ABSTRACT
Fusarium oxysporum and Fusarium solani are the two most destructive pathogens causing wilt disease in guava (Psidium guajava L.) commonly known as ‘super fruits’. These pathogens attack mainly on the root region of the plant and cause severe infection. In the present investigation the infection process was studied with scanning electron microscopy (SEM) and it was observed that the root region of the guava plant showed disintegration and necrosis in the epidermal layer, cortex tissue, and in vascular bundles, while the root region of a healthy sapling showed no such changes. The rupturing of the epidermal layer may show the entry of pathogens in the host tissue through the secretion of toxic enzymes/metabolites. These toxins have great potential to cause wilting symptoms in guava plants in the form of necrosis. Wilt disease causes huge losses in guava production in both tropical and subtropical countries. Therefore, there is a need to find the responsible factors. The present study is an attempt to understand the interaction mechanism of phyto-pathogens causing wilt disease.
Introduction
The word Fusarium is derived from the Latin word fusus, meaning ‘spindle’, i.e., a large genus of filamentous fungi found in soil and in association with plants. These fungal groups secrete mycotoxins in various crops and cause detrimental effects in humans and animals if they enter the food chain. Fusarium spp. are predominantly common soil fungi widely distributed in almost all parts of the world (Etheshamul-Haque and Ghaffar, Citation1994). Among the Fusarium species, Fusarium oxysporum and Fusarium solani are very common and the most destructive pathogens reported to cause wilt disease in guava, an important fruit crop of subtropical countries (Misra, Citation2006). The pathogens that cause these diseases are persistent because they maintain themselves in the soil, exert changes in soil quality, and develop disease symptoms on crops, which adversely affect the crop field and economy as well. Wilting may be defined as the ‘symptoms of the disease’, which causes deterioration of health of the plant leading to the drying appearance on plant parts and finally death of the plant before reaching the fruiting stage (Dwivedi and Dwivedi, Citation1999; ). The symptoms of the disease appear on whole parts of the tree, i.e., stem, roots, leaf, bark, fruits, etc. These symptoms might be attributed to the interaction between or among the pathogens at the time of infection either outside or inside the plant tissue (Chakraborty and Singh, Citation1989; Gupta et al., Citation2011).
Table 1. Symptoms on wilted guava tree.
Dwivedi (Citation1990) for the first time has reported the presence of hyphae of Macrophomina phaseolina also in the xylem vessels of roots of wilted guava trees. When the root sections thereof were inoculated onto nutrient agar plates, the colony that grew was identified as Macrophomina phaseolina. In West Bengal, both Macrophomina phaseolina and Fusarium solani were reported as the incitant of wilt either individually or in combination. Macrophomina phaseolina first attacks on phloem and destroys it. Sometimes it also attacks in xylem vessels (Chattopadhyay and Sengupta, Citation1955).
Many workers estimated the total loss of guava crop per unit land area due to wilt disease. Moreover, 5%–15% loss was estimated in 12 districts of U.P. (Singh and Lal, Citation1953). In west Bengal, the disease reduced the production by 80% (Chattopadhyay and Sengupta, Citation1955). Jhooty et al. (Citation1984) recorded that 70,000 acres of land in Andhra Pradesh under guava cultivation reduced half of the land value by presence of the disease. In Punjab and Haryana 150 to 300 acres of land is affected by guava wilt. Misra and Shukla (Citation2002) estimated 5%–60% loss in Lucknow area. Dwivedi et al. (Citation1988) reported maximum loss due to wilt disease in Varanasi, i.e., 36% and 7.2%, respectively. Naresh and Mehta (Citation1987) reported that the incidence of the disease in eight districts of India ranged from 1.97% in Sonepat to 40% in Jind with an average of 26.11%. A survey on nematode and wilt problem of guava was carried out in Allahabad region and its adjacent areas in U.P., India (Ruchi et al., Citation2002). Wilted guava plants have also been reported from South Africa (Grech, Citation1985; Schoeman et al., Citation1997), Brazil (Junqueira et al., Citation2001), Pakistan (Ansar et al., Citation1994), Bangladesh (Hamiduzzaman et al., Citation1997), and Canberra, Australia (Lim and Manicom, Citation2003).
