ABSTRACT
Commercial strawberries (Fragaria × ananassa) are one of the most investigated berries for nutritional and nutraceutical properties. A strawberry breeding program, including inter-specific back-crosses, is now producing new genotypes with increased vitamin C, folates, and phenols combined with important commercial traits. The release of new cultivars with a high content of bioactive compounds, which may contribute to health, wellness, and reduced disease risk, is expected from this program. The ultimate goal is to properly obtain and integrate new scientific knowledge from genetic, agronomic, and biomedical studies that may bring acceptance from food agencies for specific health claims on new strawberry cultivars.
Introduction
Berries play an important role in the human diet due to their chemical composition, which is naturally enriched with many nutritional and bioactive compounds, such as minerals, vitamin C, folate, and phenolic substances (Giampieri et al., Citation2012). In the last decade, and as a consequence of market demand, a high level of berry production in European Union (EU) countries has been recognized by official organizations, such as Food and Agriculture Organization of the United Nations (FAO) (Citation2007) and more recently in the framework of EU FP7 Euberry Project (Mezzetti, Citation2016; http://www.euberry.univpm.it/). Among small fruit, strawberry is the most important berry crop cultivated and consumed in the EU, with an annual fruit production exceeding 1.16 million metric tonnes cultivated on the area of 122,844 ha. The strawberry is particularly interesting, since production is widespread, with varying intensity, across all of the EU countries (FAO, Citation2007).
Moreover, strawberry production can provide economic development opportunities to rural areas due to its high value in the fresh and processed markets.
In addition, the importance of strawberry, with respect to human health benefits, is also emerging with recognition at the consumer level (Alvarez-Suarez et al., Citation2011, Citation2014; Giampieri et al., Citation2012; Romandini et al., Citation2013; Tulipani et al., Citation2014).
The strawberry crop contributes to many rural economies within the EU, especially in terms of employment and added economic value (Binard, Citation2013). Strawberries are highly perishable fruits, often sold immediately after harvest at a high price, especially when hand-picked (fresh fruit are almost exclusively hand-picked). Offering a consistently high quality fruit with superior nutritional content is an ideal way to increase consumer interest and satisfaction, and increasing strawberry consumption will contribute positively to a healthy diet. The number of studies demonstrating the benefits of berries, and in particular strawberries, for the consumers is continuously increasing (Giampieri et al., Citation2012), but it is still difficult to make consumer health claims for commercial use.
In this work, we identify the genetic variability determining traits related to human health benefits and discuss the compositional results for new cultivars with regulated claims.
Materials and methods
Five commercial cultivars (Adria, Clery, Cristina, Monterey, and Romina), three originating from the Università Politecnica delle Marche (UNIVPM) breeding program (Adria, Cristina, and Romina), and 10 selections originating from an inter-specific backcrossing program Fragaria × ananassa × F. virginiana glauca (), were planted at the P. Rosati University experimental farm, Agugliano (Ancona, Italy), in open-field conditions using the plastic hill culture production system. Fruit samples of fully red berries were harvested at the second, third, and fourth main pickings and stored at 20 °C until analyses.
Table 1. Advanced selections from Polytechnic University of Marche analyzed in this study, and corresponding parents.
Extraction methods
For the evaluation of total phenolic content (TPH), total anthocyanin content (ACY), and antioxidant capacity, a methanol extract was prepared. Briefly, for each genotype 10 ripe, commercial fruits were sampled and from each fruit two opposite slices were cut and milled into small pieces. Then 10 g of this blend were weighed and placed in a tube for extraction with 20 ml of methanol. After homogenization with an Ultraturrax T25 homogenizer (Janke and Kunkel, IKA Labortechnik, Staufen, Denmark), the suspension was continuously stirred for 30 min in the dark. Then it was centrifuged at 4500× g for 10 min (Centrifuge Rotofix, Hettich Zentrifugen, Tuttlingen, Denmark), and 3 × 1 mL of the supernatant was collected and stored in amber vials at −20 °C. The fruit pellet was extracted a second time by adding another 20 mL of methanol and repeating the previously described procedure. The supernatant was collected and added to the previous ones, and 3 × 1 ml per sample were stored in amber vials at −20 °C.
