ABSTRACT
Fruit pomace, a byproduct of the fruit juice industry, is a rich source of nutrients and bioactive components. The study explored chemical composition and bioactive potential of pomace from selected fruits. Results revealed that fruit pomace was rich in dietary fiber (insoluble fiber: 24.15–31.83%; soluble fiber: 0.43–19.71%). Both orange and sweet lemon pomace were good sources of calcium (303 mg/100 g and 581 mg/100 g). Extract yield was high in blue grapes (45.88 mg/100 g). Free radical scavenging activity was highest in blue grape pomace in methanol and aqueous extracts (78.88% and 85.99% per 4-mg extract), respectively. Thus, these pomace could be natural sources of phenolic components.
Introduction
Globally the market for processed foods is growing continuously. While a variety of foods are processed, a considerable share of market belongs to fruits and vegetables, as these are perishable commodities and processing would help in extending their shelf life. Fruit juices are becoming important nutritious beverages with good taste and provide a variety of nutrients. Fruit beverages have become popular among consumers because of their contribution towards health benefits. Thus, the beverage industry has a huge market among product categories and is the fastest growing sector. Out of the total production of fruits, nearly 76% is consumed in the fresh form, around 4% is processed, while wastage accounts for 20–22% (IHD, Citation2013). Processing and value addition is the most effective solution to reduce wastage of fresh fruits.
India is the second largest fruit and vegetable producing country in the world. The current fruit and vegetable production is around 221 million tons, of which about 4.87 million tons is processed. The processing of fruits results in high amounts of waste materials, such as peels, seeds, and residue, which is nearly 1.81 million tons (ICAR, Citation2012; FAO, Citation2012). Fruits are processed mainly to obtain pulps and juices, fruit-based ready-to-serve beverages, canned fruits, jams, squashes, marmalade, etc. They are also used to extract flavonoids, pectins, and essential oils. Pomace is the residue left after extraction of the juice. Disposal of pomace and other waste products usually presents a problem that is further aggravated by legal restriction. These unconventional resources could be a rich source of utilizable nutrients, dietary fibers, and some may also contain appreciable amounts of colorants, antioxidant compounds, or other substances with positive health effects (Schieber et al., Citation2001).
By-products of fruits contain sugars, minerals, organic acids, dietary fiber, and phenolics, which have a wide range of action, including antiviral, antimicrobial, antibacterial, antitumoral, cardioprotective, and antimutagenic activities (Djilas et al., Citation2009). These by-products also have good water-binding properties that can be used in food processing to control food texture and rheological behavior (Fischer, Citation2009).
Our earlier work demonstrated the bioactive potential of dehydrated fruit and vegetable peels and their possible utilization in product formulations (Shyamala and Prakash, Citation2011, Citation2014a, Citation2014b). In the present study, pomace of selected fruits left as residues after extraction of juice have been studied for their chemical composition and bioactive potential.
Materials and methods
Selection of sample and preparation
Fruits for the study included Myrciaria vexator McVaugh (blue grapes), Citrus medica var. limetta (sweet lemon), Citrus sinensis L. Osbeck (orange), and Ananas comosus var. comosus (pineapple), which were purchased from a local market in Mysore, India.
Processing of samples
The stalks of blue grapes were separated from the fruits, and the fruits were thoroughly washed under running tap water followed by distilled water. The washed fruits were spread on filter paper to dry. Juice was extracted with the help of a juice maker, which separates the juice and pulp. Fruits were put in the juice maker (Model Philips HR1854, Philips HR1854, Mysore, India) to obtain juice and pulp. The pulp obtained was placed in a tray and dried in hot air oven maintained at 50 ± 5 °C for 10–12 h, finely powdered, and stored in airtight containers for further analysis.
Both sweet lemon and orange were peeled and the peeled fruit slices were placed in a juice maker for extraction of the juice. Pulp was dried in a hot air oven at 50 ± 5 °C for 10–12 h, finely powdered, and stored in airtight containers for further analysis.
In the case of pineapple, the crown was removed, outer skin peeled, the edible portion separated with a sharp knife, followed by extraction of the juice in a juice maker. The residue pulp was dried in a hot air oven at 50 ± 5 °C for 10–12 h, finely powdered, and stored in airtight containers for further analysis. A record of yield of pulp waste and dry powders was maintained for all samples.
Chemical reagents
Chemicals used for the study: L-Ascorbic acid, β-carotene, and 2,2-Diphenyl-1-picrylhydrazyl (DPPH) were from Sigma Chemical Co. (Sigma-Aldrich, St. Louis, MO, USA), and all others were obtained from E-Merck (Mumbai, India) or Qualigens Fine Chemicals (Mumbai, India). Double-glass distilled water was used for all analyses, which were run in triplicate and averaged.
