ABSTRACT
Almond [Prunus dulcis (Mill.) D.A. Webb] is one of the most important nut crops worldwide. It requires chilling during winter to break dormancy and develop fruiting buds. However, late winter chilling and early spring frosts may damage the reproductive tissues leading to productivity reduction. In the present work, we evaluated the frost susceptibility by means of chlorophyll fluorescence in flower buds of almonds cultivated in two different sites in Morocco: Aknoul in the north and Sidi Bouhria in the east. Five widely grown almond cultivars, namely Marcona, Fournat de Brézenaud, Ferragnès, Ferraduel, and Tuono, were involved in this study. Flower buds were incubated in temperatures of –3, –2, –1, 0, and 25 °C during 24 h and thereafter the Fv/Fm ratio was measured at the ovary zone. Combined analysis of variance showed that cultivar was the major source of variability, while site and cultivar by site interaction effects were of lesser extent and explained together less than 20%. At 25 °C, all cultivars displayed higher scores of Fv/Fm ratio. By decreasing temperature of incubation, this ratio decreased proportionally. Kinetics of chlorophyll fluorescence in response to the frost treatment followed two patterns: A linear decrease translated by frost susceptibility for late-flowering cultivars Ferragnès and Ferraduel, and quadratic curve with an inflection point at –1 °C indicating a chilling tolerance for Tuono and the early-flowering cultivars Marcona and Fournat de Brézenaud. However, Ferragnès and Ferraduel (with later flowering date) are not likely to be affected by low temperatures at the end of spring when there is no risk of frost.
Introduction
Almond [Prunus dulcis (Mill.) D.A. Webb] is one of the oldest nut crops of Central and Western Asia. From this center of origin, almond has been widespread worldwide. Morocco is the fourth largest almond producer in the world, with a total average production of 96.523 tonnes of unshelled almonds that fluctuates considerably from year-to-year (FAOSTAT, Citation2013) as a consequence of many climatic occurrences, such as frosts and droughts. Almond cultivation was restricted to regions with low risk of spring frost, which is the limiting factor for almond production. The majority of research works in almond agronomy have focused on assessment and transmission of blooming date, blooming density, and productivity (Kodad and Socias i Company, 2006; Sánchez-Pérez et al., Citation2007; Socias i Company et al., Citation1999; Sorkheh et al., Citation2010; Valdebenito et al., Citation2017). Other works have been undertaken to assess environmental constraints such low and high temperatures (Barros et al., Citation2012; Doll and Shackel, Citation2016; Spinelli et al., Citation2016). Frost susceptibility was assessed by various authors in Spain (Kodad et al., Citation2010) and Iran (Imani et al., Citation2012).
Chlorophyll fluorescence measurement has become one of the most powerful and widely used techniques in plant ecophysiology (Maxwell and Johnson, Citation2000). This technique has many applications, such as sensing plant senescence, damages, viruses and diseases, chilling, heat, and salt stress, by using the Fv/Fm ratio as a measurement of intrinsic performance of photosystem II (PSII) reaction centers (Genty et al., Citation1989; Guo and Tan, Citation2015).
To our knowledge, there is a scarcity of information regarding frost susceptibility in almond cultivars grown in north-eastern Morocco. Therefore, the objectives of this work were: (i) to evaluate frost susceptibility in the most important growing cultivars in northeastern Morocco, and (ii) to ascertain the use of chlorophyll fluorescence as a faster and more efficient technique to evaluate frost susceptibility in flower buds.
Materials and methods
Study sites
This study was carried out at two different sites in Morocco, namely: Aknoul (60 km from Taza, 34° 39′ 0″ N, 3° 52′ 0″ W) in the north and Sidi Bouhria (40 km from Oujda, 34° 44′ 21″ N 2° 21′ 44″ W) in the east. These two sites were chosen because of their importance in regard to cultivated acreage and the availability of many commercial cultivars.
