ABSTRACT
The emergence of Fusarium wilt, caused by Fusarium oxysporum f. sp. fragariae, as a problem for strawberry production in California has been associated with delivery of fumigants to beds through drip-lines rather than flat-fumigation of an entire field. Our research shows that bed fumigation fails to eliminate propagules of the Fusarium wilt pathogen, which remain particularly abundant in bed shoulders at a depth of 30 cm. Controlled environment studies confirmed that inoculum at this depth could cause disease. Screening of currently grown strawberry cultivars documented a wide range of susceptibilities to Fusarium wilt, with some being highly resistant.
Introduction
Fusarium wilt of strawberry, caused by Fusarium oxysporum f. sp. fragariae, was first recognized in Australia (Winks and Williams, Citation1965) and shortly thereafter was identified in Japan (Okamoto et al., Citation1970). This disease has since been confirmed to occur in many other parts of the world (Koike and Gordon, Citation2015), including California, where it was identified in 2008 (Koike et al., Citation2009). Fusarium wilt is now found in all major strawberry production areas in California, which accounts for over 80% of fresh strawberries produced in the United States (California Department of Agriculture, Citation2014; USDA, Citation2015). The emergence of Fusarium wilt in California is closely associated with altered fumigation practices. This includes the absence of methyl bromide in the fumigant mix and application of fumigants to beds through drip-lines as opposed to shank-injected flat fumigation of an entire field. The initial outbreaks of Fusarium wilt in California were observed only in fields where bed fumigation had been used for several years in succession. One possible explanation for the observed lack of effective disease control is that application through drip-lines fails to deliver a lethal dose of fumigants to fungal propagules throughout the beds. It was an objective of the present study to test this hypothesis by monitoring survival of F. oxysporum fragariae at multiple locations within treated beds. We also examined the effect of bed location on the incidence of disease, the effect of inoculum depth on disease development, and explored the potential for genetic resistance to contribute to management of Fusarium wilt.
Materials and methods
Fumigation experiments
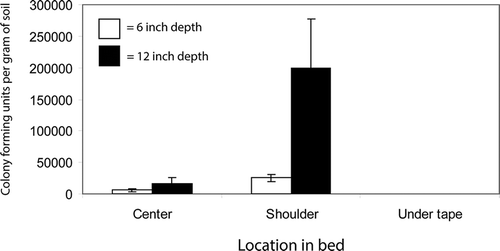
Three experiments were conducted to assess the effectiveness of standard fumigation practices. All experiments were conducted in commercial strawberry production fields with a clay loam soil in Ventura County. In all cases, fumigation treatments were applied during Aug. 2009. One experiment used laboratory-reared inoculum of F. oxysporum fragariae, which was prepared from cultures of a known pathogenic isolate (GL 1080) grown for 3 weeks at room temperature (21 to 23 °C) on plates of potato dextrose agar (PDA). Fully colonized plates were blended in sterile water (20 plates in 240 mls) and the resulting slurry was added to sand, which had been sterilized by autoclaving twice 24 h apart. The sand agar mix was kept at room temperature and stirred periodically over a period of several days until it was dry. Thereafter, 50 g of infested sand was packaged in 6 × 10 cm nylon pouches, which were buried at both 15 cm and 30 cm below the soil surface, prior to application of fumigants. At both depths, separate pouches were placed: (1) directly under a drip-line, (2) between drip-lines at the center of the bed, and (3) in the shoulder of the bed, for a total of six locations in each bed. Beds formed were 30 cm high with 170 cm distance between bed centers to accommodate four rows of strawberry plants. Two drip lines used for fumigation and later for fertigation were placed in beds 6 cm deep and 18 cm away from the two neighboring plant rows, approximately 45 cm from bed edges. These drip lines (Toro, Aqua-Traxx, El Cajon, CA, USA) delivered about 4 L/min/100 m via 20-cm-spaced emitters.