Physical characteristics of the samples
Color, bulk density (BD), and water absorption capacity (WAC)
Color is generally the first attribute that influences acceptability. Color of dried powders by visual observation was determined using Munsell color charts (Macbeth, Citation2000). Bulk densities (BD) of the samples (g/100 mL) were determined by the method of Wang and Kinsella (Citation1976). A 3.0 g of the finely powdered sample was placed in a 25-mL graduated cylinder and packed gently by tapping the cylinder on a rubber sheet until a constant volume was obtained. The bulk density was expressed as g of sample/100 ml. Water absorption capacity (WAC) of the sample (mL/100g) was determined by the centrifuge technique of Janicki and Walczak (Citation1954). A 1.0-g sample was weighed into a centrifuge tube. Then 5.0 ml of distilled water were added gently down the side and mixed with a thin glass rod. The slurry was weighed, kept aside for 30 min with gentle stirring with a glass rod every 5 min, and centrifuged at 3000 rpm for 25 min at 27 °C. The amount of water retained was calculated by measurement of the difference in the weight of the sample before and after equilibration with water. The water absorption capacity was expressed as the amount of water absorbed (ml/100 g sample).
Estimation of moisture, fat, protein, total ash, and dietary fiber
All dried powder of pulp waste was analyzed for chemical composition. Moisture, ether extractives, protein, and total ash were determined according to the Association of Official Analytical Chemists (AOAC, Citation2005) following the methods as given in parentheses. Moisture was estimated by a vacuum oven (method 926.12, 41.1.02) procedure. A known amount of sample was taken in a petri plate and dried in an oven. The dry weight of the sample was determined by repeated consistent weighing. The final weight was subtracted by initial weight divided by weight of the sample multiplied by 100. Total ether extractives were estimated by Soxhlet apparatus using petroleum ether for extraction (method 948.22, 40.1.05) with the solvent being evaporated and the residue weighed to determine the fat content. Protein was determined by Kjeldahl nitrogen determination and conversion to protein using a factor of 6.25 (method 960.52, 12.1.07). Ash was determined directly following incineration (method 942.05, 4.1.10). The greyish white residue that remains after the food sample taken in a silica crucible is charred on a hot plate, incinerated in a muffle furnace at 600 °C for 3–5 h, and weighed as total ash. The weight of ash obtained divided by the weight of the sample taken and multiplied by 100 gives total ash. Dietary fiber consisting of insoluble (IDF) and soluble fractions (SDF) was estimated by the enzymatic gravimetric (Asp et al., Citation1983) method, which is equivalent to physiologically indigestible fiber residue.
Estimation of vitamin C, carotenoids, and total anthocyanins
Ascorbic acid was estimated by a 2,6-dichlorophenol indophenol visual titration method, which is based on reduction of the dye color from blue to pale pink by ascorbic acid. To standardize the dye, 5.0 ml of standard ascorbic acid solution and 5.0 ml of HPO3 were taken in a conical flask and titrated against the dye to a pale pink color. For sample preparation, 15 g of sample were taken, blended with 3% HPO3, and made up to 100 ml with HPO3 and filtered. Next, 5–10 ml of the HPO3 extract of the sample was taken and titrated with the standard dye to get a pink end-point that persisted for at least 15 s (Ranganna, Citation1986).
For estimation of carotenoids, the powdered samples were extracted in acetone, transferred to a petroleum ether phase, and read colorimetrically at 452 nm using petroleum ether for baseline correction. ß-Carotene was separated by column chromatography using neutral aluminum oxide as an adsorbent in a 10-cm-length adsorbent column. ß-Carotene, which moves down the column prior to all the pigments, is collected till the desired pigments have moved off the column and the eluent is colorless. The eluent is made up to a known volume and the intensity of the color is measured in a spectrophotometer at 452 nm using 3% acetone in petroleum ether as blank (Ranganna, Citation1986). Total anthocyanins in a powdered sample were determined by extracting with ethanolic HCl and measurement of color at the wavelength of maximum absorption. The sample (2.0 g) was blended with 70 ml of ethanolic HCl. Extract was stored overnight in a refrigerator at 4 °C and was filtered on a Whatman No. 1 paper and made up to 100 mL. To prepare the extract for spectrophotometric measurement, 1.0 mL of sample was diluted to 10 mL. Color was measured at the maximum absorption, i.e., 545 nm, after storing the sample in the dark for 2 h (Ranganna, Citation1986).
Estimation of phosphorus, iron, and calcium
Phosphorus, iron, and calcium were determined according to standard methods described by Raghuramulu et al. (Citation2003). Phosphorus analysis was carried out by measuring the blue color, which is formed when the ash solution was treated with ammonium molybdate. The phosphomolybdate thus formed was reduced and read colorimetrically. To an aliquot of the mineral solution, 1.0 ml of ammonium molybdate, 1.0 ml of hydroquinone, and 1.0 ml of sodium sulphite solutions were added in this order, mixing well after each addition. The volume was then made up to 15 ml with glass distilled water and allowed to stand for 30 min. The absorbance was measured in a spectrophotometer against a reagent blank at 660 nm. The phosphorus content of the sample was read off from a standard curve prepared with the monobasic potassium phosphate. Iron was determined colorimetrically making use of the fact that ferric iron gives a blood red color with potassium thiocyanate. To a known volume of mineral solution, 1.0 ml each of 30% H2SO4 and 7% potassium persulphate solution, and 1.5 ml of 40% potassium thiocyanate solution were added with thorough mixing. The red color that developed was measured within 20 min at 540 nm. Similarly, a standard curve was prepared by using ferrous ammonium sulphate. The iron content of the sample was then read from the standard curve. For calcium estimation, it was precipitated as oxalate, dissolved in H2SO4, and titrated against potassium permanganate. Then, 25 ml of ash solution was taken in a 250-ml conical flask and diluted to 150 ml with water. A few drops of methyl red indicator was added and the mixture neutralized with ammonia till the pale pink color changes to yellow. The solution was heated to the boiling point, followed by the addition of 10 ml of ammonium oxalate boiled again for a few minutes. Glacial acetic acid was added till the color of the mixture was distinctly pink. It was allowed to stand at room temperature for at least 4 h or preferably overnight, filtered through Whatman No. 42 paper, and washed with warm water till the filtrate was oxalate free. Next, 5–10 ml of dilute H2SO4 was added on the filter paper, and then the filter paper was pierced with a pointed glass rod to transfer the solution to a conical flask. The solution was heated to 70 °C and titrated against 0.01 N KMnO4 to a permanent pale pink color.
