ABSTRACT
Zinc (Zn) and boron (B) disorders are common nutritional stresses in arid and semi-arid regions. In the current study, effectiveness of soil Zn amendment (0, 5.0, 10.0, and 20.0 mg kg–1 soil) using Zn-glycine chelate (Zn-Gly), a novel Zn-fertilizer, which is especially synthesized for application in calcareous soils in arid and semi-arid areas, was evaluated under different soil B levels (0, 2.5, 5.0, 10.0, and 20.0 mg kg–1 soil) for a 100-day period. Pistachio, the most valuable crop grown under arid conditions, was used as the model plant. Measurement of electrolyte leakage, H2O2, and malondialdehyde indicated the incidence of oxidative stress in the leaves of pistachio under low and high soil B concentrations. In addition, B stress caused a significant increase in lipoxygenase activity in the leaves. Plants treated with 5.0 mg B kg–1 soil showed the lowest oxidative stress injuries and lipid peroxidation. Application of 5.0 mg Zn kg–1 soil significantly alleviated the B stress damages, however, the ameliorative effect of Zn was vanished by application of higher Zn concentrations. Evaluation of the antioxidant enzymes (superoxide dismutase [SOD], catalase [CAT], or ascorbate peroxidase [APX]) and non-enzyme antioxidants (ascorbate and phenolic compounds) revealed that the protective effects of Zn-Gly against B stress are due to enhancement of cell antioxidant defense. In conclusion, application of Zn-Gly for reducing oxidative stress pressure in pistachio plants grown under B disorder was suggested.
Nomenclature
| APX | = | ascorbate peroxidase |
| B | = | boron |
| CAT | = | catalase |
| EL | = | electrolyte leakage |
| MDA | = | malondialdehyde |
| LOX | = | lipoxygenase |
| ROS | = | reactive oxygen species |
| SOD | = | superoxide dismutase |
| Zn | = | zinc |
| Zn-Gly | = | Zn-glycine chelate |
Introduction
Zinc (Zn), as an essential micronutrient, plays prominent roles in a number of plant physiological processes (i.e., synthesis of protein, DNA, RNA and plant growth regulators; bioenergetics processes; Welch, Citation2002). Chemical fertilizers are generally utilized to enhance Zn supply in soil and also to keep beneficial concentrations of this element for plants in nutrient solution cultures. Usual resources of Zn found in agricultural soils are inorganic Zn salts, and synthetic and natural organic chelates (Cakmak et al., Citation1999). Inorganic Zn fertilizers have relatively low efficiency in calcareous soils because of certain agronomic restrictions (i.e., precipitation and high impurities; Khoshgoftarmanesh et al., Citation2010). Most commercial inorganic Zn fertilizers are affiliated with the raised hazard of heavy metal toxicity such as cadmium (Afyuni et al., Citation2007). Synthetic chelates are often efficient in amending Zn deficiency though they are usually costly. It is now properly established that synthetic chelates can impair micronutrient balance and cause toxicity in plants. On the other hand, the uptake of synthesized chelates, because of the larger size, is less than free Zn cations (Vadas et al., Citation2007). Amino acids have recently been introduced as natural chelating agents to increase solubility and availability of micronutrients (Aravind and Prasad, Citation2005). Among various Zn sources, Zn-glycine complex was found to be more effective in providing Zn, enhancing plant growth, diminishing lipid peroxidation, and improving crop productivity under stress situations (Mohammadi and Khoshgoftarmanesh, Citation2014).
While of less prevalence than boron (B) deficient soils, B-rich soils are of great significance because of their association with B toxicity and decreased plant growth and productivity in different areas of the world (Nable et al., Citation1997). As in predominant ionic stresses, toxic rates of B bring about the formation of reactive oxygen species (ROS). ROS have the possibility to interact non-specifically with numerous cellular components, triggering peroxidative reactions and creating significant damages to membranes and essential macro-molecules, including photosynthetic pigments, proteins, nucleic acids, and lipids (Lin and Kao, Citation2000). For conservation against ROS-induced damages, a defense system comprised of antioxidant enzymes like superoxide dismutase (SOD), catalase (CAT), or ascorbate peroxidase (APX), and a variety of non-enzymatic antioxidants have evolved in plants (Blokhina et al., Citation2003). The Zn nutritional status of plant influences photo-oxidative damage to chloroplasts, induced by ROS. Zinc binds to ligands comprising sulfur, nitrogen, and also to a lesser extent, oxygen, and binds to the membrane proteins preferentially. Therefore, Zn plays critical functions in the cell defense system against ROS, and represents an outstanding protective agent contrary to the oxidation of particular essential cell components (Tavallali et al., Citation2010).