A 60:40 mix of chloropicrin:telone (Pic 60) was applied through drip-lines at a rate of 448 kg/ha to each of four replicate beds, 100 m in length, which were covered with black, totally impermeable plastic film. The experiment included four untreated beds, for a total of eight beds arrayed in a randomized complete block design. Pouches were recovered 21 days after fumigation and the infested sand was assayed by soil dilution plating. In this procedure, 6.25 g of sand were suspended in 100 ml of sodium hexametaphosphate, and after mixing on a magnetic stir plate for 5 min, 7.5 ml of the suspension were added to 42.5 ml of 0.1% water agar. The resulting suspension was stirred for 5 min, after which 0.25 ml were spread over the surface of each of 20 plates of Komada’s selective medium (KM) (Komada, Citation1975). Inoculated plates were incubated at room temperature under continuous fluorescent light. Five to 7 days later, colonies of F. oxysporum fragariae were identified based on their distinctive appearance and enumerated in order to quantify the viability of inoculum recovered from each pouch. The use of colony morphology on KM to identify F. oxysporum fragariae has been validated previously by testing isolates obtained from roots of strawberry plants grown in naturally infested soil. Colonies with fluffy, white, aerial mycelium; tight colony margins; and light pink coloration on the underside of the colony were tested for the presence of a DNA sequence diagnostic for F. oxysporum fragariae using PCR (Suga et al., Citation2013). The diagnostic sequence was amplified from 96% of 530 isolates that were tested (Gordon, unpublished data).
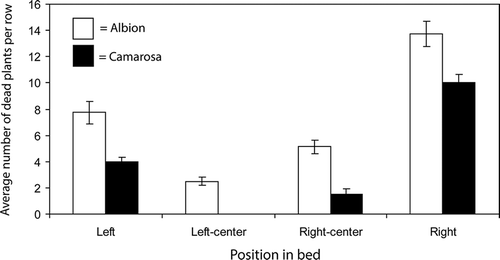
A second experiment evaluated both Pic 60 and a 1:1 mix of methyl bromide and chloropicrin (MbPic) at 448 kg/ha applied through drip-lines as described above. In this experiment, fumigant effects were evaluated based on their impact on naturally occurring inoculum of F. oxysporum fragariae in treated and untreated beds, constructed as described above. Soil samples were taken from four replicate beds corresponding to each of the three treatments (Pic 60, MbPic, and untreated). Samples were taken 21 days after fumigation from the top 5 cm of soil at the center and on the north and south shoulders of the bed. The quantity of inoculum in each sample was estimated using soil dilution plating, as described above. The third experiment tested for differential mortality by row within a bed to which pre-plant fumigation with InLine (Dow AgroSciences, Indianapolis, IN, USA; active ingredients of telone [61%] and chloropicrin [34%]) had been applied at 224 kg/ha though drip-lines (two per bed). The effect of location within the bed was evaluated by recording the number of dead plants at the end of the season (June of the year following application of the fumigant) in each of four rows (left, left center, right center, and right) in four replicate beds. Counts were taken for two cultivars (Albion and Camarosa), which were planted in separate beds in a split plot design. To confirm the cause of death, petiole sections were placed on plates of KM, which were maintained as described above. Colonies growing from petioles were identified as F. oxysporum fragariae based on their distinctive appearance, as described above.
Inoculum depth experiments
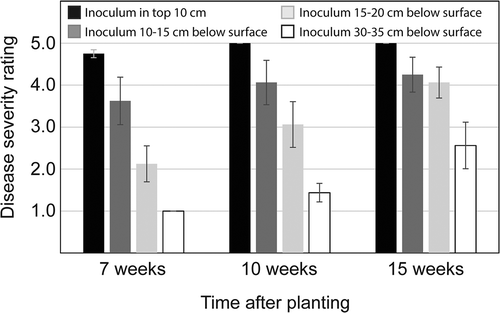
These experiments were conducted in a growth chamber set for day/night temperatures of 24 °C/18 °C, with a 12-h photoperiod. Plants (cultivar = Albion) were grown in Sunshine Mix #1 (SunGro Horticulture, Agawam, MA, USA) amended with inoculum at various depths as follows: (1) inoculum in the top 10 cm, (2) inoculum in a layer 10–15 cm below the surface, (3) inoculum in a layer 15–20 cm below the surface, (4) inoculum 30–36 cm below the surface, or (5) no inoculum (control). Inoculum was prepared by blending plates of PDA that were fully colonized by F. oxysporum fragariae isolate GL 1080 in water, and adding this slurry to sterilized sand as described above. Infested sand was blended with sterile sand and Sunshine mix to obtain an inoculum density of approximately 4 × 104 CFUs per gram of potting mix (2:1 Sunshine Mix:infested sand). Inoculum density was determined by soil dilution plating, as described above. Infested sand/potting mix was placed at the appropriate level within each pot (20 × 20 × 46 cm deep; TP818, Stuewe and Sons, Tangent, OR, USA), as described above. The remainder of the pot was filled with Sunshine Mix. Plants were rated for severity of disease at weekly intervals, using a 1–5 scale, with 1 corresponding to a healthy plant and 5 corresponding to a plant killed by Fusarium wilt. The experiment was conducted twice.