Analysis of oxalate, phytic acid, and tannin contents
Oxalates were extracted with hydrochloric acid, precipitated as calcium oxalate from the deproteinized extract and estimated by subsequent titration with potassium permanganate (Baker, Citation1952). Phytic acid was extracted and determined according to the supernatant difference method (Thompson and Erdman, Citation1982). Tannins were estimated by a colorimetric method based on the measurement of blue color formed by the reduction of phosphotungstomolybdic acid by tannin-like compounds in alkaline solution (AOAC, Citation1970).
Preparation of sample extracts in solvents
For estimation of polyphenols, flavonoids, and antioxidant activity, samples were extracted with ethanol, methanol, and in aqueous media. A 1.0-g sample was suspended with 100 mL of solvent, allowed to extract for 3 h with agitation, centrifuged at 3000 rpm, and filtered through Whatman No. 1 filter paper. All analyses were carried out in fresh extracts.
Analysis of total polyphenol and total flavonoid content
Samples were analyzed for total polyphenol content as tannic acid equivalent (TAE)/100 g of sample according to the Folin–Ciocalteu method (Matthaus, Citation2002). The total flavonoid content was determined using the Dowd method (Arvouet-Grand et al., Citation1994) using a standard curve with quercetin as the standard and expressed as mg of quercetin equivalent/100g of sample.
Antioxidant assays
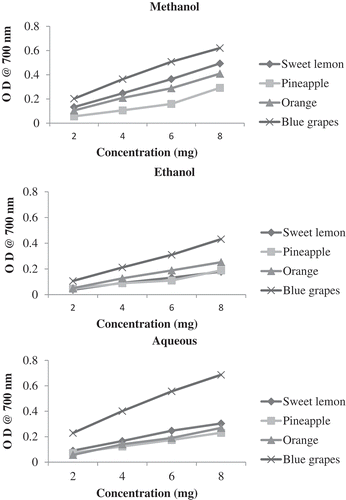
Antioxidant activity was measured by three different assays, namely, total antioxidant activity, free radical scavenging activity, and reducing power assay. Total antioxidant activity by phosphomolybdenum method is based on the reduction of Mo (VI) to Mo (V) by the sample analyte and formation of green phosphate/Mo (V) complex at acidic pH (Prieto et al., Citation1999). Free radical scavenging activity is measured using DPPH, a commercial oxidizing radical, which is reduced by antioxidants. The disappearance of the DPPH radical absorption at a characteristic wavelength is monitored by a decrease in optical density (Oktay et al., Citation2003). In reducing power assay, Fe3+/ferricyanide complex is reduced to the ferrous form by antioxidants. The Fe2+ formed is monitored by measuring the formation of Perl’s Prussian blue at 700 nm (Oyaizu, Citation1986).
Bile acid binding assay
Bile acid’s binding ability of samples was determined using a colorimetric method (Huang and Dural, Citation1995). The factor causing a colorful reaction in this method is furfural water solution. The rule of measurement is based on determination of bile acids concentration in supernatant after incubation at a temperature of 37 °C and reaction characteristic for intestinal juice.
Alpha-amylase inhibition assay
Alpha-amylase inhibition of the samples were determined using the Bernfeld (Citation1955) method and calculated using the following formula:
where Ac+, Ac–, As, and Ab are defined as the absorbance of 100% enzyme activity (only solvent with enzyme), 0% enzyme activity (only solvent without enzyme), a test sample (with enzyme), and a blank (a test sample without enzyme), respectively.
Statistical analysis
The data were analyzed for mean and standard deviation. A correlation coefficient test was applied to test the association between the antioxidant components and the antioxidant activity of the fruit pomace using a statistical package SPSS 10.0 for Windows (SPSS Base 10.0 for Windows User’s Guide, SPSS Inc., Chicago, IL). The probability level was fixed to P < 0.05.
