ABSTRACT
Thirty-one strawberry genotypes were evaluated for supporting the reproductive success of the strawberry aphid (Chaetosiphon fragaefolii), a vector of several strawberry viruses. A pure colony of C. fragaefolii was initiated from eggs collected from field strawberry leaves in Fall 2013. In Spring 2014 greenhouse-grown strawberry plants with four to five leaves were placed in screened cages (16 genotypes/cage) and five aphids were placed on each plant. After 30–32 days, the number of aphids in each of four developmental stages was counted on each plant. Total aphid numbers/plant ranged from a mean of 33 on Fragaria chiloensis CFRA 48 (PI 551459) to 279 on F. × ananassa ‘AAC Lila’. Cultivars with relatively low numbers of aphids included ‘Bounty’ (106 aphids), ‘Mira’ (114 aphids), and ‘Annapolis’ (115 aphids). This experiment, part of a larger project on aphids and virus diseases associated with the cultivated strawberry, will inform decisions in the strawberry breeding program.
KEYWORDS:
Introduction
Aphid and virus species associated with strawberry plants were investigated in Nova Scotia in the 1960s (Craig and Stultz, Citation1964; Stultz, Citation1968), a few years after the beginning of a plant certification scheme. Chaetosiphon fragaefolii (Hemiptera:Aphididae), the strawberry aphid, was present in the 1960s but in very low numbers compared to other aphid species, such as Rhodobium porosum (yellow rose aphid) and Macrosiphum euphorbiae (potato aphid). The plant certification scheme, which governed strawberry nursery plant production, was effective in preventing significant virus problems until 2012, when plants exhibiting strawberry decline symptoms were found to be infected with Strawberry mild yellow edge (SMYEV) and Strawberry mottle viruses (SMoV) (Martin and Tzanetakis, Citation2013). Symptoms are first expressed as chlorosis of the young leaves and these leaves do not reach full size. In late summer leaves may become prematurely red and fruit production is reduced in the following year. Symptomatic plants typically are infected with two or more viruses. In Nova Scotia, by 2012, C. fragaefolii, an efficient vector of these viruses, had become quite abundant in the region (Moreau and Lewis, personal communication). The feeding of the aphids, in the absence of viruses, has little negative effect on strawberry production and control measures were generally not employed. When the virus epidemic was diagnosed, ‘Mira’ was one of the most affected cultivars, so we wondered if locally important cultivars differed in their virus symptom expression or their favorability for aphids. Previous studies with older cultivars have identified Fragaria germplasm with resistance to the strawberry aphid (Barritt and Shanks, Citation1980; Crock et al., Citation1982; Shanks and Moore, Citation1995) and tolerance to several viruses (Barritt and Daubeny, Citation1982; Daubeny et al., Citation1972; Sjulin et al., Citation1986). In this report, we consider the ability of C. fragaefolii to form colonies on 31 strawberry genotypes comprised of regionally important cultivars, advanced selections from our breeding program, and additional germplasm under evaluation for use as parents.
Materials and methods
Colonies of C. fragaefolii were established with eggs collected on strawberry leaves from a commercial strawberry field in Nova Scotia in late Fall 2013 on strawberry leaves. The field was conventionally managed but had not been sprayed to control aphids. Leaves with eggs were stored at 4 °C until early February, when they were removed from the cold to stimulate hatching. Colonies were reared on F. vesca ‘UC5’ plants grown in small (30 × 30 × 30 cm) then larger (61 × 61 × 61 cm) insect cages (BioQuip Products, Inc., Rancho Dominguez, CA, USA) placed in the greenhouse, and maintained at 21 °C, 70% relative humidity with photoperiod 16 h light:8 h dark.
Cold-stored dormant plants of 31 genotypes were potted into 5ʺ fiber pots containing ProMix BX (Premier Tech Horticulture, Rivière-du-Loup, Québec, Canada) and placed in a greenhouse with supplemental light to give a 16-h day length with minimum temperatures of 22 °C day and 20 °C night. After 5 weeks, when plants had developed four or five leaves, flower stalks were removed, and plants were placed in 61 × 61 × 61 cm dacron chiffon collapsible cages (BioQuip Products, Inc., Rancho Dominguez, CA, USA) with the base fitted with a felt mat over a plastic sheet to facilitate capillary watering. Plants were watered as needed with care not to wet the leaves. Plants were moved to a greenhouse compartment on 2 May 2013 with similar temperatures but with natural day length and placed in the cages. Sixteen plants of different genotypes were arranged in a 4 × 4 pattern in each of 12 cages (). Each strawberry genotype was represented in six cages with the exception of UC5, which was in all 12 cages. The 12 cages were placed six to opposite sides of the compartment, and pairs of cages, one on each side, were considered a block containing all 31 genotypes. The experiment was laid out as a lattice design with six blocks, to ensure that genotypes had different neighboring genotypes. Aphids were added on 5 May. Five late instar nymphs were transferred from the colonies, using a fine paintbrush, to the underside of a UC5 leaflet and then placed in petri dishes and transported to the greenhouse. Leaflets were inverted and placed on one leaf of each plant of each strawberry genotype in the cages. All aphids were counted on all plant leaves, excluding the runners, after 30–32 days. Aphids were recorded according to four developmental categories: 1st–2nd instar nymphs, 3rd–5th instar nymphs, wingless adults, and winged adults. For analysis, these four categories were combined into two—immature and adult—and total aphids were calculated. The lattice design had cultivars randomized across block, cage, and rows and columns within cages. The analysis was completed using a residual maximum likelihood model (REML) where block, cage, and genotype location were the random effects. Only the cultivar was a fixed effect in the model. Cultivar differences were tested with a Fisher’s Protected Least Significant Difference (FPLSD) means comparison significant at P ≤ 0.05.