Soluble solids determination (SS)
SS content was determined using a hand-held refractometer (N-1E, ATAGO, Tokyo, Japan), and results were expressed as °Brix. Results were expressed as means of three replications ± standard deviation.
Total acidity (TA)
The TA of strawberries was determined starting from 10 mL of juice diluted with distilled water (1:2, v:v) and titrated with 0.1 N NaOH solution to pH 8.2 through a burette. Results were expressed as mEQ of NaOH per 100 g FW, means of three replications ± standard deviation.
Total antioxidant capacity (TAC)
TAC was measured through the 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) assay (Miller et al., Citation1993; Re et al., Citation1999). ABTS is a colorless substance that turns into the colored monocationic radical form when exposed to an oxidative agent. The extent of decolorization of the monocationic radical form is a function of the antioxidants present in the strawberries, and was calculated relative to the reactivity of Trolox, a water-soluble vitamin E analog. The antioxidant activity is expressed as mg Trolox equivalent per kg FW and results were expressed as means of six replications ± standard deviation.
Total phenolic content (TPH)
The fruit TPH was evaluated through the Folin-Ciocalteu’s reagent method (Slinkard and Singleton, Citation1977). Briefly, a glass test tube was filled with 7.0 mL of water and 1 mL of the diluted sample (1:20) was added, followed by the addition of 500 mL of 2N Folin Ciocalteu-Reagent (Sigma-Aldrich, St. Louis, MO, USA). The solution was vortexed and allow to react for 3 min; then 1.5 mL of a 20% sodium carbonate solution was added, and the tube was mixed again and stored in the dark for 60 min. After this incubation, the sample absorbance was measured at 760 nm and data were expressed as mg gallic acid per kg FW. Results were obtained as means of six replications ± standard deviation.
Total anthocyanin content (ACY)
Total fruit ACY was measured using the pH differential shift method (Giusti and Wrolstad, Citation2001). Each sample was diluted 1:10 with two different solutions: potassium chloride (pH 1.00) and sodium acetate (pH 4.50) solutions. Then the absorbance for both of the solutions was measured at 500 and 700 nm. Data were expressed as mg pelargonidin-3-glucoside (molar extinction coefficient 15,600 L mol–1 cm–1, molecular weight 433.2 g mol–1) per kg FW. Calculations of anthocyanins as mg of pelargonidin-3-glucoside per kg FW were calculated through the following equation:
where A is absorbance; MW is molecular weight of pelargonidin-3-glucoside; F is dilution factor; d is cell path lengths; ε is molar extinction coefficient of pelargonidin-3-glucoside; E is sample weight; and 1000 is factor for mg. Results were obtained as means of six replications ± standard deviation.
Vitamin C content (vit C)
For vitamin C quantification, the extracting solution consisted of MilliQ water containing 5% meta-phosphoric acid and 1 mM ethylenediaminetetraacetic acid (EDTA). Vit C was extracted by sonication of 1 g of frozen strawberries in 4 ml of extracting solution for 5 min, after a previous homogenization using an Ultraturrax T25 homogenizer (Janke and Kunkel, IKA Labortechnik, Staufen, Denmark) at medium-high speed for 2 min. After a centrifugation at 2500 rpm for 10 min at 4 °C, the supernatant was filtered using a 0.45 μm NY (nylon) filter into 1.8 mL high pressure liquid chromatography (HPLC) vials, and immediately analyzed (Tulipani et al., Citation2008). Vit C (ascorbic acid) was measured as previously described (Helsper et al., Citation2003). Strawberry extracts were subjected to HPLC analysis immediately after the extraction procedure. The HPLC system was comprised of a Jasco PU-2089 Plus controller and a Jasco UV-2070 Plus ultraviolet (UV) (Jasco Inc., Easton, MD, USA) detector set at absorbance of 260 nm. The HPLC column used was a Supelcosil LC8 150 × 4.6 mm (Sigma-Aldrich Corp., St. Louis, MO, USA). The elution was isocratic with 50 mM of potassium phosphate (KH2PO) in MilliQ (MQ) water, leading to pH 3.2 (below the pKa of the ascorbic acid) by adding orthophosphoric acid, and analysis consisted of a 10-min run, after which the column was cleaned with 50% acetonitrile. Quantification of the vit C content was carried out through a calibration curve prepared by running standard concentrations of vit C. Results are expressed as mg vit C per 100 g FW and were obtained as means of three replications ± standard deviation.