Although some researches have been accomplished on Zn and B interaction, no reports were found that assess the influence of green synthesized zinc-amino complex on the protection system of plant under B-deficient or B-excess conditions. Hence, in the current study a zinc-amino acid chelate was synthesized by complexation of Zn with glycine and its effectiveness on activity of antioxidant enzymes and molecules in pistachio seedlings (Pistacia vera L. ‘Badami’) under boron stress was evaluated. Pistachio, the model plant used in this study, is the most valuable crop grown in arid areas, which are prone to B and Zn disorders. Our purpose was to assign whether Zn-glycine (Zn-Gly) chelate is involved in regulation of antioxidant enzymes and lipid peroxidation under B toxicity, and to elucidate the physiological mechanisms by which boron toxicity is alleviated by Zn-Gly chelate in pistachio seedlings.
Material and methods
Plant material and growth conditions
The experiment was repeated twice, in which similar results and the data are offered as the average of the 2-year replications. Soil in this study was a loam obtained from 0 to 40-cm depth of Chitgar series soils (fine-loamy, carbonatic, thermic Typic Calcixerepts). The physicochemical properties of the soil are given in . The soil samples were air-dried and smashed to pass through a 2-mm sieve. Zinc (0, 5, 10, and 20 mg Zn kg–1 soil as Zn-Gly) was combined thoroughly with the soil and put in 8-L plastic pots. Four germinated seeds of pistachio (P. vera L. cv. Badami) were planted in each pot and the pots were irrigated with deionized water twice a week. Nitrogen and P at the rate of 50 mg kg–1 soil, and Cu and Mn at the rate of 5 mg kg–1 soil were applied uniformly to the pots as NH4NO3, KH2PO4, CuSO4.5H2O, and MnSO4.H2O, respectively. After 25 days, the seedlings at the four-leaved stage were thinned to two uniform seedlings per pot. Seven days later, B treatments (0, 2.5, 5.0, 10.0, and 20.0 mg B kg–1 soil as boric acid) were added to the pots at 3-day intervals using 0.5-L irrigation water. Further, 100 days after boron treatment, various analyses were performed. Twenty treatments were organized in a factorial experiment established on completely randomized design with four replications.
Table 1. Some physic-chemical properties of the soil used in the experiment.
Synthesis of Zn(II)-amino acid chelate
The zinc-amino acid chelate was synthesized using the amino acid glycine (Gly), the most abundant amino acid in the soil (Werdin-Pfisterer et al., Citation2009), as a complexing agent. A solution of Gly (2 mM) in 5 mL of distilled water was gradually added to a solution of ZnSO4 (1 mM) in 2 mL of distilled water. The mixture was made hotter at reflux temperature for 2 h while being stirred vigorously. The disappearance of solvent at room temperature gave light brown microcrystals of Zn-glycine chelate. The microcrystals were washed with cold ethanol followed by diethyl ether and air-dried (Mohammadi and Khoshgoftarmanesh, Citation2014). The complexes were characterized by various analytical techniques. A Perkin-Elmer 2400 CHNS elemental analyzer (Waltham, MA, USA) was used to determine the quantity of carbon (C), hydrogen (H), nitrogen (N), and sulfur (S) in various operating modes. The Fourier transform infra-red (FT-IR) spectra were measured with a FT-IR BRUKER 460 spectrophotometer (Billerica, MA, USA) over KBr pellet in 4000–400 cm−1 range.
Determination of electrolyte leakage
Electrolyte leakage (EL) from fresh leaf tissues was assessed using a conductivity meter (Ohm 419) according to the procedure of Sairam et al. (Citation1997).
Ascorbic acid content
Ascorbic acid was extracted with 6% trichloroacetic acid from 100 mg of frozen leaf tissue. Four milliliters of the extract were mixed with 2% dinitrophenylhydrazine (2 mL), followed by the addition of one drop of 10% thiourea solution (in 70% ethanol). The mixture was boiled for 15 min in a water bath and after cooling to 0 °C, treated with 5 mL of 80% (v/v) H2SO4. The absorbance of the solution including the hydrazone complex was measured at 530 nm using a spectrophotometer (Shimadzu, spectrophotometer 160 A, Kyoto, Kyoto Prefecture, Japan; Mukherjee and Choudhuri Citation1985).