Plant material and sampling
The widely grown almond cultivars, namely: Marcona, Fournat de Brézenaud, Ferragnès, Ferraduel, and Tuono were involved in this study. According to Khanizadeh and DeEll (Citation2001), at 53 phenological stage on the general Biologische Bundesanstalt, Bundessortenamt und CHemische Industrie (BBCH) scale, 10 flower buds were collected from three tagged trees of each cultivar in the two sites stated above. Thereafter, samples were quickly brought to the laboratory in moist polyethylene bags. The sampled buds were incubated in temperatures of –3, –2, –1, 0, and 25 °C during 24 h.
Chlorophyll fluorescence (CF) measurements
CF measurements were made at the ovary zone of the flower buds where frost injury occurs following protocol given by Khanizadeh and DeEll (Citation2001). In order to avoid water stress, flower buds were amply moistened during the experiment. After 20 mn of dark adaptation, CF measurements were made at room temperature with a hand-held chlorophyll fluorometer (OS-30p, Opti-Science Inc., Hudson, NH, USA) using the Fv/Fm test, where Fv (variable fluorescence of dark-adapted tissue) = Fm (maximal fluorescence) – Fo (minimal fluorescence). The OS-30p has a pre-adjusted saturation pulse light source with a 0.8 s duration and an intensity of 3000 μmol m–2 s–1 (provided by an array of three light emitted diodes in the sensor) to reliably measure Fm. The measurements were done in a dark room without windows, with a single green 40-watt safe light used, providing a low level of illumination during the measurements.
Statistical analyses
For each temperature treatment, 10 bud flowers were considered for measurements of CF and thereafter averaged. Three replicates per cultivar and per site were regarded for statistical analysis. Combined analyses of variance (ANOVA) were calculated using the general linear model procedure over sites. Least significance differences (LSD) were computed at 5% as probability level. All statistical analyses were carried out by means of STATGRAPHICS package version XVI (Statpoint Technologies, Inc., Virginia, USA).
Results
Analyses of variance
Mean squares from the combined analyses of variance for Fv/Fm ratio at various temperatures are listed in . Over 72% of total variance was assigned to cultivar effect, while site effect was of lesser extent and accounted for about 13% of data variability. The magnitude of site × cultivar interaction explained only 7% of variance.
Table 1. Mean squares of the combined analyses of variance for chlorophyll fluorescence at –3, –2, –1, 0, and 25 °C in five almond cultivars grown at two different sites in Morocco: Aknoul in the north and Sidi Bouhria in the east.
Mean comparison between sites
shows mean values of Fv/Fm ratio for the whole tested temperatures. The LSD test did not detect significant differences between sites for most temperature treatments except for T = –1 °C and T = –2 °C, where Sidi Bouhria had higher values of Fv/Fm. Moreover, by increasing temperature of incubation the Fv/Fm ratio also increased. Values of Fv/Fm in cold temperatures (T = –3, –2, –1, and 0 °C) were lower than that of 25 °C. In fact, the lowest value (0.439) was exhibited in Aknoul at T = –3 °C, but the highest one was recorded at Sidi Bouhria (0.867) at T = 25 °C.
Table 2. Mean site values for chlorophyll fluorescence at –3, –2, –1, 0, and 25 °C in five almond cultivars grown at two different sites in Morocco: Aknoul in the north and Sidi Bouhria in the east.
Mean comparison among cultivars
Cultivar mean values averaged between sites for Fv/Fm ratio are summarized in . Significant variations were found between the five cultivars in terms of CF for most temperatures. Regarding the cold treatment, the lowest value of Fv/Fm (0.380) was recorded in Fournat de Brézenaud at T = –3 °C. For this temperature, Ferragnès exhibited the highest value of CF (0.523). For T = –2, Fv/Fm ranged from 0.573 in Tuono to 0.632 in Marcona and Ferragnès. On the other hand, at T = –1 and 0 °C, Marcona showed higher values of Fv/Fm, which were 0.783 and 0.798, respectively. Nevertheless, the lowest scores of Fv/Fm at –1 °C and 0 °C were recorded in Ferraduel (0.706) and Tuono (0.785). If we consider the temperature of 25 °C, Ferragnès was superior in terms of Fv/Fm (0.915), while the lowest value at the same temperature was 0.835 in Marcona.