Folates content
For folate extraction, 5 g of frozen strawberries were added to 15 ml of extraction buffer (0.1 M phosphate buffer containing 1.0% of L(+)-ascorbic acid (w/v) and 0.1% 2,3-Dimercapto-1-propanol (v/v) at pH 6.5, freshly prepared) and homogenized using an Ultraturrax T25 homogenizer at medium-high speed for 2 min. The capped tube was then placed in a water bath at 100 °C for 10 min, and then rapidly cooled on ice. The tube was then centrifuged at 4000× g for 20 min at 4 °C. The supernatant was collected in a 25-ml volumetric flask, and filled up to 25 ml exact volume with extraction buffer. From this flask, 5 ml of this solution was taken and placed in another centrifuge tube. For deconjugation of polyglutamylated folates, 150 μl of folate conjugase from rat serum was added to the 5 ml of solution, and incubated on a shaking oven at 37 °C for 2 h. Then, an additional treatment of 5 min at 100 °C was carried out to inactivate the enzyme, again followed by cooling on ice. The samples were then centrifuged again at 4000× g for 20 min at 4 °C. The final supernatant was then filtered using 0.45-μm filter pore size, 25-mm inner diameter, nylon disposable syringe filters, and the filtrates were purified through solid-phase extraction on strong anion-exchange Isolute cartridges as described previously (Iniesta et al., Citation2009; Jastrebova et al., Citation2003). The chromatographic separation method previously described was used, with slight modification. An HPLC system (Jasco PU-2089 Plus), consisting of a gradient binary pump, an UV detector (Jasco UV-2070 Plus), a fluorescence detector (FLD; Jasco FP-2020 Plus, Jasco Inc., Easton, MD, USA), and a computer running ChromNAV software (Jasco Inc., Easton, MD, USA) was used. The separation of folates was performed on a Mediterranea Sea18 (Teknokroma, Sant Cugat del Vallés, Barcelona, Spain) 250 × 4.6 mm, at room temperature. The flow rate was 0.4 ml/min. The injection volume was 20 µl, with a total running time of 42 min. For the detection and quantification of folates, a FLD (excitation/emission = 290/360 nm) was used. Peak purity and identity were confirmed by a comparison of relative peak areas in both detectors with external standards. The mobile phase was a binary gradient mixture of 30 mM potassium phosphate buffer at pH 2.3 and acetonitrile. Results are expressed as µg of each folate per 100 g FW. Results were obtained with three replications ± standard deviation.
Principal component analysis (PCA)
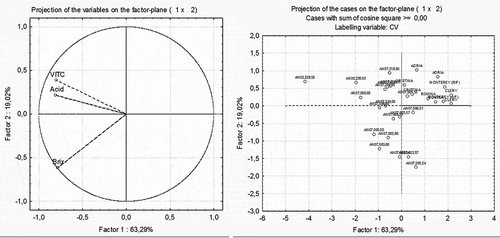
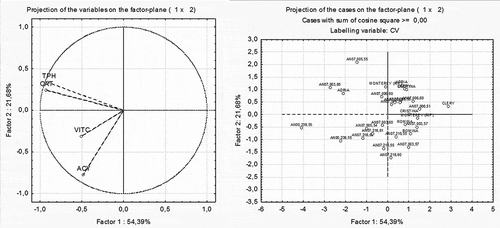
PCA was used to evaluate the levels of association among the various quality and nutritional parameters. The factor loading values are the correlations of each variable with the PC and are represented as vectors in the space represented by the axes of the PCA bi-plots ( and ). In the graphs, the closer the variables (left part of and ) and observations (right part of and ) are to each other in the same geometric plane of the bi-plot, the more interrelated they are. Similarly, the greater the distance of a vector from the origin of the axis, the higher the correlation of the variable with the PC represented in that axis. Analyses were performed using STATISTICA software (StatSoft Inc., Tulsa, OK, USA).