Hydrogen peroxide assay
Leaf hydrogen peroxide (H2O2) content was determined as described by Velikova et al. (Citation2000). Leaf tissue was homogenized in an ice bath with trichloroacetic acid (TCA). The homogenate was centrifuged at 13,000 rpm and 0.5 mL of the supernatant was supplemented to 0.5 mL of 10 mM potassium phosphate buffer (pH 7) and 1 mL of 1 M KI. The absorbance of the supernatant was measured at 390 nm.
Concentration of phenolic compounds
Phenolic compounds were extracted with 80% methanol. To 200 μL of the extract, we added 1 mL of Folin-Ciocalteau reagent and 800 μL of sodium carbonate solution (7.5%) reagent. Subsequently, the mixture was incubated in the dark for 45 min. At last, the absorbance was measured at 790 nm according to AOAC (Citation1990). The concentration of the phenolic compound was reported as gallic acid equivalents using the linear equation based on a standard curve.
Lipid peroxidation
Lipid peroxidation was determined by evaluating the amount of malondialdehyde (MDA) formed using the thiobarbituric acid reactive substances as described by Heath and Parker (Citation1968). Leaf samples were homogenized in TCA and the homogenates were centrifuged. To a 1.0-mL aliquot of the supernatant, 4.0 mL of 0.5% thiobarbituric acid in 20% TCA was supplemented. The mixture was then heated at 95 °C for 30 min. After centrifuging, the absorbance of the supernatant was determined at 532 and 600 nm. The MDA content (nmol g–1 FW) was calculated utilizing an extinction coefficient of 155 mM cm–1 after subtracting the non-specific absorbance at 600 nm.
Lipoxygenase activity assay (LOX, E.C. 1.13.11.12)
Plant material (300 mg of leaf sample) was homogenized in 50 mM sodium phosphate buffer (pH 7.0), 1 mM EDTA, 0.1 mM phenylmethylsulfonyl fluoride (PMSF), 2% (w/v) polyvinylpyrrolidone (PVP), 1% (v/v) glycerol, and 0.1% (v/v) Tween 20. The extract was centrifuged at 15,000 g for 20 min and the supernatant was immediately used to assay for lipoxygenase activity, according to Ederli et al. (Citation1997). LOX activity was measured at room temperature by the addition of 1 mM linoleic acid in 0.1 M sodium acetate buffer (pH 5.6) to the extract and by reading the rise in absorbance at 234 nm. The extinction coefficient of (25 mM L–1)–1 cm–1 was utilized to convert absorbance values to micromoles of conjugated diene. One unit of activity was defined as the amount of enzyme that catalyzed the synthesis of 1 μmol of hydroperoxide min–1. Enzyme activity was expressed in units (1 mol of substrate conversion/s).
Antioxidant enzymes activity
Plant material (1 g of frozen leaf sample) was ground in 50 mM sodium phosphate buffer; pH 7.8 for SOD and pH 7.0 for CAT and APX. The homogenates were centrifuged at 12,000 g for 20 min at 4 °C. The supernatants were used to measure the activity of SOD, CAT, and APX. The protein content in the supernatant was determined according to Lowry et al. (Citation1951). The superoxide dismutase activity assay was based on the procedure described by Giannopotitis and Ries (Citation1977). One unit of enzyme activity was defined as the amount of enzyme required to result in 50% inhibition of the rate of nitro blue tetrazolium reduction measured at 560 nm. Enzyme activity was expressed in units.
Catalase activity was determined according to Cakmak and Marschner (Citation1992). The reaction mixture, in a total volume of 2 mL, contained 25 mM sodium phosphate buffer (pH 7.0) and 10 mM H. The reaction was initiated by the addition of 100 μL of enzyme extract and the activity was measured by determining the initial rate of disappearance of H at 240 nm (E = 39.4 mM−1 cm−1) for 30 s. Enzyme activity was expressed in units.
Ascorbate peroxidase activity was determined in accord with Nakano and Asada (Citation1981). The reaction mixture in a total volume of 2 mL consisted of 25 mM sodium phosphate buffer (pH 7.0), 0.1 mM EDTA, 0.25 mM ascorbate, 1.0 mM H2O2, and 100 μL of enzyme extract. H2O2-dependent oxidation of ascorbate was followed by a decline in the absorbance at 290 nm (E = 2.8 mM−1 cm−1). Enzyme activity was expressed in units.