In this article, an interaction process by Fusarium oxysporum and Fusarium solani was studied by scanning electron microscopy (SEM) to understand the penetration and establishment of infection in guava root.
Materials and methods
Samples were prepared following the method of Hayat (Citation1981) for SEM. Healthy and infected plant part (roots) of guava saplings (one year old grown in net house) were collected and washed twice with sterile water, fixed in 2.5% glutaraldehyde for 2–6 h. Fixed plant parts were washed three times with phosphate buffer and then dehydration process in 30%–100% ethanol series and kept in dry acetone and dried by the CO2 critical point method. Samples were subsequently mounted on aluminum stubs with double-coated carbon conductive tape and sputtered with gold. Observations and microphotographs were taken under SEM (model Jeol JSM-6490 LV; JEOL USA, Peabody, MA).
Result and discussion
Histopathological studies of healthy and wilted root samples were done using scanning electron microscopy (–). It was evident from the scanning electron microscopic observations that Fusarium blocked the vascular bundles giving much insight into the fungal infestation in the Fusarium-infested guava saplings, whereas the healthy saplings showed clear vascular bundles (). These results confirm the findings of Singha et al. (Citation2011) who reported that Fusarium oxysporum f. sp. lycopersici blocked the vascular bundles of healthy plant of tomato also (Lycopersicon esculentum) and caused wilt disease.
Figure 1. (A–E) Histopathological observations of roots of healthy and wilted guava plant by scanning electron microscope (SEM). (A) Healthy guava root. (B) Infection in root tissues. (C) Blocking and rupturing of xylem vessels. (D) Necrosis with distortion of vascular bundle and cortex region. (E) Rupturing of epidermis with necrosis symptoms.
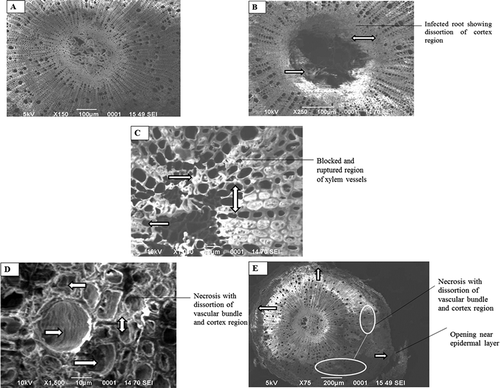
The observation of wilted guava root also showed disintegration of the epidermal layer and cortex tissues. Epidermal layer of wilt-affected roots were found to be fragile and disrupted at several points compared to healthy roots (). The disintegration of the epidermal layer may be due to the entry of pathogens. These results confirm the observation made by Chattopadhyay and Bhattacharjya (Citation1968) and Gupta et al. (Citation2011). They reported that the disintegration of epidermis and increasing gap between the external layers might be attributed to the secretion of enzyme and toxic metabolites by the causal pathogens. The observations of wilted guava roots also showed disintegration and necrosis in cortex tissues (–). Edward (Citation1960) and Chattopadhyay and Bhattacharjya (Citation1968) also reported that the cortex region of the stem and root showed light brown discoloration and damage has been noticed in vascular tissues. During their histopathological studies, Gupta et al. (Citation2011) reported no presence of pathogen in infected regions; hence, the effect was observed only in the form of necrosis and disintegration of tissues.
However, Sohi (Citation1983a, Citation1983b) and Pandey and Dwivedi (Citation1985) reported the presence of fungal mycelium in vascular tissues in their histopathological studies.