Differences in cultivar susceptibility to Fusarium wilt
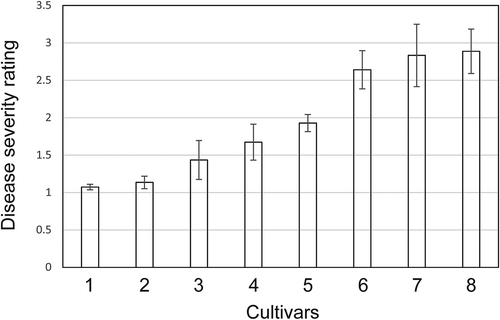
Eight strawberry cultivars from the University of California breeding program were tested for susceptibility to Fusarium wilt. This was accomplished by immersing roots of plants in an aqueous suspension of pathogen spores for 5 min. A spore suspension was prepared from plates of PDA that were fully colonized by one of four isolates of F. oxysporum fragariae. Spores were washed from each plate using sterile distilled water and adjusted by the addition of sterile water to obtain a density of 106 spores/ml. A spore suspension of each isolate was used to inoculate 400 mL of potato dextrose broth (PDB) in a 1-L flask (a separate flask for each isolate), which was maintained on a rotary shaker at 90 rpms for 7 days at room temperature. Colonized PDB from each flask was filtered through three layers of sterilized cheesecloth to remove mycelium. Sterile 0.1% water agar was added to the filtrate as needed to obtain a final density of 5 × 106 spores per ml, with each of five isolates representing 25% of the total. Experiments were conducted at the University of California, Wolfskill Experimental Orchard in Solano Co., California. Plants were inoculated in October and immediately transplanted into plots that had been flat-fumigated with 2 methyl bromide:1 chloropicrin at 392 kg/ha. Two replicate plots of five plants per plot were established for each entry. Each replication of inoculated plants was paired with two non-inoculated plants for comparison. In spring of the year following inoculations, plants were rated 4–5 times starting with the onset of symptoms and given a disease severity rating on the 1 to 5 rating scale described above. The final rating for each cultivar was the average of all ratings taken during the season. The same set of cultivars was evaluated in each of 5 years: 2010, 2011, 2012, 2013, and 2014.
Results
Fumigation experiments
In the buried pouch experiment, colonies of F. oxysporum fragariae were identified by their distinctive appearance on KM, as described above. In most cases, no other fungi were recovered from sand in buried pouches. The only exception was Trichoderma sp., which was readily distinguished from colonies of F. oxysporum fragariae. For pouches buried directly beneath a drip-line at depths of either 15 or 30 cm, F. oxysporum fragariae was not detectable (threshold = 21 CFUs/gram). Pathogen inoculum was viable in sand recovered from pouches at all other positions, with the highest levels found in bed shoulders at a depth of 30 cm (). In tests based on naturally occurring inoculum, measurable pathogen populations were confirmed in all locations of the untreated beds. Both Pic 60 and MbPic were effective in reducing inoculum of the Fusarium wilt pathogen to below detectable levels at the center and shoulders of the bed in the top 5 cm of the soil profile (data not shown).