Results
Chemical composition of fruit pomaces
The results of the study are summarized in – and –. includes the data on physical and chemical characteristics of dried fruit pomace. The color of the fruit pomace varied from pale yellow to very dusky red (). Yield of pomace as whole fruit varied from 6.33 to 13 g/100g and as dry powder ranged from 15.28 to 31.36 g/100g. WAC of pomace was least among the samples analyzed for blue grapes (295 ml/100g) and the highest in sweet lemon pomace (637.5 ml/100g). Bulk densities for all of the fruit pomace were between 60.0 to 68.21 g/100g.
Table 1. Physical and chemical characteristics of selected fruit pomace.
Table 2. Extract yield, polyphenols, flavonoids, and total antioxidant activity of fruit pomace in different extracts.
Table 3. Correlation coefficients between antioxidant components and antioxidant activity of fruit pomace (R value).
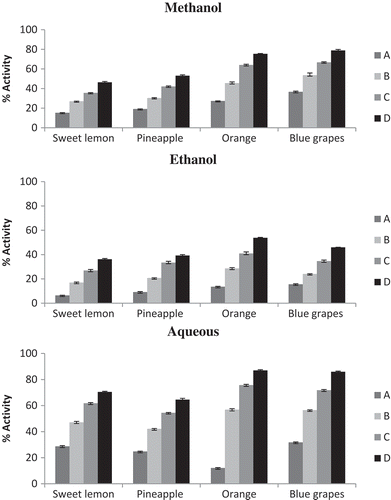
Figure 1. Free radical scavenging activity of fruit pomace by DPPH method in different extracts. Concentration: Sweet lemon, pineapple, orange: A–5, B–10, C–15, and D–20 mg. Blue grapes: A–1, B–2, C–3, and D–4 mg.
Among the fruit pomace, the moisture content was highest in blue grapes (85.45%) and lowest in sweet lemon pomace 80.39% (). The moisture content of dry powders ranged between 7.02% and 7.67%. Orange pomace had the highest protein content of 8.45% followed by sweet lemon pomace (7.27%). Blue grapes and pineapple pomace had significantly lower protein contents. The IDF of pomace was between 24 to 31 g/100g whereas SDF was between 12.78 to 19.71 g/100g except for pineapple pomace, which had least SDF (0.43 g/100g). Significant differences were noted between the pomace of orange and sweet lemon.
Fruits are poor sources of fat, hence, the ether extractives ranged from 1.44 to 2.16 g/100g in fruit pomace. Ether extractives significantly differed between orange and pineapple pomace. However, no differences were noted between orange and blue grapes or orange and sweet lemon pomace.
The ash content of fruit pomace was comparatively less ranging from 1.2 to 4.15 g/100g () with significant differences noted between samples. The ash content of pineapple pomace was lowest among the pomaces analyzed, i.e., 1.2 g/100g. Iron content was almost similar for sweet lemon and pineapple pomace (6.16 and 5.86 mg/100g). Significant differences were noted between orange and the other three fruit pomace for iron content. Blue grapes had high phosphorus content, i.e., 161 mg/100g. Sweet lemon pomace was a good source of calcium (581 mg/100g). Between the fruit pomace samples there were significant differences between phosphorus and calcium content.
Fruit pomace contained total carotenes ranging from 354 to 760 µg/100g. It was high in orange pomace (760 µg/100g). Ascorbic acid was also high in orange pomace (29.71 mg/100g) and was lowest in blue grapes pomace (13.72 mg/100g). The anthocyanin content of blue grapes pomace was 401.72 ± 0.86 mg/100g on dry weight basis. Tannins were high in blue grapes pomace (2391 mg/100g) and low in pineapple pomace (392 mg/100g; ).
Oxalates and phytic acid are mineral binding anti-nutrients present in many foods, though fruits contain small amounts. Total oxalates and water soluble oxalates were less in fruit pomace indicating that these could not possibly interfere with mineral absorption (). Similarly, phytate content was also less ranging from 48.45 to 73.90 mg/100g in different fruit pomace samples. Significant differences were observed between orange, pineapple, blue grapes, and sweet lemon for oxalates and phytic acid.
Polyphenol and flavonoid content
Both agricultural and industrial residues are attractive sources of natural antioxidants. In methanol extracts, pineapple pomace had the least polyphenol content (140 mg/100g) and the highest was seen for orange pomace (1032 mg/100g). Blue grapes pomace had the highest content of polyphenol in methanol extract followed by aqueous and ethanol extract (836, 613, and 592 mg TAE/100g of sample). Among the fruit pomace, flavonoid content was high in blue grapes in both methanol and ethanol extracts (1.57 and 1.24 mg QE/100g of sample). The other fruit pomace, such as pineapple, sweet lemon, and orange, had low flavonoid content ().