Statistical analysis
The experiment was repeated twice with similar results and the data are offered as the average of the 2-year replications. Data were subjected to analysis of variance (ANOVA) and means were compared using Tukey’s honest significant difference (HSD) test at the 5% probability level. Statistical analyses were performed using SPSS v.19.0 software (SPSS, Inc., Chicago, IL, USA). Data are presented as the means for each treatment (n = 4).
Results
Characteristics of Zn-Gly chelate
The complex used in this research was synthesized in great yield. The results of elemental analysis uphold the forming of Zn-Gly complex with 2:1 ligand to metal molar proportion (). The computational results recommended that the glycine ligands arranged in proper order to Zn(II) ion via their nitrogen and oxygen atoms and corroborate the accord mode from IR spectroscopy. The FT-IR spectra of Zn-Gly chelates reveal an absorption style in a 4000–400 cm−1 area that is similar to glycine. In comparison to the free glycine, the vibration of N‒H bands seems to be switched in the direction of higher frequency in the Zn-Gly chelate confirming the commitment of the amine group in the complex formation. The carboxylate ion of glycine integrates to Zn(II) as a unidentate arrangement. The C=O group of Zn-Gly chelate had an approximately equivalent frequency around 1594–1695 cm−1 and it can be perceived that the ʋ(CO) is metal reactive. As the result of chelate effect, the development constants of Zn-Gly chelate was high ().
Table 2. Analytical data for zinc-glycine chelate.
Table 3. Picked out IR bands (cm–1) of zinc-glycine [Zn(Gly)2] chelate.
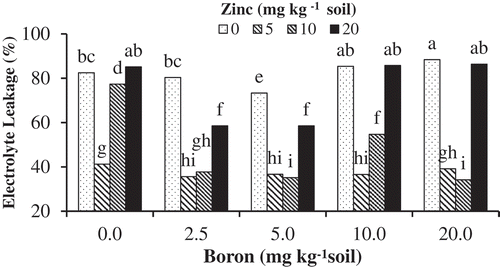
Electrolyte leakage
The lowest EL was observed in plants treated with 5.0 mg kg−1 soil B. Higher doses of B (10 and 20 mg kg−1 soil) impaired cell membrane permeability by increasing EL. Higher EL was also observed in boron deficient seedlings. Zinc supplementation at 5 and 10 mg kg−1 soil reduced EL of tissue in boron-treated plants ().
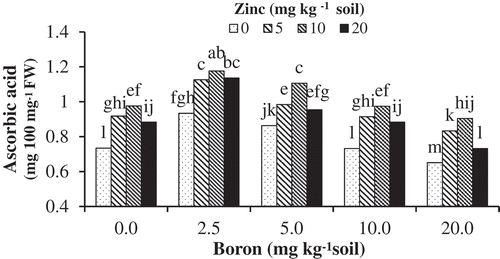
Ascorbic acid content
shows the effects of Zn and B interaction on leaf ascorbic acid concentration. With increasing B up to 5 mg kg−1 soil, ascorbic acid content was increased significantly in leaves compared to the control. However, the reduction in the ascorbic acid content was indicated when higher B concentrations were utilized. Zn-Gly supply significantly improved ascorbic acid content. This parameter in the zinc-deficient seedlings was lower than in those supplied with Zn, particularly at higher boron levels. The concentration of ascorbic acid in seedlings supplied with 10 mg Zn kg−1 soil was approximately 1 mg 100 mg−1 DW, followed by a slight decrease with 5 and 20 mg Zn kg−1 soil.
Concentration of H2O2, MDA, and phenolic compounds
Boron treatments without application of Zn did not affect H2O2 in the leaves of pistachio seedlings. Application of Zn-Gly chelate significantly affected the H2O2 content in boron stressed and non-stressed leaves (). In the control treatment (0 mg B kg−1 soil), supplemental Zn at 5 mg kg−1 soil significantly reduced leaf H2O2 content. The interactive effect of Zn-Gly at 5 and 10 mg Zn kg−1 soil and 2.5–10 mg B kg–1 soil resulted in a significant decrease in H2O2 content in comparison with no Zn or 20 mg Zn kg−1 soil treatments.
