration in the osmotic solution (P ≤ 0.05), while the increment of glucose syrup concentration caused an increase in DPPH radical scavenging activity (P ≤ 0.05). Polyphenol compounds found in cantaloupe are a type of antioxidant containing a polyphenolic or natural phenol substructure. In addition, the Maillard reaction products might be also responsible for its powerful antioxidant capacity. The use of high concentration of sorbitol might promote a high leaching of soluble antioxidant components during the osmotic dehydration process, resulting in the reduction of DPPH radical scavenging activity. On the other hand, increasing glucose syrup in the osmotic solution decreased water loss, leading to the reduction in leaching of antioxidant components into the osmotic solution.
According to the result, it was hypothesized that the main factor affecting the loss of soluble components from the fruit cell during osmotic dehydration was water loss. The utilization of low molecular weight osmotic agent enhanced the water loss during osmotic dehydration, resulting in high leaching of soluble nutritional components as mentioned previously. The loss of soluble components, such as hydrophilic phenolic compounds and vitamin C, was also previously reported by several researchers (Stojanovic and Silva, Citation2007; Nadia et al., Citation2013; Azoubel et al., Citation2009; Santos and Silva, Citation2008). They reported that all mentioned soluble compounds could migrate from the fruit sample to the osmotic solution, resulting in a decrease in polyphenol and vitamin C contents. In addition, they also revealed that the leaching of phenolic compounds and vitamin C was promoted by the factors that enhanced the loss of water during osmotic dehydration. This could explain the loss of soluble components, particularly hydrophilic phenolic and vitamin C, which is highly soluble in water by leaching with water. On the other hand, the application of glucose syrup in the osmotic solution did not affect the loss of vitamin C and phenolic compounds. Santos and Silva (Citation2008) reported that the viscosity of osmotic solution might be responsible for this phenomenon. More viscous osmotic solution might limit soluble components transfer. The other reason was based on the sugar concentrated layer formed at the periphery of the sample. This layer may act as a barrier to the soluble components transfer, resulting in a higher retention of it on the final product. Additionally, the phenolic and vitamin C contents may be lost during the drying process, however, a similar drying temperature was applied for all treatments. Thus, the loss of phenolic content caused by leaching during soaking in the osmotic solution might be mainly responsible for lower phenolic and vitamin C contents in the dried product. Moreover, a positive correlation between nutritional components, especially phenolic compounds and vitamin C, and antioxidant properties is normally found. Therefore, low vitamin C and phenolic content in the final product resulted in a reduction in the antioxidant activity as evaluated by DPPH radical scavenging activity.
Effect of osmotic solutions on the sensory qualities of osmo-dried cantaloupe
The sensory evaluation of osmo-dried cantaloupe was shown in . It can be seen that the use of humectant in the osmotic dehydration process of cantaloupe affected the color, texture, and overall acceptability (P ≤ 0.05). No difference in appearance and flavor scores was observed in all treatments (P > 0.05). Higher mean scores of color, texture, and overall acceptability were observed in all samples immersed in the osmotic solution containing sucrose and humectants (glucose syrup and sorbitol) when compared to the control treatment. This was due to the fact that use of humectant in the osmotic solution could reduce brown color and hard texture of osmo-dried cantaloupe. The result of sensory evaluation was in agreement with the result of color and hardness. Moreover, no difference in mean score of all sensory attributes was found in the samples that immersed in sucrose-glucose syrup mixture and sucrose-sorbitol mixture.
Table 4. Acceptance scores on the different attributes of osmo-dried cantaloupe obtained from the 9-Point hedonic scale.
Conclusion
Cantaloupe slices were osmotically dehydrated using osmotic solutions containing various ratios of sucrose and humectant solutions (sorbitol and glucose syrup). Increasing the amount of the sorbitol solution in the osmotic solution could significantly increase water loss and solid gain while the use of glucose syrup in the osmotic solution caused a decrease in water loss and solid gain. Increasing the amount of the humectants (sorbitol and glucose syrup) in the mixture could decrease aw and hardness of the final product. Moreover, the use of sorbitol could reduce reducing sugar, total sugar, and HMF content and improve sensory qualities, especially color and texture. However, the loss of nutritional components, such as vitamin C and phenolic compounds, during osmotic dehydration process should be considered when increasing sorbitol in the osmotic solution. Further work is needed to establish the possible method to reduce leaching of nutritional components when sorbitol was applied as an osmotic agent.
Funding
The authors are grateful to the Faculty of Agricultural Product Innovation and Technology, Srinakharinwirot University for the financial support.
Literature cited
- Azoubel, P.M., A.A. El-Aouar, R.V. Tonon, L.E. Kurozawa, G.C. Antonio, F.E.X. Murr, and K.J. Park. 2009. Effect of osmotic dehydration on the drying kinetics and quality of cashew apple. Intl. J. Food Sci. Technol. 44:980–986.
- Binsan, W., S. Benjakul, W. Visessanguan, S. Roytrakul, M. Tanaka, and H. Kishimura. 2008. Antioxidative activity of Mungoong, an extract paste, from the cephalothorax of white shrimp (Litopenaeus vannamei). Food Chem. 106:185–193.
- Brochier, B., L.D.F. Marczak, and C.P.Z. Norena. 2015. Osmotic dehydration of yacon using glycerol and sorbitol as solutes: Water effective diffusivity evaluation. Food Bioprocess. Technol. 8:623–636.