Table 3. Mean genotypic values for chlorophyll fluorescence at –3, –2, –1, 0, and 25 °C in five almond cultivars grown at two different sites in Morocco: Aknoul in the north and Sidi Bouhria in the east.
Kinetics of chlorophyll fluorescence in response to the frost treatment
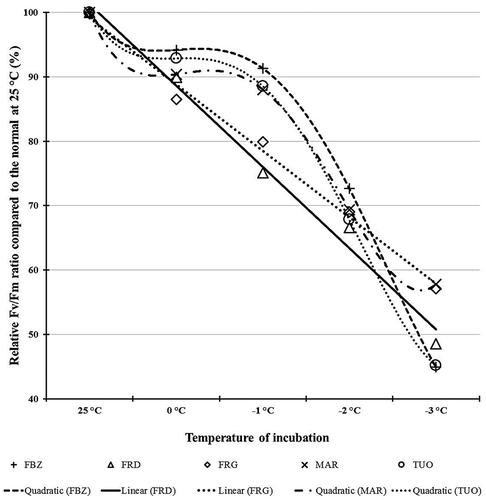
The recorded values of Fv/Fm were averaged over sites for each cultivar. Changes in the kinetics of chlorophyll fluorescence in response to the frost treatment were expressed as % of reduction compared to Fv/Fm at 25 °C as indicated in . By decreasing the incubation temperature, Fv/Fm relative values also decreased in all cultivars; however, the slopes were not identical (). These reductions followed two patterns: quadratic or linear depending on the chilling tolerance of each cultivar. Marcona, Fournat de Brézenaud, and Tuono showed a quadratic decrease of their relative Fv/Fm with −1 °C as the inflection point. This means that until this temperature was reached, the reduction in Fv/Fm was not significant and these cultivars should be resistant to temperatures between 0 °C and −1 °C. Ferraduel and Ferragnès exhibited a linear decrease in Fv/Fm and would be the least tolerant to chilling.
Figure 1. Relative Fv/Fm ratio (%) compared to the normal at 25 °C as functions of incubation temperatures in five almond cultivars grown at two different sites in Morocco: Aknoul in the north and Sidi Bouhria in the east. FBZ = Fournat de Brézenaud, MAR = Marcona, FRG = Ferragnès, FRD = Ferraduel, and TUO = Tuono.
Discussion
It is well known that low temperatures affect both reproductive and vegetative organs in plant species. In our results, incubation of bud flowers in low temperatures affected significantly CF for all studied cultivars in agreement with other works in strawberry (Khanizadeh and DeEll, Citation2001) and almond (Kodad et al., Citation2010). By decreasing the temperature of incubation, CF decreased proportionally resulting in a reduction of photosynthetic performance of Photosystem II (PSII). This could be due to structural alterations at the primary reaction centers of PSII as pointed out by Schreiber and Bilger (Citation1987). Steponkus and Webb (Citation1992) explained the decline in maximum quantum yield of photosystem II by the breakdown of compartmentalization due to membrane damage. In our findings, 13% of CF variability was attributed to site effects. Sidi Bouhria exhibited higher values of Fv/Fm for all incubation probably because of the mild temperatures during bud development. Such environmental effects on CF performance were reported by several authors (Elhani et al., Citation2000; López-Climent et al., Citation2008; Zhou et al., Citation2016). Previous environmental conditions to which the plants were exposed were determinants for frost tolerance as reported by Badeck and Rizza (Citation2015). The main source of CF variability in our work was genotypic in line with other reports (Abid et al., Citation2016; Brennan and Jefferies, Citation1990; Elhani et al., Citation2000). Vernalization and photoperiod genes were behind genotypic behavior difference as pointed out by Rizza et al. (Citation2016) who demonstrated that genotypic differences regarding CF were attributed to polymorphism in VRN-H1 intron 1 region gene. Alisoltani et al. (Citation2015) constructed a regulatory network gene in response to cold stress, which includes SIZ1, ICE1, CBF/DREB1, WRKY21, HOS1, and ANK. Additionally, significant differential gene expression patterns were observed between frost tolerant and frost-sensitive