ABSTRACT
Common strawberry cultivars are hermaphrodites, producing both anthers and pistils in their flowers. However, octoploid Fragaria species are trioecious and different genotypes can be female with pistillate flowers, hermaphrodites, or males with staminate flowers. One female selection, F. virginiana High Falls 22 and three hermaphrodite selections, N8688, RH23, and Montreal River 10 were hybridized with five June-bearing cultivars. Seedlings of each family were planted in Ontario and Michigan. Two F. chiloensis selections Pigeon Point (female) and FRA1267 (hermaphrodite), were hybridized with 14 F. virginiana selections. Seedlings from each family were planted in Ontario and Minnesota. In all progeny, the presence or absence of anthers was recorded and a subjective scale used to estimate the percentage of fruit set (1–10 representing 10% intervals). Also, FRA1267 was crossed with one F. × ananassa selection and the progeny intercrossed and grown in Ontario. In the sib-crosses gender was recorded. In two female parents, the female progeny had on average a higher fruit set than the hermaphrodite progeny. F. virginiana genotypes varied in their fruit set, which suggested that there are a number of alleles involved in the fertility of hermaphrodites. In crosses, FRA1267 produced 80% females when used as a female parent and 100% hermaphrodites when used as a male parent. It is thought that the trait is inherited cytoplasmically or that the F. chiloensis alleles involved are epistatic when F. virginiana or F. × ananassa is used as a male parent.
Introduction
Octoploid strawberries (Fragaria × ananassa Duch.), F. virginiana Miller, and F. chiloensis (L.) Miller are trioecious and have female, male, and hermaphrodite flowers. In the genes for gender, the alleles for female flowers are dominant over those for hermaphrodite flowers, which are dominant over those for male flowers (Ahmadi and Bringhurst, Citation1991). Also, an allele for female flowers, which is recessive to hermaphrodite flowers, has been reported in cultivar Brighton (Bentvelsen and Sterk, Citation1996) and in F. vesca subsp. Bracteata (Ashman et al., Citation2015).
Recently, studies in F. virginiana have shown that there are sex chromosomes in strawberries that have two linked loci, one for androecial function (A), and one for gynoecial function (G). Here, staminate (male) sterility is dominant over staminate fertility, and pistillate (female) fertility is dominant over pistillate sterility. Consequently, sterile plants can be formed (Spigler et al., Citation2008). However, this could not be confirmed in F. chiloensis (Goldberg et al., Citation2010).
Anecdotally, hermaphrodites in F. chiloensis have been seen to set fruit uniformly within an inflorescence, whereas in F. virginiana fruit set within an inflorescence can be very variable.
Further, it appears that female plants can be more fertile than hermaphrodites within progeny of a single F. virginiana family (Spigler et al., Citation2010). They postulate that females increase their seed fertility/fruit set to invade and establish within a population (Bishop et al., Citation2010). The function of the sex-determining genes is possibly influenced by additional genetic factors, e.g., variable methylation (Gorelick, Citation2003), or accumulation of transposons in non-recombining regions of the sex chromosomes (Zhang et al., Citation2008).
Cytoplasmic staminate sterility systems are common in the genus Fragaria. Genes for staminate organs in F. chiloensis may be inactivated by the cytoplasm of F. virginiana (Staudt, Citation1997). Also, cytoplasmic staminate sterility has been observed in progenies of F. vesca L. and F. virginiana (Staudt, Citation1967, Citation1984).
The goal of this article is to further investigate the expression of gender, and its effect on fruit set along the inflorescence within the octoploid species of Fragaria. Three expressions of gender were evaluated: female receptivity, variations in hermaphrodism, and the inheritance of gender in progeny of one F. chiloensis clone. Here, we have used the term ‘female receptivity’ to mean the consistent uniform fruit set along an inflorescence in female plants within a single family.
Methods
Female fertility and variability in the fruit set of hermaphrodites
In 1994, one female selection, F. virginiana High Falls 22, and three hermaphrodite selections, N8688, RH23, and Montreal River 10, were hybridized with five June-bearing cultivars: Chandler, Totem, Glooscap, Cardinal, and Governor Simcoe. Seedlings of each family were planted in late May 1994 at Simcoe, Ontario and late July 1994 at Benton Harbor, Michigan. The experimental details are given in Hancock et al. (Citation2002).