Antioxidant activity
Samples were analyzed for their antioxidant components and antioxidant activity and further, correlation was also established between the two (). Antioxidant activity was determined by three methods. The phosphomolybdenum method was used to measure the total antioxidant activity. High total antioxidant activity was observed in aqueous extracts for fruit pomace compared to solvent extracts (). Aqueous extracts followed the order: Pineapple > blue grapes > orange > sweet lemon pomace. In methanol and ethanol extracts the order was blue grapes > pineapple > orange > sweet lemon. In the case of total carotene/anthocyanins a positive correlation R value of 0.54 and 0.85 in methanol and ethanol extracts were observed, whereas a negative correlation was seen in aqueous extracts. Solvent extracts of tannins and polyphenols showed weak positive correlation with the total antioxidant activity.
shows the effect of the different fruit pomace extracts on the DPPH free radicals. Blue grape pomace showed activity at a low concentration of 1 to 4 mg. Considering the antioxidant effect of the different extracts of the pomace, it was obvious that the extracts obtained from blue grapes pomace by extraction with methanol and aqueous showed the strongest effect on the DPPH radicals (85.99% and 78.88%), respectively. Also having strong effects on the DPPH radicals were extracts from orange pomace in the order: methanol > aqueous > ethanol. Sweet lemon and pineapple pomace showed the highest activity in aqueous extracts (70.52% and 64.62%), respectively. Thus, it can be said that extracts of blue grapes pomace were by far the most suitable for stabilization of the DPPH radicals. The IC50 values in methanol, ethanol, and aqueous extracts, respectively, for different fruit pomace were as follows: sweet lemon—16, 38, and 9 mg; pineapple pomace—13, 27, and 10 mg; orange pomace—9, 18, and 20 mg; and blue grapes—1.5, 4, and 1.5 mg. A correlation coefficient was tested between antioxidant components and antioxidant activity (). In the free radical scavenging activity method, total carotene/anthocyanins showed high correlation in all three extracts (R > 0.9). A positive correlation was observed with tannins, which was high in solvent extracts (R > 0.7) compared to aqueous extracts (R > 0.5). Flavonoids (R > 0.5) and polyphenols (R < 0.5) also had positive correlation in all three extracts.
Blue grapes pomace had the highest reducing power compared to the other fruit pomace both in the solvent and aqueous extracts suggesting that the blue grapes pomace phenolics possess strong electron donating capacity. The other fruit pomace, such as pineapple, sweet lemon, and orange, had almost similar reducing power in all three extracts (). Total carotene/anthocyanins exhibited high correlation in methanol (R > 0.8), ethanol (R > 0.9), and aqueous (R > 0.9) extracts. High correlation of antioxidant activity was observed with tannins, polyphenols, and flavonoids (). Tannins had the highest correlation in ethanol (R > 0.8) extract followed by methanol (R > 0.7) and aqueous extracts (R > 0.6). Reducing power of pomace had high correlations with flavonoids in solvent (R > 0.7) as well as in aqueous extracts (R > 0.6).
Bile acid binding activity
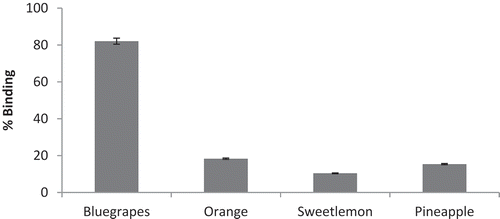
Foods rich in fiber also have the capacity of binding bile acids, metabolites of cholesterol, which play an important role in the digestion and absorption of lipids in the small intestine. Cholestyramine was used as a standard for comparison. Cholestyramine bound more than 60% of bile acids. Acid concentration with 6 mmol/L was used for the experiment. Blue grapes pomace had the highest (82%) cholic acid binding compared to the other three fruit pomace (). The least binding was observed with sweet lemon pomace. Both orange and pineapple pomace had 18.31% and 15.39% binding, respectively.
α-Amylase inhibition assay
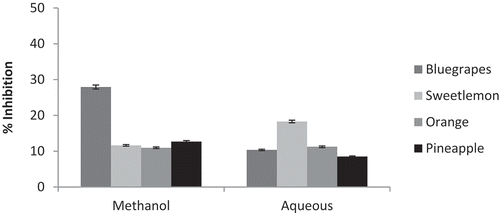
Chronic degenerative diseases, such as diabetes mellitus, is increasing at a rapid rate with changing dietary patterns associated with sedentary and stressful life styles. One of the therapeutic approaches for decreasing hyperglycemia is to retard absorption of glucose by inhibiting carbohydrate hydrolyzing enzymes, such as alpha-amylase and alpha-glucosidase. In the present study the alpha-amylase inhibitory effect of the fruit pomace were determined. Methanol and aqueous extracts were tested for the α-amylase inhibition assay to detect activity, which might be extrapolated to detect a potential antidiabetic effect using this in vitro method. Acarbose, the positive control, demonstrated an IC50 value of 45 µg/ml. Acarbose used as standard in 20, 40, 60, 80, and 100 µg/ml concentrations exhibited inhibition of α-amylase in aqueous media to the extent of 15.0%, 36.33%, 50.50%, 76.5%, and 99.86%, respectively.
The α-amylase inhibitory activity by methanol extract of blue grape pomace was high compared to the other fruit pomace. This could be due to high tannins in the sample. The other fruit pomace viz., sweet lemon, orange, and pineapple, showed 10–12% inhibition. The highest α-amylase inhibition in aqueous extracts was recorded in sweet lemon pomace (18%). The remaining pomace, such as orange, pineapple, and blue grapes, showed the inhibition between 8–11% ().