Figure 3. The effects of Zn-Gly application and different soil Boron concentrations on (A) hydrogen peroxide (H2O2), (B) malondialdehyde (MDA), and (C) phenolics contents in pistachio leaf. *Mean separation by THSD at P ≤ 0.05.
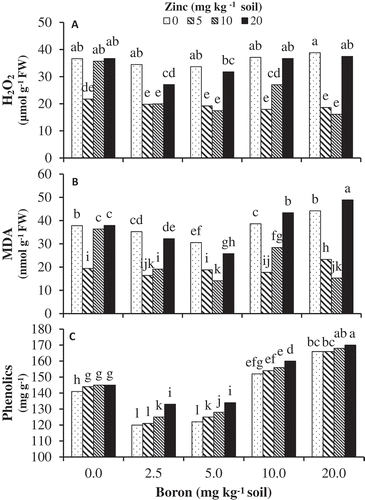
MDA significantly increased at high B levels above 5 mg kg−1 soil and also in non-B condition. The interaction of supplemental Zn-Gly with all boron levels resulted in significant declines in MDA content, as compared with B treatments without Zn. The effects of Zn on reducing MDA levels were especially noticeable at concentrations of 5 and 10 mg kg−1 soil ().
Increasing B concentration in soil significantly increased phenolics content. However, control plants contained significantly higher phenolics content than those exposed to 2.5 and 5 mg B kg−1 soil. Zn-glycine chelate application caused a significant enhancement in phenolics content. Such an increase was greater in the presence of B treatment. The highest phenolic increase occurred at 20 mg Zn kg−1 soil ().
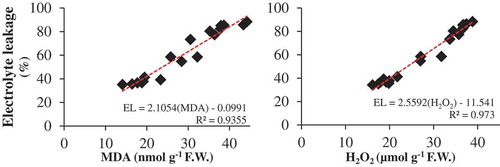
Zn-Gly treatment influenced the relationship between MDA and EL, and between H2O2 and EL. Positive correlations between EL and MDA (r2 = 0.935) and H2O2 content (r2 = 0.973) were observed ().
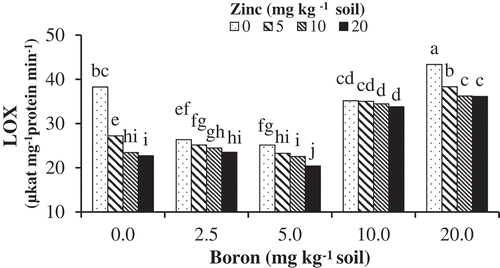
Lipoxygenase (LOX) activity
Plants grown under high B concentration (10 and 20 mg kg−1 soil) showed higher values of lipolytic enzyme activity compared to the lower B concentrations (2.5 and 5 mg kg−1 soil). B treatment resulted in 100% increase in LOX activity. LOX activity was significantly reduced in boron-treated seedlings supplemented with Zn-Gly (especially at 20 mg Zn kg−1 soil). Plants treated with Zn-Gly chelate showed only a slight increase in LOX activity ().
Antioxidant enzyme activity
Analysis of the antioxidant enzymes SOD, CAT, and APX revealed a higher increase in their activity in B-treated plants supplemented with Zn-Gly than in plants treated with B alone. Higher SOD activity was observed under B toxicity at 10 and 20 mg kg−1 soil and non-B-treated plants; however, B at 2.5 and 5 mg kg−1 soil significantly reduced SOD activity. Zn-Gly supplement caused a noticeable increase in SOD activity, in contrast to boron stress alone, with 20 mg Zn kg−1 soil being more effective ().
Figure 6. The effects of Zn-Gly application and different soil Boron concentrations on (A) superoxide dismutase (SOD), (B) catalase (CAT), and (C) ascorbate peroxidase (APX) activity in pistachio leaf. *Mean separation by THSD at P ≤ 0.05.
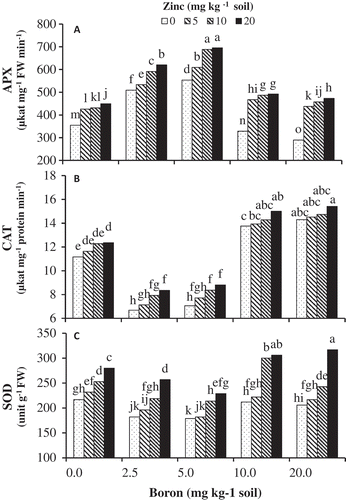
The trend for CAT activity in the presence and absence of Zn in B-treated plants was similar to those observed for SOD. As the levels of Zn were increased in soil, the enzyme activity increased; however, there were no significant differences between Zn-Gly treatments (). On the other hand, APX activity was reduced significantly under high and lack of B conditions (). Plants treated with both B and Zn had significantly higher APX activity than those exposed to B alone. This increase was more pronounced in 2.5 and 5 mg B kg−1 treatments.