Misra et al. (Citation2003) and Misra and Gupta (Citation2010) reported the pathogenic diversity of the pathogen causing wilt disease of guava. Variability in the strains of pathogens, involvement of several pathogens with the disease, and subsequent variation in the time taken for wilting indicate several reasons, which may be of physical presence of pathogen or toxic metabolites released by different pathogens. Hence, necrosis of the tissue, disintegration, and loosening of tissue may be one of the expressions of wilt occurrence due to toxic metabolites/enzyme secreted by the different pathogens involved in the wilt disease of guava.
It is well known that Fusarium spp. are highly effective/virulent and has the ability to infect the plant successfully and causes histopathological changes very efficiently. In the infection process some important steps are common for all strategies including adhesion and penetration to the surface of the plant and absortion of required nutrients from the plant cells (Dwivedi Citation1991; Hardham, Citation2001; Schoeman et. al., Citation1997). Chakrabarti (Citation2005) reported that combined artificial inoculation with Fusarium oxysporum and Macrophomina phaseolina produced the maximum wilt incidence in guava. This might be attributed to Macrophomina phaseolina, which secretes cell wall degrading enzymes that degrade epiblema, cortical cells, and xylem vessels and thus offered an easy passage for Fusarium oxysporum f. sp. psidii.
Acknowledgments
We are thankful to the Head, Department of Environmental Science, Baba Saheb Bhim Rao Ambedkar (A Central) University, Lucknow, India for providing the necessary research facilities.
Literature cited
- Ansar, M., A. Saleem, and A. Iqbal. 1994. Cause and control of guava decline in Punjab (Pakistan). Pak. J. Phytopathol. 6:41–44.
- Benade, E., G.H.J. Kemp, M.J. Wingfield, and J.F.L. Kock. 1991. Comparison of Acremonium diospyri with the guava wilt pathogen in South Africa. Phytophylactica. 137:98.
- Chakrabarti, D.K. 2005. Complexity in the wilt disease of guava (Psidium guajava L.). J. Mycol. Plant Pathol. 35(1):92–94.
- Chakraborty, D.K., and R.N. Singh. 1989. Guava wilt correlation between variation in disease syndrome and edaphic factors. Indian Phytopathol. 42:310–310.
- Chattopadhyay, S.B., and S.K. Bhattacharjya. 1968. Formation of Tylosis in xylem vessels by xylem invading pathogens. Indian J. Agr. Sci. 38:65–72.
- Chattopadhyay, S.B., and S.K. Sengupta. 1955. Studies on wilt of Psidium guajava L. in West Bengal. Indian J. Hort. 12:76–79.
- Dwivedi, S.K. 1990. Guava wilt incited by Macrophomina phaseolina. Natl. Acad. Sci. Lett. 13(8):301–303.
- Dwivedi, S.K. 1991. Population dynamics of micro-fungi including pathogenic forms in the beds of completely healthy partially wilted and completely wilted guava trees grown on a line. Intl. J. Trop. Plant Dis. 9:95–109.
- Dwivedi, S.K., and P. Dwivedi. 1999. Wilt disease of Guava a national problem. J. Appl. Hort. 1(2):151–154.
- Dwivedi, S.K., R.C. Mishra, and R.S. Dwivedi. 1988. Incidence of wilt disease of guava (Psidium guajava L.) in Varanasi, India. Intl. J. Trop. Plant Dis. 6(2):213–216.
- Edward, J.C. 1960. Penetration and establishment of Fusarium oxysporum f. sp. psidii in Guava root. Indian Phytopathol. 13(1):168–170.
- Etheshamul-Haque, S., and A. Ghaffar. 1994. New records of root infecting fungi from Pakistan. Pakistan J. Phytopathol. 6:50–57.
- Grech, N.M. 1985. First report of guava rapid death syndrome caused by Septofusidium sp. in South Africa. Plant Dis. 69:726–726.
- Gupta, V.K., A.K. Misra, and B.K. Pandey. 2011. Histological changes in guava root during wilting induced by Fusarium spp. Arch. Phytopathol. Plant Prot. 45:1–4.