- Chauhan, O.P., A. Singh, A. Singh, P.S. Raju, and A.S. Bawa. 2011. Effect of osmotic agents on colour, textural, structural, thermal and sensory properties of apple slices. Intl. J. Food Prop. 14:1037–1048.
- Devic, E., S. Guyot, J. Daudin, and C. Bonazzi. 2010. Effect of temperature and cultivar on polyphenol retention and mass transfer during osmotic dehydration of apples. J. Agr. Food Chem. 58:606–616.
- El-Aouar, A.A., M.P. Azoubel, L.J. Barbosa, and X.E.F. Murr. 2006. Influence of osmotic agent on the osmotic dehydration of papaya (Carica papaya L.). J. Food Eng. 75:267–274.
- Guimares, R., L. Barros, C.M.J. Barreira, J.M. Sousa, M.A. Carvalho, and C.F.R.J. Ferreira. 2010. Targeting excessive free radicals with peels and juices of citrus fruits: Grapefruit, lemon, lime and orange. Food Chem. Toxicol. 48:99–106.
- Heredia, A., I. Peinado, C. Barrera, and A. Ande. 2009. Influence of process variables on colour changes, carotenoids retention and cellular tissue alteration of cherry tomato during osmotic dehydration. J. Food Compos. Anal. 22:285–294.
- Ismail, H.I., K. Chan, A.A. Mariod, and M. Ismail. 2010. Phenolic content and antioxidant activity of cantaloupe (Cucumis melo) methanolic extracts. Food Chem. 119:643–647.
- Korsrilabut, J., C. Borompicaicharkul, and K. Duangmal. 2010. Effect of invert sugar on the drying kinetics and water mobility of osmosed-air dried cantaloupe. Intl. J. Food Sci. Technol. 45:1524–1531.
- Lin, S.D., C.F. Hwang, and C.H. Yeh. 2003. Physical and sensory characteristics of chiffon cake prepared with erythritol as replacement for sucrose. J. Food Sci. 68:2107–2110.
- Matusek, A., B. Czukor, and P. Meresz. 2008. Comparison of sucrose and fructo-oligosaccharides as osmotic agents in apple. Inno. Food Sci. Emerg. 9:365–373.
- Nadia, D.M., B.M. Nourhene, K. Nabil, C. Francis, and B. Catherine. 2013. Effect of osmo-dehydration conditions on the quality attributes of pears. J. Food Process. Technol. 4:1–6.
- Naknean, P. 2012. Factors affecting mass transfer during osmotic dehydration of fruit. IFR J. 19:7–18.
- Naknean, P., M. Rattanawedee, and K. Aekkasak. 2013. Effect of different osmotic agents on the physical, chemical and sensory properties of osmo-dried cantaloupe. Chiang Mai J. Sci. 40:427–429.
- Ozen, B.F., L.L. Dock, M. Ozdemir, and J.D. Floros. 2002. Processing factors affecting the osmotic dehydration of diced green peppers. Intl. J. Food Sci. Technol. 37:497–502.
- Pattanapa, K., N. Therdthai, W. Chantrapornchai, and W. Zhou. 2010. Effect of sucrose and glycerol mixtures in the osmotic solution on characteristics of osmotically dehydrated mandarin cv. (Sai-Namphaung). Intl. J. Food Sci. Technol. 45:1918–1924.
- Pereira, L.M., C.C. Ferrari, S.D.S. Matrantonio, A.C.C. Rodrigues, and M.D. Hubinger. 2006. Kinetic aspects, texture, and color evaluation of some tropical fruits during osmotic dehydration. Drying Technol. 24:475–484.
- Phisut, N., M. Rattanawedee, and K. Aekkasak. 2013. Effect of osmotic dehydration process on the physical, chemical and sensory properties of osmo-dried cantaloupe. IFR J. 20:189–196.
- Rangana, S. 1986. Handbook of analysis and quality control for fruit and vegetable products. McGraw-Hill, New Delhi, India.
- Rattanathanlerk, M., N. Chiewchan, and W. Srichumpoung. 2005. Effect of thermal processing on the quality loss of pineapple juice. J. Food Eng. 66:259–265.
- Ronda, F., M. Gomez, A.C. Blanco, and A.P. Caballero. 2005. Effects of polyols and nondigestible oligosaccharides on the quality of sugar-free sponge cakes. Food Chem. 90:549–555.
- Santos, P.H.S., and M.A. Silva. 2008. Retention of vitamin C in drying processes of fruits and vegetables—A review. Drying Technol. 26:1421–1437.
- Shi, X.Q., P. Fito, and A. Chiralt. 1995. Influence of vacuum treatment on the mass transfer during osmotic dehydration of fruits. Food Res. Intl. 28:445–454.
- Solval, M.K., S. Sundararajan, L. Alfaro, and S. Sathivel. 2012. Development of cantaloupe juice powder using spray drying technique. LWT–Food Sci. Technol. 46:287–293.
- Sritongtae, B., T. Mahawanich, and K. Duangmal. 2011. Drying of osmosed cantaloupe: Effect of polyols on drying and water mobility. Drying Technol. 29:527–535.
- Stojanovic, J., and J.L. Silva. 2007. Influence of osmotic concentration, continuous high frequency ultrasound and dehydration on antioxidants, colour and chemical properties of rabbiteye blueberries. Food Chem. 101:898–906.
- Wangcharoen, W. 2013. Development of dried chewy longan arils. Maejo Intl. J. Sci. Technol. 7:497–477.