Figure 1. The effect of bed fumigation with a 60:40 mix of chloropicrin:telone applied through drip-lines on viability of Fusarium oxysporum f. sp. fragariae at various locations within the bed. Values represent means of four replications and error bars correspond to 2× the standard error of the mean.
Where fumigants (telone + chloropicrin) were applied through drip-lines, the effect of position within a bed on plant mortality caused by Fusarium wilt was significant (P < 0.001), with higher mortality in rows nearest the edge of the bed (right and left rows) than in the two center rows (). Mortality was significantly higher for Albion than for Camarosa (P < 0.001). The interaction between cultivar and location was not significant (P = 0.9115).
Inoculum depth experiments
In both replications of the experiment, plants grown in containers with no inoculum of F. oxysporum fragariae remained healthy for the duration of the experiment (15 weeks). Disease developed most rapidly where inoculum was placed in the top 10 cm of soil, with all plants being either dead or severely diseased by 7 weeks after initiation of the experiment (). At this time, all plants were healthy in treatments where inoculum was present only below 30 cm, but symptoms of Fusarium wilt were evident in some plants in each of the other treatments (). Disease became progressively more severe in all treatments over time, and at the final rating (15 weeks after planting) symptoms were apparent even in plants exposed to inoculum only below 30 cm (). The results of ANOVA, conducted separately for data at each rating interval (7, 10, and 15 weeks), indicated that the effect of planting depth on disease severity was significant (P < 0.001 in each case).
Differences in cultivar susceptibility to Fusarium wilt
Root-dip inoculation experiments revealed significant differences in susceptibility to Fusarium wilt (P < 0.001). Averaged over tests conducted in each of 5 years, both San Andreas and Portola had disease severity ratings very close to 1.0 (), indicating that all plants were free of symptoms or showed at most slight stunting. Albion and Camarosa were the most susceptible, with mean disease severity scores >2.6 (). Although there was some variation in cultivar response to disease across years, the effect of inoculation year on severity scores was not significant (P = 0.067). Likewise, the interaction between inoculation year and cultivar was not significant (P = 0.158), indicating that the ranking of the cultivars was consistent across years.
Figure 4. Mean disease severity scores, on a 1–5 scale with 1 corresponding to a healthy plant and 5 to a plant killed by the disease, for eight UC cultivars (1 = San Andreas, 2 = Portola, 3 = Ventana, 4 = Camino Real, 5 = Palomar, 6 = Camarosa, 7 = Albion, 8 = Monterey) subjected to root-dip inoculations in each of 5 years. Error bars correspond to 2× the standard error of the mean.
Discussion
The emergence of Fusarium wilt as a problem in California was closely associated with the adoption of bed fumigation. This suggested that the disease may have become problematic because the application method failed to effectively distribute fumigants, allowing for survival of pathogen propagules within treated beds. The results of this study support that hypothesis by showing that where fumigants are applied to a bed through two drip-lines, F. oxysporum fragariae can survive at many locations, with especially high levels being detectable at a depth of 30 cm in bed shoulders (). This pattern is consistent with a higher rate of plant mortality in rows near the edges of a bed as compared to rows near the center (). To the extent that inoculum is eliminated from the upper soil profile, a delay in the onset of disease can be expected. However, based on results of controlled environment experiments (), even where inoculum is only present below 30 cm, a depth commonly exceeded by strawberry roots under field conditions (Strand, Citation2008), Fusarium wilt is still likely to be problematic.
In the absence of fully effective fumigation, genetic resistance to disease will be of central importance in management of Fusarium wilt of strawberry. Fortunately, tests conducted in various parts of the world have documented significant differences in susceptibility to this disease (Koike and Gordon, Citation2015). For example, breeders in Asia have released a number of cultivars with resistance to Fusarium wilt (Kim et al., Citation1982; Kodama, Citation1974; Mori et al., Citation2005) and breeding lines and cultivars developed in Mexico have been shown to be resistant (Davalos-Gonzales et al., Citation2006). Likewise, we found UC cultivars, San Andreas and Portola, to be highly resistant to Fusarium wilt in root-dip inoculation trials conducted over a period of 5 years. Ventana appeared to be relatively resistant in our tests, but this cultivar was reported to be susceptible to Fusarium wilt based on studies conducted in Spain (Arroyo et al., Citation2009). This discrepancy could reflect differences in virulence of isolates used to inoculate plants and/or the influence of environmental conditions. In support of an environmental effect on susceptibility, we did see variation in disease severity ratings for Ventana, which ranged from 1.0 to 2.5 across all replications and years. In contrast, resistance in San Andreas appeared to be quite stable, with no mean severity rating above 1.4 in any of the 5 years in which tests were conducted. Furthermore, a test conducted in 2015 confirmed the resistance of San Andreas in a field naturally infested with F. oxysporum fragariae (Gordon, unpublished data).