Results and discussion
For most fruit breeding programs within the EU, fruit quality components have increased in importance as targets for new cultivars in recent years (EUBerry project, Program EU FP7 KBBE–2010-4, Grant agreement no. 265942; GoodBerry project, Program H2020–SFS-05-2015, Grant agreement no. 679303). These quality components comprehend both agronomic (adaptability to different environmental conditions and cultivation techniques) and quality (commercial, sensorial, and in particular nutritional quality) traits, and the ultimate goal for breeders and commercial growers is a plant variety that combines these traits that can be grown sustainably under future climate scenarios projected for the EU. These findings are supported by the specific challenge and scope of the topic ‘SFS-05-2015: Strategies for crop productivity, stability and quality’ as part of the “Horizon 2020 Societal Challenge 2 Focus Area: Sustainable Food Security,” which demonstrate that EU aimed to improve knowledge and procedures to facilitate development of well accepted and desirable fruits with improved plant adaptation and high fruit quality, even under a changing climate, e.g., higher temperature. Breeding for the enhancement of one or more beneficial phytochemicals in strawberry fruit is likely to be achievable by using selected germplasm as source material in specific breeding programs to combine them with the commercial traits.
The analyses of the SS and TA in the varieties and selections of the present study indicate that the selections AN07,003,60, AN07,005,54, and AN00,239,55 had a higher sugar content (11.10, 10.50, and 10.10 °Brix, respectively, ). Among these selections, AN00,239,55 also showed a very high value of TA (18.90 meq NaOH/100 g FW, ), while AN07,005,54 and AN07,003,60 showed lower values (11.50 and 10.20 meq NaOH/100 g FW, respectively, ). AN07,216,60 and AN07,216,55 also showed very high values of TA (16.70 and 15.40 meq NaOH/100 g FW, ), but lower SS (9.90 and 8.60 meq NaOH/100 g FW) relative to AN07,003,60, AN07,005,54, and AN00,239,55.
Table 2. RSR, TA, TPH, ACY, TAC, and VIT C of the analyzed cultivars.z
The phenolics in fruit encompass a broad chemical range across subgroups, such as anthocyanins, flavonols, flavan-3-ols, ellagitannins, procyanidins, hydroxycinnamic acids and their esters, etc. (Tulipani et al., Citation2009a). In this study, the selection AN00,239,55 showed higher total phenols, with 2321 mg GA/Kg FW, followed by AN07,003,60 and the cultivar Adria, with 2233 and 2110 mg GA/Kg FW, respectively.
Among phenolic compounds, berries contain significant levels of anthocyanins compared to other fruit, and in planta these are responsible for the fruit colors (Diamanti et al., Citation2015). They have often been suggested to have a positive impact on human health (Tsuda, Citation2012). This idea supports the selection of anthocyanins for fruit breeding and product development, with a view to human health maintenance and improvement. In this study the highest concentration of total anthocyanins was found in the selection AN07,216,60, with a concentration of 893 mg PEL-3-GLU/Kg FW, followed by AN00,239,55 (844 mg PEL-3-GLU/Kg FW) and AN07,005,54 (796 mg PEL-3-GLU/Kg FW; ). As with many of the phenolic subclasses, anthocyanin composition varies among berry fruit crops (Beattie et al., Citation2004; Scalzo et al., Citation2005; Wu et al., Citation2006), leading to a variation in the associated antioxidant activity (Tulipani et al., Citation2008, Citation2009b). In fact, TAC changes greatly considering the different analyzed genotypes; in particular, AN00,239,55 showed the highest TAC with a value of 28.2 mgTE/Kg FW. Then AN07,003,60 and ‘Adria’ showed the highest values of TAC, with 26.3 and 24.9 mgTE/Kg FW, respectively (). The in vitro antioxidant activity was extensively considered in strawberries and the role of the genotype in affecting the phytochemical composition is already well established (Azodanlou et al., Citation2003; Giampieri et al., Citation2012; Wang et al., Citation2002).