In 1996, one female F. chiloensis selection, Pigeon Point, from California was hybridized with 14 F. virginiana selections. The 14 F. virginiana selections were two males, Frederick 9 and Hemlo 2, and two hermaphrodites, Eagle 14 and Montreal River 10, from Ontario; five hermaphrodites, LH10-6, LH28-1, LH30-4, LH39-15, and LH40-4, from Montana; and five hermaphrodites, RH18, RH23, RH30, N8688, and N8417, from New York, Minnesota, Alberta, and Alaska (Hancock et al., Citation2002; Luby et al., Citation2008). Seedlings from each family were planted in late May at Simcoe, Ontario and in late July at Becker, Minnesota (Luby et al., Citation2008).
Among the characteristics recorded was the presence or absence of anthers and a subjective scale was used to estimate the percentage fruit set (1–10 representing 10% intervals). These ratings were used to designate the plants as female (anthers absent or small and infertile), hermaphrodite (anthers present and at least a score of 1 for fruit set), or male (anthers present but a score of 0 for fruit set).
To study female receptivity in the two female clones, F. virginiana High Falls 22 and F. chiloensis Pigeon Point, the female and hermaphrodite plants were separated and the number of seedlings in each % fruit set class for the five crosses with the hermaphrodite June-bearing F. × ananassa cultivars were added together.
To study variations in fruit set among progeny of the hermaphrodites, the number of seedlings in each percent fruit set class for the five crosses of each individual F. virginiana clone and hermaphrodite June-bearing F. × ananassa cultivars were added together.
The number of fruits in each percent fruit set class was analyzed for gender over two locations using a general linear model (SAS/STAT, version 9.2, SAS Institute Inc., Citation2009).
Inheritance of gender in progeny of one F. chiloensis clone
In 1996, FRA1267, a very fertile hermaphrodite from British Columbia, was crossed to 12 of the 14 F. virginiana listed above (excluding Eagle 14 and LH30-4). It was used as a female parent with 10 genotypes and used as a male parent with RH30 and N8417. Seedlings of each family were planted in late May 1994 at Simcoe, Ontario and late July at Benton Harbor, Michigan.
In 2009, FRA1267 was used as a female parent and crossed with a hermaphrodite day-neutral F. × ananassa. The resulting progeny segregated for females and hermaphrodites. Five of these females (6F, 7F, 9F, 10F, 14F) were crossed in all combinations with three of the hermaphrodites (1H, 2H, 15H). The three hermaphrodites were selfed and two of them crossed together. Also, three of the five females and one of the hermaphrodites were backcrossed to F. × ananassa 11S41S4, a day-neutral, hermaphrodite genotype selfed for four generations.
For each plant of the F. virginiana crosses, the gender was recorded as female, hermaphrodite, or male. In the sib-crosses, gender was recorded as female, hermaphrodite with fully developed anther, hermaphrodite with aborted anthers, and male.
To study the inheritance of gender in progeny of FRA 1267, number of seedlings in each gender type within each family was counted, and whether FRA 1267 was used as the female or male parent noted. Since FRA1267 is hermaphrodite, where it was used as a female parent, the expected gene model was assumed to be a single recessive gene. The 3 hermaphrodite:1 female ratio was tested for significance by Chi2.
Results
Female fertility
For F. virginiana High Falls 22 the number of plants differed significantly for the interactions between percent fruit set * gender (P < 0.0001) and fruit set * gender * location (P < 0.0002). Location * gender did not differ significantly (P < 0.0001). For F. chiloensis Pigeon Point all three interactions varied significantly (P < 0.0001).
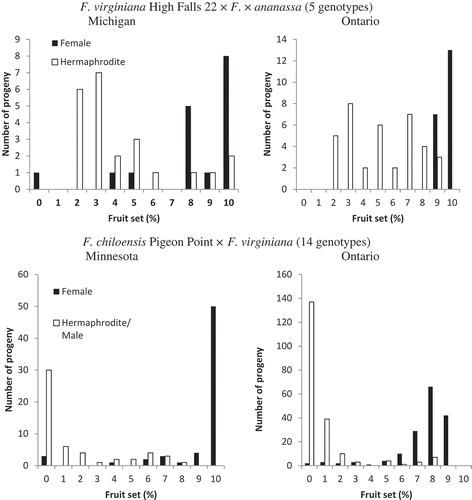
In families from both F. virginiana High Falls 22 and F. chiloensis Pigeon Point the female plants had on average a greater percent fruit set than the plants with hermaphrodite flowers, both in Ontario and Michigan. For High Falls 22, the percent fruit set was 8.4 and 9.8 for females, and 4.9 and 5.6 for hermaphrodites in Michigan and Ontario, respectively, and for Pigeon Point, 9.0 and 7.5 for females, and 3.2 and 2.5 for hermaphrodites in Minnesota and Ontario, respectively (). Also, both female genotypes produced sterile plants. Pigeon Point also had many male progeny, which were infertile (0 for fruit set) at both locations.