Discussion
The interaction between a micronutrient and a stressor may possibly be important for understanding, analyzing, and developing plant defense strategies through different factors. B deficiency and/or toxicity can promote the ROS formation (Lin and Kao, Citation2000). This is evident from the results of lipid peroxidation, which show that high levels of B increased MDA level in the leaves (). The increase in LOX activity due to B stress suggests higher lipolytic activity of the membrane and oxidation of membrane-bound fatty acids, which promote lipid peroxidation (Lacan and Baccou, Citation1998). The increases in EL in response to B deficiency and toxicity treatments () was in parallel with the increase in MDA and H2O2 in the leaves (). These observations suggest that B stress could damage the integrity of the cell membrane, as well as cell components, such as lipids. Zn-Gly amendment reduced EL and MDA content, thereby alleviating the oxidative damages caused by boron stress. These results are consistent with other researches, which found higher H2O2 and MDA concentrations in the leaves of apple rootstock and grape subjected to B toxicity (Gunes et al., Citation2006; Molassiotis et al., Citation2006). As reported by Wen et al. (Citation2015), the elevated levels of H2O2 and O2·- caused by stress facilitate the formation of highly reactive hydroxyl radicals (OH·-). The OH·- radicals are generally considered to be the most likely ROS to initiate the peroxidation destruction of lipids that leads to membrane damage.
Zn-Gly application reduced lipid peroxidation, electrical conductivity, and LOX activity under B stress ( and ). Studies have definitively revealed that most of the antioxidant activity may be ascribed to phenolic compounds (Rice-Evans et al., Citation1997). The evidence suggests that application of Zn-Gly chelate increased phenolic content in B-affected pistachio seedlings, thus mitigating B deficiency and toxicity (). Lower H2O2 content was observed as well when Zn-Gly was added under B stress (). These results suggest that Zn-Gly complex can partially prevent oxidative damage in pistachio seedlings due to B stress. Zn has a unique property of living in a divalent state, without any redox cycling, and according to that is stable in a biological medium in which the oxido-reductive potential is exposed to successive flux (Vallee and Falchuk, Citation1993). These properties provide protection for membrane lipid packing against ROS peroxidation, which in turn preserves membrane integrity (Li et al., Citation2006). Zn binds preferably to the –SH groups of the membrane protein moiety, and protects phospholipids and proteins from thiol oxidation and disulfide formation (Aravind and Prasad, Citation2004), either by direct binding, binding to a site close to the sulfhydryl group, or by conformational change resulting in obvious stability of the enzymes, membrane proteins, and the lipid structure (Sharma et al., Citation1994).
Meanwhile, to scavenge ROS, plants possess another specific mechanism, which includes non-enzymatic antioxidants, such as free amino acids. Glycine alleviates oxidative stress in plant tissues via improving osmotic solutes and phenolic contents and abating lipid peroxidation (Sadak and Abdelhamid, Citation2015). Moreover, free amino acids were established as important osmotically active organic solutes, and these compounds may be involved in osmotic adjustment, ion transport, enzyme synthesis, scavenging free radicals, and redox homeostasis (Rai, Citation2002). Thomas et al. (Citation2009) stated that application of amino acids enhanced the uptake and accumulation of N elements to be involved in different biological processes leading to the establishment of protective compounds, such as amino acids, against abiotic stress condition. Our results are in agreement with recent studies indicating that glycine alleviates oxidative stress in plant tissues by alleviating oxidative stress pressure under B deficiency/toxicity (Sadak and Abdelhamid, Citation2015).
Insufficiency and/or toxicity of a nutrient are recognized to trigger oxidation of NADPH, leading to superoxide radical production (O2·–; Kawano et al., Citation2001). The protective effect of Zn has been declared being due to its capability to prevent NADPH oxidation (Cakmak and Marschner, Citation1988). Elevated O2·– formation is deleterious, which ultimately produces a potent oxidant OH·– radical by simply reacting with H2O2. This kind of reaction, known as the site-specific Haber-Weiss reaction, is definitely catalyzed by Fe(III) reduction to Fe(II), followed simply by the reduction of H2O2 by Fe(II; Fridovich, Citation1986).