genotypes. Moreover, the Pddof4 transcript shows significant over-expression in tolerant genotypes compared to sensitive genotypes during cold stress. In our work, the reduction of the relative Fv/Fm ratio (%) as functions of incubation temperatures was different from one cultivar to another and followed two patterns. The first one is linear with accelerated CF reduction translated by frost susceptibility (Hakam et al., Citation2000; Khanizadeh and DeEll, Citation2001), it is the case for the later flowering cultivars (Ferragnès and Ferraduel). The second pattern of the relative Fv/Fm ratio reduction is quadratic with –1 °C as inflection point; this means that from 25 to –1 °C, Fv/Fm reduction was not significant and bud flowers of such cultivars could be considered as frost tolerant. This second pattern fit the earlier flowering cultivars (Marcona and Fournat de Brézenaud) and Tuono. Similar results were highlighted by Khanizadeh and DeEll (Citation2001) for 64 strawberry cultivars grown in Canada and also by Kodad et al. (Citation2010) for 12 almond cultivars from Spain.
Conclusion
It has been proven that chlorophyll fluorescence is a powerful and faster technique to assess frost tolerance in almond. From our results, it could be concluded that the earlier blooming cultivars (Marcona and Fournat de Brézenaud) were relatively frost tolerant; however, Ferragnès and Ferraduel (later blooming date) are not likely to be affected by low temperatures at the end of spring when there is no danger of frost. These findings also suggest the use of chilling tolerant cultivars as parents in a breeding program to improve this trait and extended almond cultivation to high frost zones. Further information is required about eventual correlations between chlorophyll fluorescence and the visual degree of frost damage in almond cultivars.
Acknowledgments
We are thankful to the almond growers in Aknoul (northern Morocco) and Sidi Bouhria (eastern Morocco) for allowing access to their orchards and flower buds sampling.
Literature cited
- Abid, G., M. M’hamdi, D. Mingeot, M. Aouida, I. Aroua, Y. Muhovski, K. Sassi, F. Souissi, K. Mannai, and M. Jebara. 2016. Effect of drought stress on chlorophyll fluorescence, antioxidant enzyme activities and gene expression patterns in faba bean (Vicia faba L.). Arch. Agron. Soil Sci. 63(4):536–552.
- Alisoltani, A., B. Shiran, H. Fallahi, and E. Ebrahimie. 2015. Gene regulatory network in almond (Prunus dulcis Mill.) in response to frost stress. Tree Genet. Genomes 11(5):1–15.
- Badeck, F.W., and F. Rizza. 2015. A combined field/laboratory method for assessment of frost tolerance with freezing tests and chlorophyll fluorescence. Agronomy 5(1):71–88.
- Barros, P.M., N. Gonçalves, N.J. Saibo, and M.M. Oliveira. 2012. Cold acclimation and floral development in almond bud break: Insights into the regulatory pathways. J. Exp. Bot. 63(12):4585–4596.
- Brennan, R.M., and R.A. Jefferies. 1990. The use of chlorophyll fluorescence in assessment of low temperature hardiness in blackcurrant (Ribes nigrum L.). Ann. Appl. Biol. 117(3):667–672.
- Doll, D., and K. Shackel. 2016. Drought management for California almonds. Crops Soils 49(2):28–35.
- Elhani, S., Y. Rharrabti, L.F. Roca, and L.F. García del Moral. 2000. Evolution of chlorophyll fluorescence parameters in durum wheat as affected by air temperature. CIHEAM-Opt. Med. 40:275–277.
- FAOSTAT. 2013. FAO Statistical yearbook. World Food and Agriculture, FAO, Rome.
- Genty, B., J.M. Briantais, and N.R. Baker. 1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta. 990(1):87–92.