Figure 1. Fruit set on a scale of 1–10 in pistillate, and staminate flowered progeny of crosses of female F. virginiana High Falls 22 with five F. × ananassa genotypes, and female F. chiloensis Pigeon Point with 14 F. virginiana genotypes, each cross planted at two different locations of Benton Harbor, MI; Becker, MN; and Simcoe, ON.
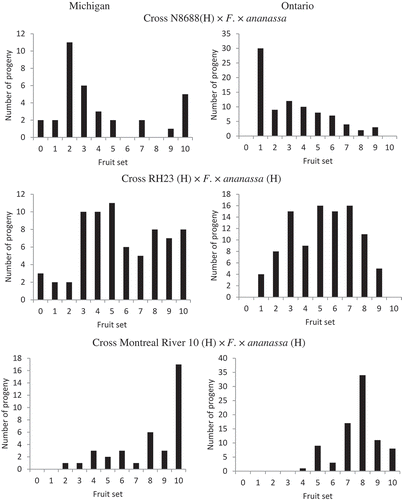
Variability in the fruit set of hermaphrodites
The F. virginiana hermaphrodites appeared to have three separate expressions of fruit set. The families from N8688 had on average a low fruit set (4.0 and 3.3 in Michigan and Ontario, respectively), RH23 an intermediate fruit set (6.5 and 5.2 in Michigan and Ontario, respectively), and Montreal River 10 had a high fruit set (8.0 and 7.7 in Michigan and Ontario, respectively). Male plants were observed in the N8688 and RH23 families in Michigan, but not in Ontario ().
Inheritance of gender in progeny of one F. chiloensis clone
When hermaphrodite F. chiloensis FRA1267 was crossed with F. × ananassa and F. virginiana genotypes, gender was expressed differently in the seedlings depending on whether it was used as a female or male parent. When it was used as a female parent, on average 80% of the offspring were female, whereas when it was used as a male parent 0% of the offspring were female (). Where the F. × ananassa crosses were female parents, the hermaphrodites segregated for fertile or sterile anthers, and where they were the male parents, all of the hermaphrodites had fertile anthers. None of the ratios tested fitted the model for a single recessive gene.
Table 1. Gender ratio of the progeny of F. chiloensis FRA 1267 crossed with F. × ananassa and F. virginiana when used as a female or male parent.
Discussion
Here, we have demonstrated three expressions of gender in Fragaria: (1) female plants of F. virginiana and F. chiloensis are more fertile than their hermaphrodite siblings, (2) hermaphrodite F. virginiana produce progeny with at least three levels of flower fertility, and (3) in F. chiloensis gender also can be inherited cytoplasmically.
The presence of sterile plants in both F. virginiana and F. chiloensis confirms the two-gene model of Spigler et al. (Citation2008). However, the presence of a high proportion of infertile male progeny in Pigeon Point suggests that other genetic factors, such as epistasis, may affect the expression of gender. Also, the variability of fertility in the hermaphrodites suggests that the expression fertility in hermaphrodites could be controlled polygenetically.
Environment also can influence the expression of gender and hence complicate our understanding of the underlying genetics. For example, in our Pigeon Point progeny, the expression of fruit set in Minnesota matched the expression of a genetic population between a female and male F. × ananassa subsp. cunifolia plant from Oregon and the expression of fruit set in Ontario matched that of a different population between a female and hermaphrodite plant (Govindarajulu et al., Citation2013). Also, female and hermaphrodite genotypes can produce anthers or have infertile anthers, respectively, when transferred from the field to the greenhouse. Spigler and Ashman (Citation2011) suggested that the proportion of females in F. virginiana populations was related to soil nitrogen. However, they did not test whether this proportion changed when the populations were grown in different soil nitrogen environments.
Female genotypes are more fertile than hermaphrodites. Previously, F. virginiana was found to adjust female investment via changes in fruit and seed set than ovule number (Bishop et al., Citation2010). Also, there can be many possible mechanisms to control this: possibly, females require lower levels of auxin to set fruit, or pistillate flowers could be more attractive to insects or females easily increase their seed fertility to better establish in a population (Bishop et al., Citation2010).
Fruit set within the hermaphrodites appeared to be controlled polygenetically. We conclude this because F. virginiana RH23, which only sets terminal fruits, and Montreal River 10, which sets many fruits on the inflorescence, had very different fruit set patterns in their progeny.