ROS-scavenging antioxidant enzymes, like SOD, CAT, and APX, play a fundamental role in eliminating ROS under B stress. The results of the present study showed that the pistachio seedlings treated with Zn-Gly had higher SOD, CAT, and APX activity (). It could be ascribed to very effective antioxidative ability of Zn as it is an important component of Zn-SOD and Cu-Zn-SOD, which enhance SOD activity under oxidative stress. Similarly, its deficiency in control treatment resulted in lower SOD activity in pistachio seedlings. Aravind and Prasad (Citation2004) documented an increase in SOD activity under an optimal zinc supply. Moreover, Zn-Gly application increased the APX activity. The APX catalysis breaks down H2O2 into H2O and O2. Zn treatment increases the SOD activity, which catalysis conversion of O2·– into H2O2 (Alscher et al., Citation2002). Zn brings about a higher level of H2O2 in plant cells, which might have initiated the APX activity. Application of Zn-Gly also enhanced the CAT activity and vice versa. Although Zn is not a cofactor required for CAT activity, the reason for a decrease in CAT activity could be that Zn deficiency is related to inhibition of CAT by elevated O2·– formation (Cakmak and Marschner, Citation1988).
In pistachio seedlings treated with 10 and 20 mg B kg−1 soil and non-B (without Zn supplementation), SOD and CAT activity increased; in contrast, APX activity was declined. B toxicity effects on enzyme activities in leaves are different, depending on the plant species. Cervilla et al. (Citation2007) reported that with 2 mM B, CAT, APX, and SOD activities increased significantly in tomato. In barley, Karabal et al. (Citation2003) found that excess B in the medium significantly increased APX activity but not that of SOD. In an apple rootstock, it was reported that an increase of B concentration in the culture medium from 0.1 to 6 mM reduced CAT activity, while inducing SOD and peroxidase activities in leaves (Molassiotis et al., Citation2006). Our results are consistent with those of Gunes et al. (Citation2006) who have declared that B toxicity enhances CAT and SOD activity at the same time as it reduces that of APX.
Aside from the antioxidant enzymes, there are alternative non-enzymatic routes for the conversion of O2·– to H2O2 using an antioxidant agent such as ascorbic acid (Noctor and Foyer, Citation1998). In the current study, upon increasing B up to 5 mg kg−1 soil, a significant increase in the total level of ascorbic acid was observed; however, the higher B concentrations caused a significant decrease in ascorbic acid content (). B application is known to elevate the ascorbate concentration in plant tissues as compared with B-deficient conditions (Blevins and Lukaszewski, Citation1998). Raised ascorbate concentration by foliar applications of B was reported in potato (Solanum tuberosum; Mondy and Munshi, Citation1993). More recently, Keles et al. (Citation2004), studying orange, observed a significant increase in this antioxidant under conditions of excess B in the medium, proposing that this could be important against ROS formation. Zn-induced enhancement of ascorbic acid content is very beneficial for plants, facilitating the detoxification of O2·–.
On the other hand, glycine, the ligand of the Zn-Gly chelate, could counteract negative effects of boron stress on pistachio seedlings. Ortiz-Lopez et al. (Citation2000) stated that the positive effect of Zn-Gly on root and shoot growth of lettuce might be due to the growth motivating effect of glycine. Glycine enhanced antioxidant enzyme activities of lettuce by increasing concentration of proteins and soluble sugars (Wang and Jin, Citation2007). Mohammadi and Khoshgoftarmanesh (Citation2014) demonstrated that elevated K and Ca contents in root and shoot of lettuce supplied with Zn-Gly in comparison with Zn-sulfate under salt stress. Their results confirm the role of glycine in improvement of root membrane integrity and function. Zn-Gly supplied a greater amount of nitrogen compared with Zn-sulfate for the plants. These results may be attributed to the potential role of glycine as a free radical scavenger (Sadak and Abdelhamid, Citation2015).