Discussion
Physical characteristics of food ingredients are some of the properties that define their visual characteristics and basic functionality. Since the samples selected for the study were fruit pomace, it was thought essential to record some basic characteristics that would be beneficial in understanding the nature of the substance. Also, as these materials have to be converted to powder form for ease of utilization, the yield, color, and bulk density of the powders were recorded. Since these by-products also represent the structural matrices, they are mostly comprised of celluloses, hemicelluloses, and related material; hence, they may have a higher water absorbing capacity (WAC). The acceptability of most food products is greatly affected by the overall appearance, particularly by color. In the present study, color of the fruit pomace varied from pale yellow to dusky red.
Fiber with strong hydration properties could increase stool weight and potentially slow the rate of nutrient absorption from the intestine (Gallaher and Schneeman, Citation2001). Fruits are rich source of dietary fiber, which can retain water in its matrix. Figuerola et al. (Citation2005) reported the highest water retention capacity (2.09–2.26 g water/g) in grape fruit pomace. However, in the present study sweet lemon pomace had the highest WAC (637.5 ml/100g). This could be due to their high fiber content. Though fruits are not a good source of protein, a study by Nassar et al. (Citation2008) reported a protein content of 4.75 g/100g in orange pulp, which was much lower compared to our study on pomaces. Orange pomace had the highest protein content of 8.45%. For health benefits, it is said that 30–50% SDF and 50–70% IDF are well-balanced proportions. In a study by Swanson et al. (Citation2002), apple pomace had 79% of total dietary fiber, whereas grape pomace contained 55% of total dietary fiber. These values were much higher than our study. In another study by Ajila et al. (Citation2008), mango peel powder had 51.2% of total dietary fiber of which SDF was 19.0% and IDF was 32.1%, which was similar to our study except for the pineapple pomace, which had considerably less SDF (0.43 g/100g). A higher dietary fiber content in fruit pomace can be exploited as a potential source of natural fiber for value addition in dietary supplements. The differences could be due to different cultivars and different fruit growing conditions. Lipid content of the fiber concentrates was 4.46 g/100g in ‘Granny Smith’ apple (Figuerola et al., Citation2005), which was much higher than analyzed values for pomaces in the present study.
The ash content of orange pomace was 2.65 g/100g and this was in agreement with the value obtained by Nassar et al. (Citation2008) for orange pulp, i.e., 2.60 g/100g. Grigelmo-Miguel and Martin-Belloso (Citation1999) reported an ash content of 2.6–3.1 g/100g in oranges, and Fernández-López et al. (Citation2009) reported values of 3.7 g/100g in citrus fruit peels. In another study Figuerola et al. (Citation2005) obtained 3.91 g/100g for Fino 49 lemon fiber concentrate, which was closer to our value for sweet lemon pomace (4.15 g/100g). Although fruits are considered to only be a fair source of iron, the pomace did exhibit a considerable quantity considering that the bioavailability from fruits would be higher due to the presence of vitamin C and carotenoids.
In general, fruit pomace were rich in antioxidant components indicating that even after juice extraction, a considerable amount of bioactive components remained in the residue portion. Larrauri et al. (Citation1997) in their study showed that drying temperatures of 100 °C and 140 °C resulted in a significant reduction in both total extractable and condensed tannins, resulting in a decrease of 28–50% antioxidant activity in red grape pomace peels. Generally, fruits contain small amounts of phytates in comparison to grains, which contain high amounts. A study by Ma et al. (Citation2005) showed that grain had the highest phytate content (223 to 1419 mg/100g). In the present study the value ranged from 48.45 to 73.90 mg/100g, which is much less compared to whole grains. As the pomace analyzed contain a negligible amount of oxalates and phytic acid, they may not exert a negative effect on mineral absorption; hence, they can be incorporated in different products for value addition.
Fruits, vegetables, and beverages are the major source of phenolic compounds in the human diet. The food and agricultural products processing industries generate substantial quantities of phenolics-rich by-products, which could be valuable natural sources of antioxidants. In a study by Ajila et al. (Citation2007) the total polyphenols content in 80% acetone extract of raw and ripe mango peels ranged from 90–110 mg/g and 55–100 mg/g, respectively, which was similar to values obtained in the present study in methanol extracts for pineapple pomace. Accordingly, these pomaces are attractive sources of natural antioxidants.
Fruits and vegetables have received much attention as a source of biologically active substances because of their antioxidant properties though controversial reports have been found on this subject. Some authors found a positive correlation between the polyphenol content and the antioxidant activity, whereas others found no such relationship. The quality of natural extracts and their anti-oxidative performances depends not only on the quality of the original plant, the geographic origin, climatic condition, harvesting date, and storage, but also on environmental and technological factors, which affect the activities of antioxidants from residual sources (Moure et al., Citation2001). Therefore, it was of interest to measure the antioxidant capacity of the fruit pomace. When measured as total antioxidant activity, high activity was observed in aqueous extracts for fruit residues compared to solvent extracts. Blue grapes pomace had the highest activity with methanol and aqueous extracts (78.88% and 85.99%), respectively. Also having strong effects on the DPPH radicals were extracts from orange pomace in the order: methanol > aqueous > ethanol. Blue grapes pomace had the highest reducing power compared to the other fruit pomace both in the solvent and aqueous extracts suggesting that the blue grapes pomace phenolics possess a strong electron donating capacity.