- Guo, Y., and J. Tan. 2015. Recent advances in the application of chlorophyll a fluorescence from photosystem II. Photochem. Photobiol. 91(1):1–14.
- Hakam, N., J.R. DeEll, S. Khanizadeh, and C. Richer. 2000. Assessing chilling tolerance in roses using chlorophyll fluorescence. HortScience 35:184–186.
- Imani, A., M. Ezaddost, F. Asgari, S. Masoumi, and I. Raeisi. 2012. Evaluation the resistance of almond to frost in controlled and field conditions. Int. J. Nuts Related Sci. 3:29–36.
- Khanizadeh, S., and J. DeEll. 2001. Chlorophyll fluorescence: A new technique to screen for tolerance of strawberry flowers to spring frost. Small Fruits Rev. 1(3):61–67.
- Kodad, O., F. Morales Iribas, and R. Socias i Company. 2010. Evaluación de la tolerancia de las flores de almendro a las heladas por la fluorescencia de clorofila. Inform. Técnica Econ. Agraria 106(2):142–150.
- Kodad, O., and R. Socias i Company. 2006. Influence of genotype, year and type of fruiting branches on the productive behavior of almond. Sci. Hort. 109(3):297–302.
- López-Climent, M.F., V. Arbona, R.M. Pérez-Clemente, and A. Gómez-Cadenas. 2008. Relationship between salt tolerance and photosynthetic machinery performance in citrus. Environ. Exp. Bot. 62(2):176–184.
- Maxwell, K., and G.N. Johnson. 2000. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 51(345):659–668.
- Rizza, F., I. Karsai, C. Morcia, F.W. Badeck, V. Terzi, D. Pagani, T. Kiss, and A.M. Stanca. 2016. Association between the allele compositions of major plant developmental genes and frost tolerance in barley (Hordeum vulgare L.) germplasm of different origin. Mol. Breed. 36(11):156.
- Sánchez-Pérez, R., E. Ortega, H. Duval, P. Martínez-Gómez, and F. Dicenta. 2007. Inheritance and relationships of important agronomic traits in almond. Euphytica 155(3):381–391.
- Schreiber, U., and Bilger, W. 1987. Rapid assessment of stress effects on plant leaves by chlorophyll fluorescence measurements, p. 27–53. In: J.D. Tenhunen, F.M. Catarino, O.L. Lange, and W.D. Oechel (eds.). Plants response to stress. Springer, Berlin.
- Socias i Company, R., A.J. Felipe, and J.G. Aparisi. 1999. A major gene for flowering time in almond. Plant Breed. 118(5):443–448.
- Sorkheh, K., B. Shiran, M. Khodambashi, H. Moradi, T.M. Gradziel, and P. Martínez-Gómez. 2010. Correlations between quantitative tree and fruit almond traits and their implications for breeding. Sci. Hort. 125(3):323–331.
- Spinelli, G.M., R.L. Snyder, B.L Sanden, and K.A. Shackel. 2016. Water stress causes stomatal closure but does not reduce canopy evapotranspiration in almond. Agr. Water Manage. 168:11–22.
- Steponkus, P.L., and M.S. Webb. 1992. Freeze-induced dehydration and membrane destabilisation in plants, p. 338–362. In: G.N. Somero, C.B. Osmond, and C.L. Bolis (eds.). Water and life. Springer, Berlin.
- Valdebenito, D., S. Tombesi, A. Tixier, B. Lampinen, P. Brown, and S. Saa. 2017. Spur behavior in almond trees (Prunus dulcis [Mill.] D.A. Webb): Effects of flowers, fruit, and “June drop” on leaf area, leaf nitrogen, spur survival and return bloom Sci. Hort. 215:15–19.
- Zhou, R., K.H. Kjær, E. Rosenqvist, X. Yu, Z. Wu, and C.O. Ottosen. 2016. Physiological response to heat stress during seedling and anthesis stage in tomato genotypes differing in heat tolerance. J. Agron. Crop Sci. 203(1):68–80.