The availability of cultivars with resistance to Fusarium wilt can make an immediate contribution to management of Fusarium wilt while also serving as a source of genes for resistance that can be introgressed into a broader array of genetic backgrounds. Of course, deployment of resistant cultivars may promote selection for pathogen strains that overcome resistance, compromising this mode of disease control over time. The risk of new pathotypes emerging can be reduced by limiting opportunities for growth and reproduction of the pathogen. More effective delivery of fumigants can contribute to this effort, and deployment of multiple drip lines at different depths would be among the strategies worthy of further study.
Funding
The authors gratefully acknowledge support from the California Strawberry Commission.
Additional information
Funding
Literature cited
- Arroyo, F.T., Y. Llergo, A. Aguado, and F. Romero. 2009. First report of Fusarium wilt caused by Fusarium oxysporum on strawberry in Spain. Plant Dis. 93:323.
- California Department of Agriculture. 2014. Crop Report. https://www.cdfa.ca.gov/statistics/.
- Davalos-Gonzalez, P.A., A.E. Jofre-Garfias, A.R. Hernandez-Razo, J. Narro-Sanchez, J. Castro-Franco, N. Vazquez-Sanchez, and R. Bujanos-Muniz. 2006. Strawberry breeding for the Central Plateau of Mexico. Acta Hort. 708:547–552.
- Kim, C.H., H.D. Seo, W.D. Cho, and S.B. Kim. 1982. Studies on varietal resistance and chemical control to the wilt of strawberry caused by Fusarium oxysporum. Korean J. Plant Prot. 21:61–67.
- Kodama, T. 1974. Characteristics of strawberry yellows disease caused by Fusarium and cultivar resistance. Bull. Nara. Agr. Exp. Sta. 6:68–75.
- Koike, S.T., S.C. Kirkpatrick, and T.R. Gordon. 2009. Fusarium wilt of strawberry caused by Fusarium oxysporum in California. Plant Dis. 93:1077.
- Koike, S.T., and T.R. Gordon. 2015. Management of Fusarium wilt of strawberry. Crop Prot. 73:67–72.
- Komada, H. 1975. Development of a selective medium for quantitative isolation of Fusarium oxysporum from natural soils. Rev. Plant Prot. Res. 8:114–125.
- Mori, T., H. Kitamura, and K. Kuroda. 2005. Varietal differences in Fusarium wilt resistance in strawberry cultivars and the segregation of this trait in F1 hybrids. J. Jpn. Soc. Hort. Sci. 74:57–59.
- Okamoto, H., S. Fujii, K. Kato, and A. Yoshioka. 1970. A new strawberry disease ‘Fusarium wilt’. Plant Prot. 24:231–235.
- Strand, L. 2008. Integrated pest management for strawberries, 2nd ed. ANR publication number 3351. University of California, Davis, CA.
- Suga, H., Y. Hirayama, M. Morishima, T. Suzuki, K. Kageyama, and M. Hyakumachi. 2013. Development of PCR primers to identify Fusarium oxysporum f. sp. fragariae. Plant Dis. 97:619–625.
- USDA. 2015. Economic Research Service Fruit and Tree Nut Yearbook. http://www.ers.usda.gov/data-products/fruit-and-tree-nut-data/yearbook-tables.aspx.
- Winks, B.L., and Y.N. Williams. 1965. A wilt of strawberry caused by a new form of Fusarium oxysporum. Queensland J. Agr. Anim. Sci. 22:475–479.