Dietary fiber acts as a protective agent against cardiovascular diseases, diverticulosis, constipation, irritable colon, colon cancer, and diabetes (Rodríguez et al., Citation2006). The insoluble fraction of the fiber (IF) seems to be related to the intestinal regulation, whereas the soluble fiber (SF) is associated with the decrease of cholesterol levels and the adsorption of intestinal glucose (Lunn and Buttriss, Citation2007). The degree of absorption of common bile acids, lithocholic, deoxycholic, and cholic acids, and cholesterol by fiber from plant food depends on the kind of raw material, conditions of processing, and type of bile acids (Górecka et al., Citation2002). In the present study blue grape pomace had the highest (82%) cholic acid binding compared to the other three fruit pomace.
Diabetes mellitus is one of the chronic degenerative diseases that is increasing at a rapid rate owing to the changing dietary patterns associated with sedentary and stressful life styles. One of the therapeutic approaches for decreasing hyperglycemia is to retard absorption of glucose by inhibiting carbohydrate hydrolyzing enzymes, such as alpha-amylase and alpha-glucosidase. Methanol and aqueous extracts were tested for the α-amylase inhibition assay to detect activity, which might be extrapolated to detect a potential antidiabetic effect using this in vitro method. Blue grape pomace showed high α-amylase inhibitory activity in methanol extract compared to the other fruit pomace.
Conclusion
Fruit pomace, a byproduct of the fruit juice industry, if utilized fully could be a major source of natural phenolic compounds. The pomace analyzed from selected fruits contained polyphenols, anthocyanins, and carotenoids, and all three extracts exhibited good antioxidant activity by effectively scavenging various free radicals, such as DPPH radical, and reducing the ferric to ferrous ion in different antioxidant systems. The antioxidant potential of fruit pomace could be due to synergistic actions of bioactive components present in them. Thus, fruit pomace can be an abundant source of phenolics having high antioxidant activity. The nutritional compositional analysis of fruit pomace indicated that they were a good source of protein, dietary fiber, in particular, the soluble fiber, calcium, and ascorbic acid. There was a positive correlation between the phenolic compounds and the methods describing the antioxidant activity of the extract. Hence, these fruit pomace could be a source for further utilization in formulating value-added products with high nutritional value.
Funding
The source of funding for this study, the Council of Scientific and Industrial Research (CSIR), New Delhi, India, is gratefully acknowledged.
Additional information
Funding
Literature cited
- Ajila, C.M., K.A. Naidu, S.G. Bhat, and U.J.S. Prasada Rao. 2007. Bioactive compounds and antioxidant potential of mango peel extract. Food Chem. 105:982–988.
- Ajila, C.M., K. Leelavathi, and U.J.S. Prasada Rao. 2008. Improvement of dietary fiber content and antioxidant properties in soft dough biscuits with the incorporation of mango peel powder. J Cereal Sci. 48(2):319–326.
- AOAC. 1970. Estimation of tannins. In: Official methods of analysis. 11th ed. Association of Official Analytical Chemists, Washington, D.C.
- AOAC. 2005. Determination of moisture, ash, protein and fat. In: Official methods of analysis. 18th ed. Association of Official Analytical Chemists, Washington, D.C.
- Arvouet-Grand, A., B. Vennat, A. Pourrat, and P. Legret. 1994. Standardisation d’un extrait de propoliset identification des principaux constituants. J. Pharmacie Belgique 49:462–468.
- Asp, N.G., C.G. Johansson, H. Hallmer, and M. Siljestrom. 1983. Rapid enzymatic assay of insoluble and soluble dietary fiber. J. Agric. Food Chem. 31(3):476–482.
- Baker, C.J.L. 1952. The determination of oxalates in fresh plant material. Analyst 77:340–344.
- Bernfeld, P. 1955. Amylases α and β, p. 149–153. In: S.P. Colowick and N.O. Koplan (eds.). Methods in enzymology. Vol. I. Academic Press, New York, NY.
- Djilas, S., C.B. Jasna, and G. Cetkovic. 2009. By-products of fruits processing as a source of phytochemicals. Chem. Ind. Chem. Eng. Q. 15(4):191–202.
- Fernández-López, J., E. Sendra, C. Navarro, M.E. Sayas-Barberá, M. Viuda-Martos, and J.A. Pérez-Alvarez. 2009. Storage stability of a high dietary fibre powder from orange by-products. Int. J. Food Sci. Technol. 44:748–756.
- Figuerola, F., M.L. Hurtado, A.M. Estevez, I. Chiffelle, and F. Asenjo. 2005. Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chem. 91:395–401.
- Fischer, J. 2009. Fruit fibers, p. 427–438. In: S.S. Cho and P. Samuel (eds.). Fiber ingredients: Food applications and health benefits. CRC Press, Taylor & Francis Group, Boca Raton, FL.