- Hamiduzzaman, M.M., M.B. Meah, and M.U. Ahmad. 1997. Effect of Fusarium oxysporum and nematode interaction on guava wilt. Bangladesh J. Plant Pathol. 13(1–2):9–11.
- Hardham, A.R. 2001. The cell biology behind Phytophthora pathogenicity. Aust. Plant Pathol. 30:91–98.
- Hayat, N.I.A. 1981. Principles and techniques of electron microscopy. University Park Press, Baltimore, MD, 412 p.
- Jhooty, J.S., J.N. Chand, and V. Krishnamurthy. 1984. Report of committee constituted by ICAR on guava decline in Punjab and Haryana. ICAR, New Delhi, India.
- Junqueira, N.T.V., L.R.M. De Andrade, M. Pereira, M.M. Lima, and R.C. Chaves. 2001. Diseases of guava (Psidium guajava L.) cultivated in Brazilian Cerrdos. Circ. Tech. Embrapa. Cerrados, 15:31.
- Lim, T.K., and B.Q. Manicom. 2003. Diseases of guava, p. 275–289. In: R.C. Ploetz (ed.). Diseases of tropical fruit crops. CABI Publication, Wallingford, UK.
- Misra, A.K. 2006. Wilt of guava—A disease of national importance. Indian Phytopathol. 59:269–280.
- Misra, A.K., and V.K. Gupta. 2010. Pathogenicity of Fusarium spp. isolates of guava wilt. J. Mycol. Plant Pathol. 40:72–77.
- Misra, A.K., and B.K. Pandey. 2000. Progressive natural wilting of guava plants during different months. Indian Phytopathol. 53:423–427.
- Misra, A.K., B.K. Pandey, B. Prasad, and S.K. Shuklam. 2003. Pathogenic diversity in the cause of wilt disease of guava. Indian Phytopathol. 56:314.
- Misra, A.K., and S. Shukla. 2002. National Seminar on Production and Post-Harvest Technology of Guava, Kanpur, India, 9–10 Jan. 2002.
- Naresh, M., and N. Mehta. 1987. Distribution of guava wilt in relation to age, soil type, management practices and varieties grown in Haryana. Plant Dis. Res. 2(2):116–119.
- Pandey, R.R., and R.S. Dwivedi. 1985. Fusarium oxysporum f. sp. psidii as a pathogen causing wilt of guava in Varanasi District, India. Phytopathol. Z. 114(3):243–248.
- Rangaswami, G. 1984. Diseases of crop plants in India. 4th. ed. Rekha Printers (PVT) Ltd. New Delhi, India.
- Ruchi, L., B.K. Dwivedi, B.P. Dwivedi, D.N. Shukla, and R. Logani. 2002. Nematode and wilt problems of guava in and around Allahabad region. Curr. Nematol. 13(1–2):23–26.
- Schoeman, M.H., E. Benade, and M.J. Wingfield. 1997. The symptoms and cause of Guava wilt in South Africa. J. Phytopathol. 145:37–41.
- Singh, B.K., and S.B. Lal. 1953. Wilt of guava. Agr. Animal Husban. 3:78–79.
- Singha, I.M., B.G. Umi, Y. Kakoty, J. Das, S.B. Wann, L. Singh, and M.C. Kalita. 2011. Evaluation of in vitro antifungal activity of medicinal plants against phytopathogenic fungi. Arch. Phytopathol. Plant Prot. 44(11):1033–1040.
- Sohi, H.S. 1983a. Diseases of tropical and subtropical fruits and their control. In: A. Husain, K. Singh, B.P. Singh, and V.P. Agnihotri (eds.). Recent advances in plant pathology. Lucknow: Print House, Lucknow, India, p. 73–86.
- Sohi, H.S. 1983b. Studies on wilt disease of guava. Annual report. Indian Institute of Horticulture Research, Hassarghatta, Banglore, India, p. 100–102.
- Tandon, R.N., and A.P. Singh. 1969. Studies on the anthracnose of guava and its control. Indian Phytopathol. 22:322–326.