Results and discussion
The aphids responded well to the cage environment and reproduced rapidly. The mean number of immature aphids at the end of the experiment was 125.6 ± 32.3 (mean ± SE) and ranged from 25.0 on Fragaria chiloensis CFRA 48 (PI 551459) to 218.9 on F. × ananassa ‘AAC Lila’ (). The mean number of adult aphids at the end of the experiment was 32.7 ± 9.9 and ranged from 5.6 on CFRA 48 to 60.7 on ‘AAC Lila’. Only 3.4% of the adult aphids were winged. Strawberry genotypes with higher numbers of immature aphids also tended to have more adults (r = 0.81). REML analysis identified significant cultivar effects for immature aphids (P = 0.0011), adult aphids (P = 0.0160), and total aphids (P = 0.0012).
Table 1. Number of immature and adult aphids on 31 strawberry genotypes.
That CFRA 48 was less preferred as a host for C. fragaefolii is evident in . Four of the six CFRA 48 plants supported ≤5 aphids at the end of the experiment, indicating that a successful colony was not established. Among the three F. chiloensis genotypes in this study, only CFRA 48 showed this aphid response (). This aphid response to CFRA 48 has been previously reported in GRIN-Global (National Plant Germplasm System, USDA) and other F. chiloensis clones have been shown to be poor habitats for Chaetosiphon (Crock et al., Citation1982). CFRA 48 is interesting as a breeding parent because, in addition to its aphid response, it is resistant to red stele root rot, spider mites, and it is very late flowering, but it produces few berries. We have created seven seedling families with CFRA 48 as pollen parent to incorporate these traits into our program of strawberry genetic enhancement.
Figure 2. The total number of aphids (158.4 ± 40.2; mean ± SE) on each genotype at the end of the experiment. The sample size of each genotype listed was six plants.
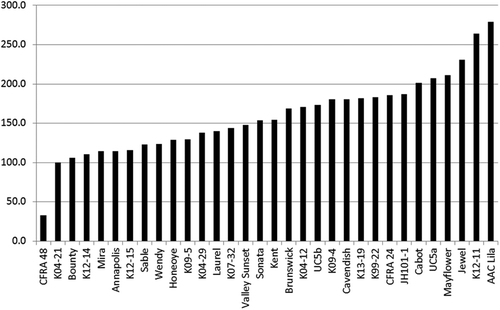
Our initial idea that Mira was preferred to other cultivars by C. fragaefolii was not supported by the data. It may be that Mira is more symptomatic of SMYEV and SMoV than other regionally adapted cultivars, but comparative studies of symptoms have not been done.
Our experimental design allowed for aphid movement; they could walk from plant to plant. This may have contributed to the experimental error, but this design was more consistent with the reality of the strawberry field, especially matted rows. The strawberry aphid typically is found on young succulent tissue so cultivar differences in the supply of newly emerging leaves and runners may also have contributed to experimental error. The low numbers of aphids on CFRA 48 suggests that there may be a biochemical explanation related to the quality of the habitat (Underwood, Citation2007). Research is underway to identify chemicals unique to CFRA 48 isolated from plant sap, or volatiles given off by leaves, which may have a negative impact on aphid attraction or reproduction.
Acknowledgment
We thank the National Clonal Germplasm Repository, Corvallis, Oregon for provision of accessions of Fragaria chiloensis.
Literature cited
- Barritt, B.H., and C.H. Shanks, Jr. 1980. Breeding strawberries for resistance to the aphids Chaetosiphon fragaefolii and C. thomasi. HortScience 15:287–288.
- Barritt, B.H., and H.A. Daubeny. 1982. Inheritance of virus tolerance in strawberry. J. Amer. Soc. Hort. Sci. 107:278–282.
- Craig, D.L., and H.T. Stultz. 1964. Aphid dissemination of strawberry viruses in Nova Scotia. Can. J. Plant Sci. 44:235–239.
- Crock, J.E., C.H. Shanks, Jr., and B.H. Barritt. 1982. Resistance in Fragaria chiloensis and F. × ananassa to the aphids Chaetosiphon fragaefolii and C. thomasi. HortScience 17:959–960.
- Daubeny, H.A., R.A. Norton, and B.H. Barritt. 1972. Relative differences in virus tolerance among strawberry cultivars and selections in the Pacific Northwest. Plant Dis. Rep. 56:792–795.
- Martin, R.R., and I.E. Tzanetakis. 2013. High risk strawberry viruses by region in the United States and Canada: Implications for certification, nurseries and fruit production. Plant Dis. 97:1358–1362.
- Shanks, Jr., C.H., and P.P. Moore. 1995. Resistance to twospotted spider mite and strawberry aphid in Fragaria chiloensis, F. virginiana, and F. × ananassa clones. HortScience 30:596–599.
- Sjulin, T.M., J. Robbins, and B.H. Barritt. 1986. Selection for virus tolerance in strawberry. J. Amer. Soc. Hort. Sci. 111:458–464.
- Stultz, H.T. 1968. Aphids on strawberry in Nova Scotia. Can. Entomol. 100:869–878.
- Underwood, N. 2007. Variation in and correlation between intrinsic rate of increase and carrying capacity. Amer. Nat. 169:136–141.