Within progeny of F. chiloensis FRA 1267 we have shown cytoplasmic inheritance of gender. Previous studies have shown that genes for staminate organs in F. chiloensis may be inactivated depending on the cytoplasm of F. virginiana (Staudt, Citation1997). Also, cytoplasmic staminate sterility has been observed in progenies of F. vesca L. and F. virginiana (Staudt, Citation1967, Citation1984). However, in our case the apparent cytoplasmic effect is coming from a hermaphrodite parent. It is unclear why FRA 1267 is fertile.
In conclusion, gender in strawberries appeared to be controlled by two closely linked genes in F. virginiana and F. chiloensis, and it is partially regulated by epistasis or cytoplasmic genes. There are other genes, many as yet undefined, which also affect the expression of the gender, in particular, fruit set.
Literature cited
- Ahmadi, H., and R.S. Bringhurst. 1991. Genetics of sex expression in Fragaria species. Amer. J. Bot. 78(4):504–514.
- Ashman, T.L., J.A. Tennessen, R. Dalton, R. Govindarajulu, M.H. Koski, and A. Liston. 2015. Multilocus sex determination revealed in two populations of gynodioecious wild strawberry, Fragaria vesca subsp. bracteata. Genes, Genomes, Genet. 5:2759–2773.
- Bentvelsen, G.C.M., and W. Sterk. 1996. Fragaria plants and seeds. U.S. Patent US005585540A.
- Bishop, E.J., R.B. Spigler, and T.L. Ashman. 2010. Sex-allocation plasticity in hermaphrodites of sexually dimorphic Fragaria virginiana (Rosaceae). Botany 88:231–240.
- Goldberg, M.T., R.B. Spigler, and T.L. Ashman. 2010. Comparative genetic mapping points to different sex chromosomes in sibling species of wild strawberry (Fragaria). Genetics 186:1425–1433.
- Gorelick, R. 2003. Evolution of dioecy and sex chromosomes via methylation driving Muller’s ratchet. Biol. J. Linn. Soc. 80:353–368.
- Govindarajulu, R., M. Parks, J.A. Tennessen, A. Liston, and T.L. Ashman. 2013. Comparison of nuclear, plastid, and mitochondrial phylogenies and the origin of wild octoploid strawberry speciesm. Amer. J. Bot. 102(4):1–11.
- Hancock, J.F., J.J. Luby, A. Dale, P.W. Callow, S. Serc, and A. El-Shiek. 2002. Utilizing wild Fragaria virginiana in strawberry cultivar development: Inheritance of photoperiod sensitivity, fruit size, gender, female fertility and disease resistance. Euphytica 126:177–184.
- Luby, J.J., J.F. Hancock, A. Dale, and S. Serce. 2008. Reconstructing Fragaria ananassa utilizing wild F. virginiana and F. chiloensis: Inheritance of winter injury, photoperiod sensitivity, fruit size, female fertility and disease resistance in hybrid progenies. Euphytica 163:57–65.
- SAS Institute Inc. 2009. SAS Users Guide. SAS/STAT, Version 9.2. SAS Institute Inc., Cary, NC.
- Spigler, R.B., and T.L. Ashman. 2011. Sex ratio and subdioecy in Fragaria virginiana: The roles of plasticity ANF gene flow examined. New Phytol. 190:1058–1068.
- Spigler R.B., K.S. Lewers, A.L. Johnson, and T.L. Ashman. 2010. Comparative mapping reveals autosomal origin of sex chromosome in octoploid Fragaria virginiana. J. Hered. S107–117.
- Spigler, R.B., K.S. Lewers, D.S. Main, and T.L. Ashman. 2008. Genetic mapping of sex determination in wild strawberry, Fragaria virginiana, reveals earliest form of sex chromosome. Heredity 101:507–517.
- Staudt, G. 1967. The genetics and evolution of heteroecy in the genus Fragaria I. The interspecific crosses F. vesca × F. orientialis and F. viridis × F. orientalis, Zeitschrift fur Pflanzenzuchtung 58:309–322.
- Staudt, G. 1984. The cytological evidence of double restitution in Fragaria. Plant Syst. Evol. 146:171–179.
- Staudt, G. 1997. Reconstitution of Fragaria × ananassa: The effect of Fragaria virginiana cytoplasm. Acta Hort. 439:55–62.
- Zhang, W., X. Wang, Q. Yu, R. Ming, and J. Yang. 2008. DNA methylation and heterochromatinization in the male-specific region of primitive Y chromosomes in papaya. Genome Res. 18:1938–1943.