Table 3. Folates identification and quantification attempt in three Polytechnic University of Marche cultivars and one Polytechnic University of Marche selection.z
The European Food Safety Authority (EFSA) evaluates the applications for health and nutritional claims on food (http://www.efsa.europa.eu/en/topics/topic/nutrition.htm). Health claims for vitamin C can be accepted for food that qualifies as a source of vitamin C as listed in the Annex to Regulation (EC) No. 1924/2006. To bear the “source” claim, the respective food has to contain “significant amounts” of vitamin C. “Significant amounts” is defined as containing at least 15% of recommended daily allowance in a 100-g serving. The European Recommended Daily Intake RDA value for vitamin C is 80 mg (Commission directive 2008/100/EC), so a food source containing at least the 15% of 80 mg of vitamin C in a 100-g serving could be considered as a claiming source of vitamin C. The EFSA has released claims for vitamin contents in food and their positive activity regarding human health. For example, the following vitamin C health claims for different effects on human health, have been released: reduction of tiredness and fatigue (ID 139, 2622); restoring normal psychological functions (ID 140); regeneration of the reduced form of vitamin E (ID 202); contribution to normal energy-yielding metabolism (ID 2334, 3196); maintenance of the normal function of the immune system (ID 4321); and protection of DNA, proteins, and lipids from oxidative damage (ID 3331). A similar approach can also be thought of for new strawberry cultivars. Therefore, new breeding programs can select certain strawberry genotypes containing a high amount of vitamin C for functional claim application according to article 13.1 and will thus provide clear and transparent information to the consumer.
As an example, new cultivars that can be claimed for a significant amount of vitamin C are the selections and varieties released by the Ancona University breeding program. Almost all of them have a stable content of vitamin C higher than 30 mg/100 g of fresh fruit (), double the minimal RDA requested for a claim on significant amount of vitamin C. In particular, AN00,239,55 showed a value of 88.7 mg/100 g FW, followed by AN07,006,60 and AN07,003,60, with 61.9 and 57.7 mg/100 g FW (). Giving the importance of vitamin C as a putative characteristic for functional claim application, its correlation with other qualitative and nutritional parameters was evaluated through the PCA. Titratable acidity and vitamin C seems to be well correlated, and also the selection AN00,239,55 differs from all of the other genotypes for vitamin C and titratable acidity. Also in Figure 1, a correlation between vitamin C and titratable acidity was confirmed, but not among these two parameters and soluble solids (, left). Likewise in this case, the selection AN00,239,55 is far from all of the other genotypes (, right). Finally, in , TPH and TAC seem to be strongly related to each other, more than ACY and vitamin C (, left). Again, in the right side of , AN00,239,55 results are slightly different from the other cultivars and selections.
In case of new claims based on articles 13.1 (general function claim), 13.5 (new claims based on proprietary data), and 14.1 (reduction of disease risk claims) it is necessary to present a larger dossier to EFSA. To assess health claims the EFSA has a two-step procedure (see EFSA guidelines: http://www.efsa.europa.eu/en/nda/ndaguidelines.htm). In the first step, it is evaluated if the food constituent is well defined, if a cause and effect relationship could be established between the consumption of the food and the claimed effect for the consumers under the proposed condition of use. In the second step, it is determined if the food can be consumed within a balanced diet. The substantiation of the claim depends on a favorable outcome of both steps. For example, if the recommended dose of folate for a pregnant woman is 0.4 mg/day (Scientific Committee for Food, Citation1993), it is possible to declare that the strawberry eliminates the risk of folate deficiency during this period only after the verification of these two steps. In the first step, the stability of the concentration of folates must be verified in fruits of the same cultivars, while in the second step it must be established that the strawberry consumption in a balanced diet effectively provides the required amount of folates necessary for avoiding the complications related to their deficiency. In that study, an attempt of measuring the folates in strawberry was made, but the results must be repeated and the stability must be verified. The most important aspect that could be underlined is that a big difference in folate concentrations seems to exist among the evaluated cultivars ().