- Food and Agriculture Organization (FAO). 2012. FAO statistics database. http://www.fao.org/statistics/en/
- Gallaher, D. and B.O. Schneeman. 2001. Dietary fiber, p. 805. In: B. Bowman and R. Russel (eds.). Present knowledge in nutrition. 8th ed. ILSI, Washington, DC.
- Górecka, D., J. Korczak, E. Balcerowski, and K. Decy. 2002. Sorption of bile acids and cholesterol by dietary fiber of carrots, cabbage and apples. J. Polish Agric. Univ. 5:2.
- Grigelmo-Miguel, N. and O. Martin-Belloso. 1999. Comparison of dietary fibre from byproducts of processing fruits and greens and from cereals. Lebensm. Wiss. Technol. 32:503–508.
- Huang, C.M. and N.H. Dural. 1995. Adsorption of bile acids on cereal type food fibres. J. Food Process Eng. 18:243–266.
- Indian Council of Agricultural Research (ICAR). 2012. Agricultural research data book. ICAR, New Delhi, India.
- Indian Horticulture Database (IHD). 2013. Indian Horticulture Production at a glance. National Horticulture Board, Ministry of Agriculture, Government of India. IG Printer Pvt Ltd, New Delhi, India.
- Janicki, N.A. and J. Walczak. 1954. Wateriness in meat and methods for its determination. Adv. Food Res. 10:355–394.
- Larrauri, J. A., P. RupeÂrez, and F. Saura-Calixto. 1997. Effect of drying temperature on the stability of polyphenols and antioxidant activity of red grape pomace peels. J. Agric. Food Chem. 44:1390–1393.
- Lunn, J. and J.L. Buttriss. 2007. Carbohydrates and dietary fibre. Nutr. Bull. 32:21–64.
- Ma, G., Y. Jin, J. Piao, F. Kok, B. Guusje, and E. Jacobsen. 2005. Phytate, calcium, iron, and zinc contents and their molar ratios in foods commonly consumed in China. J. Agric. Food Chem. 53:10285–10290.
- Macbeth, G. 2000. Munsell color charts. Gretag Macbeth, New Windsor, NY.
- Matthaus, B. 2002. Antioxidant activity of extracts obtained from residues of different oilseeds. J. Agric. Food Chem. 50:3444–3452.
- Moure, A., J.M. Cruz, D. Franco, J.M. Dominguez, J. Sineiro, H. Dominguez Nunez, M. Jose, and J.C. Parajo. 2001. Natural antioxidants from residual sources. Food Chem. 72:145–171.
- Nassar, A.G., A.A. AbdEl-Hamied, and E.A. El-Naggar. 2008. Effect of citrus by-products flour incorporation on chemical, rheological and organoleptic characteristics of biscuits. World J. Agric. Sci. 4(5):612–616.
- Oktay, M., I. Culcin, and O.I. Kufrevioglu. 2003. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. Lebensm. Wiss. Technol. 36:263–271.
- Oyaizu, M. 1986. Studies on product of browning reaction prepared from glucose amine. Jpn. J. Nutr. 44:307–315.
- Prieto, P., M. Pineda, and M. Aguilar. 1999. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of Vitamin E. Anal. Biochem. 269:337–341.
- Raghuramulu, N.M., K. Nair, and S. Kalyansundaram. 2003. A manual of laboratory techniques. National Institute of Nutrition, ICMR, Jamai-Osmania, Hyderabad, India.
- Ranganna, S. 1986. Handbook of analysis and quality control for fruit and vegetable products. 2nd ed., p. 84, 94, 105. Tata McGraw-Hill, New Delhi, India.
- Rodríguez R., A. Jiménez, J. Fernández-Bolaños, R. Guillen, and A. Heredia. 2006. Dietary fibre from vegetable products as source of functional ingredients. Trends Food Sci. Technol. 17:3–15.
- Schieber, A., F.C. Stintzing, and R. Carle. 2001. By-products of plant food processing as a source of functional compounds—Recent developments. Trends Food Sci. Technol. 12:401–413.
- Shyamala, B.N. and J. Prakash. 2011. Chemical composition and antioxidant potential of peels from three varieties of banana. Asian J. Food Agric. Ind. 4(1):31–46.
- Shyamala, B.N. and J. Prakash. 2014a. Utilizing dehydrated peels of ridge gourd (Luffa Acutangula) for development of a high fiber snack product. Ind. J. Nutr. Diet. 51(2):173–182.
- Shyamala, B.N. and J. Prakash. 2014b. Chemical composition and bioactive potential of dehydrated peels of Benincasahispida, Luffa acutangula, and Sechiumedule. J. Herbs Spices Med. Plants 20(2):193–202.
- Swanson, K.S., C.M. Grieshop, G.M. Clapper, Jr, R.G. Shields, T. Belay, N.R. Merchen, and G.C. Fahey, Jr. 2002. Fruit and vegetable fiber fermentation by gut microflora from canines. J. Anim. Sci. 79:919–926.
- Thompson, D.B. and J.W. Erdman, Jr. 1982. Phytic acid determination in soybeans. J. Food Sci. 47:513–517.
- Wang, J. and J.E. Kinsella. 1976. Functional properties of novel proteins: Alfalfa leaf proteins. J. Food Sci. 41:18–23.