Conclusions
Strawberries with higher amounts of various phytochemical compounds exist, and it can be easily proved by scientific data (Diamanti et al., Citation2014; Tulipani et al., Citation2008) and also by studies like the present work, which demonstrates that the UNIVPM intra and interspecific breeding program is generating strawberries with great qualitative and nutritional characteristics, nearly combined to other important commercial traits (yield, berry size, and firmness). Nevertheless, the way to obtain a market recognition is still long. The existing rules for the release of health claims for new commercial products and, in particular, on fresh fruit (including strawberry), are rigorous and important to save the consumer from fraud and misleading commercial and marketing propaganda. Therefore, while the rules cannot be changed, scientists should understand them and work in order to produce the scientific data requested by the food safety authorities for the evaluation and approval of new commercial claims on berry health benefits. In the future, scientists may focus their research to an interdisciplinary approach able to provide the information required for the claim evaluation and consequently for providing science-based details on the composition and health benefits of a new commercial strawberry.
Funding
The research work was supported by the EU FP7 EUBerry project No. 265942 and by the National Natural Science Foundation of China (Grant No. 31201579).
Additional information
Funding
Literature cited
- Alvarez-Suarez, J.M., D. Dekanski, S. Ristić, N.V. Radonjić, N.D. Petronijević, F. Giampieri, P. Astolfi, A.M. González-Paramás, C. Santos-Buelga, S. Tulipani, J.L. Quiles, B. Mezzetti, and M. Battino. 2011. Strawberry polyphenols attenuate ethanol-induced gastric lesions in rats by activation of antioxidant enzymes and attenuation of MDA increase. PLoS One 6(10):e25878.
- Alvarez-Suarez, J.M., F. Giampieri, S. Tulipani, T. Casoli, G. Di Stefano, A.M. González-Paramás, C. Santos-Buelga, F. Busco, J.L. Quiles, M.D. Cordero, S. Bompadre, B. Mezzetti, and M. Battino. 2014. One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J. Nutr. Biochem. 25:289–294.
- Azodanlou, R., C. Darbellay, J. Luisier, J. Villettaz, and R Amadò. 2003. Quality assessment of Strawberry (Fragaria species). J. Agr. Food Chem. 51:715–721.
- Beattie, J., A. Crozier, and G.G. Duthie. 2004. Potential health benefits of berries. Curr. Nutr. Food Sci. 1:71–86.
- Binard, P. 2013. Strawberry in perspective “Fact and figures in Europe and in the world.” 2nd Intl. Strawberry Congr., Antwerp, Belgium, 4–6 Sept. 2013.
- Diamanti, J., F. Balducci, L. Di Vittori, F. Capocasa, C. Berdini, A. Bacchi, F. Giampieri, M. Battino, and B. Mezzetti. 2015. Physico-chemical characteristics of thermally processed purée from different strawberry genotypes. J. Food Compos. Anal. 43:106–118.
- Diamanti, J., L. Mazzoni, F. Balducci, R. Cappelletti, F. Capocasa, M. Battino, G. Dobson, D. Stewart, and B. Mezzetti. 2014. Use of wild genotypes in breeding program increases strawberry fruit sensorial and nutritional quality. J. Agr. Food Chem. 18:3944–3953.
- Food and Agriculture Organization of the United Nations (FAO). 2007. FAOSTAT: Crops. <http://faostat.fao.org/site/567/default.aspx#ancor>.
- Giampieri, F., S. Tulipani, J.M. Alvarez-Suarez, J.L. Quiles, B. Mezzetti, and M. Battino. 2012. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 28:9–19.
- Giusti, M., and R.E. Wrolstad. 2001. Characterization and measurement of anthocyanins by UV-visible spectroscopy, p. F1.2.1–F1.2.13. In: Current protocols in food analytical chemistry. John Wiley and Sons, Hoboken, NJ.
- Helsper, J.P.F.G., C.H. Ric de Vos, F.M. Maas, H.H. Jonker, H.C. van den Broeck, W. Jordi, C. Sander Pot, L.C.P. Keizer, and A.H.C.M. Schapendok. 2003. Response of selected antioxidants and pigments in tissues of Rosa hybrida and Fuchsia hybrida to supplemental UV-A exposure. Physiol. Plant. 117:171–178.
- Iniesta, M.D., D. Perez-Conesa, J. Garcia-Alonso, G. Ros, and M.J. Periago. 2009. Folate content in tomato (Lycopersicon esculentum): Influence of cultivar, ripeness, year of harvest, and pasteurization and storage temperatures. J. Agr. Food Chem. 57:4739–4745.
- Jastrebova, J., C. Witthoft, A. Grahn, U. Svensson, and M. Jagerstad. 2003. HPLC determination of folates in raw and processed beetroots. Food Chem. 80:579–588.
- Mezzetti, B. 2016. The sustainable improvement of European berry production, quality and nutritional value in a changing environment: Strawberries, currants, blackberries, blueberries and raspberries - the EUBerry project. Acta Hort. 1117:309–314.
- Miller, N.J., C. Rice-Evans, and M.J. Davis. 1993. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 83:407–412.
- Re, R., N. Pellegrini, A. Proteggente, A. Pannala, and M. Yang. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med. 26:1231–1237.
- Romandini, S., L. Mazzoni, F. Giampieri, S. Tulipani, M. Gasparrini, T.Y. Forbes-Hernandez, N. Locorotondo, M. D’Alessandro, B. Mezzetti, S. Bompadre, and J.M. Alvarez-Suarez. 2013. Effects of an acute strawberry (Fragaria × ananassa) consumption on the plasma antioxidant status of healthy subjects. J. Berry Res. 3(3):169–179.
- Scalzo, J., A. Politi, B. Mezzetti, and M. Battino. 2005. Plant genotype affects total antioxidant capacity and phenolic contents in fruit. Nutrition 21:207–213.
- Scientific Committee for Food (SCF). 1993. Nutrient and energy intakes for the European Community. Reports of the Scientific Committee for Food, Thirty First Series. European Commission, Luxembourg.
- Slinkard, K., and V.L. Singleton. 1977. Total phenol analysis: Automation and comparison with manual methods. Amer. J. Enol. Vitic. 28:49–55.
- Tsuda, T. 2012. Dietary anthocyanin-rich plants: Biochemical basis and recent progress in health benefits studies. Mol. Nutr. Food Res. 56:159–170.
- Tulipani, S., B. Mezzetti, F. Capocasa, S. Bompadre, J. Beekwilder, C.H. Ric de Vos, E. Capanoglu, A. Bovy, and M. Battino. 2008. Antioxidants, phenolic compounds and nutritional quality in different strawberry genotypes. J. Agr. Food Chem. 56:696–704.
- Tulipani, S., B. Mezzetti, and M. Battino. 2009a. Impact of strawberries on human health: Insight into marginally discussed bioactive compounds for the Mediterranean diet. Publ. Health Nutr. 12(9A):1656–1662.
- Tulipani, S., S. Romandini, M. Battino, S. Bompadre, F. Capocasa, and B. Mezzetti. 2009b. Variation in strawberry micronutrients, phytochemical and antioxidant profiles: The combined effect of genotype and storage. Acta Hort. 842:867–872.
- Tulipani, S., T. Armeni, F. Giampieri, J.M. Alvarez-Suarez, A.M. Gonzalez-Paramás, C. Santos-Buelga, F. Busco, G. Principato, S. Bompadre, J.L. Quiles, B. Mezzetti, and M Battino. 2014. Strawberry intake increases blood fluid, erythrocyte and mononuclear cell defenses against oxidative challenge. Food Chem. 156:87–93.
- Wang, S.Y., W. Zheng, and G.J. Galletta. 2002. Cultural system affects fruit quality and antioxidant capacity in strawberries. J. Agr. Food Chem. 50:6534–6542.
- Wu, X., G.R. Beecher, J.M. Holden, D. Haytowitz, S. Gebhardt, and R.L. Prior. 2006. Concentrations of anthocyanins in common food in the United States and estimation of normal consumption. J. Agr. Food Chem. 54:4069–4075.