Conclusions
Here, a clear positive effect of adequate B and Zn application and their synergistic effect of these elements when applied in combination on enzymatic and non-enzymatic antioxidants of pistachio was demonstrated. Sinha et al. (Citation2000) also reported a synergistic interaction between Zn and B in mustard (Brassica nigra) when both of the nutrients were either in low or excess supply. The results revealed that application of Zn-Gly chelate improved the B stress (deficiency/toxicity) tolerance of pistachio seedlings by improving activity of antioxidative enzymes, ascorbate, and phenolics contents. The results emphasize the potential of applying Zn-amino acids complex in sustainable agriculture in arid and semiarid areas, which are prone to Zn deficiency and B toxicity.
Literature cited
- Afyuni, M., A.H. Khoshgoftarmanesh, V. Dorostkar, and R. Moshiri. 2007. Zinc and cadmium content in fertilizer commonly used in Iran. Int. Conf. Zinc Crops, Istanbul, Turkey, 24–28 May.
- Alscher, R.G., N. Erturk, and L.S. Heath. 2002. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 53:1331–1341.
- AOAC. 1990. Official methods of analysis of the Association of Official Analytical Chemists, 15th ed., 703 p., K. Helrich (ed.). AOAC, Arlington, VA.
- Aravind, P., and M.N.V. Prasad. 2004. Zinc protects chloroplasts and associated photochemical functions in cadmium exposed Ceratophyllum demersum L., a fresh water macrophyte. Plant Sci. 166:1321–1327.
- Aravind, P., and M.N.V. Prasad. 2005. Cadmium-induced toxicity reversal by zinc in Ceratophyllum demersum L. (a free floating aquatic macrophyte) together with exogenous supplements of amino and organic acids. Chemosphere 61:1720–2173.
- Blevins, D.G., and K.M. Lukaszewski. 1998. Boron in plant structure and function. Ann. Rev. Plant Physiol. Plant Mol. Biol. 49:481–500.
- Blokhina, O., E. Virolainen, and K.V. Fagerstedt. 2003. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 91:179–194.
- Cakmak, I., and H. Marschner. 1988. Enhanced superoxide radical production in roots of zinc-deficient plants. J. Exp. Bot. 39:1449–1460.
- Cakmak, I., and H. Marschner. 1992. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascrobate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 98:1222–1227.
- Cakmak, I., M. Kalayci, H. Ekiz, H.J. Braun, Y. Kilinc, and A. Yilmaz. 1999. Zinc deficiency as a practical problem in plant and human nutrition in Turkey: A NATO science for stability project. Field Crops Res. 60:175–188.
- Cervilla, L.M., B. Blasco, J.J. Rios, L. Romero, and J.M. Ruiz. 2007. Oxidative stress and antioxidants in tomato (Solanum lycopersicum) plants subjected to boron toxicity. Ann. Bot. 100:747–756.
- Ederli, L., S. Pasqualini, P. Batini, and M. Antonielli. 1997. Photoinhibition and oxidative stress: Effects on xanthophyll cycle, scavenger enzymes and abscissic acid content in tobacco plants. J. Plant Physiol. 151:422–428.
- Fridovich, I. 1986. Biological effects of the superoxide radical. Arch. Biochem. Biophys. 247:1–11.
- Giannopotitis, C.N., and S.K. Ries. 1977. Superoxide dismutase in higher plants. Plant Physiol. 59:309–314.
- Gunes, A., G. Soylemezoglu, A. Inal, E.G. Bagci, S. Coban, and O. Sahin. 2006. Antioxidant and stomatal responses of grapevine (Vitis vinifera L.) to boron toxicity. Sci. Hort. 110(3):279–284.
- Heath, R.L., and L. Parker. 1968. Photoperoxidation in isolated chloroplast. 1. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 125:189–198.
- Karabal, E., M. Yucel, and H.A. Oktem. 2003. Antioxidant responses of tolerant and sensitive barley cultivars to boron toxicity. Plant Sci. 164:925–933.
- Kawano, T., N. Kawano, S. Muto, and F. Lapeyrie. 2001. Cation-induced superoxide generation in tobacco cell suspension culture is dependent on ion valence. Plant Cell Environ. 24:1235–1241.
- Keles, Y., I. Oncel, and N. Yenice. 2004. Relationship between boron content and antioxidant compounds in citrus leaves taken from fields with different water source. Plant Soil 265:345–353.
- Khoshgoftarmanesh, A.H., R. Schulin, R.L. Chaney, B. Daneshbakhsh, and M. Afyuni. 2010. Micronutrient-efficient genotypes for crop yield and nutritional quality in sustainable agriculture. A review. Agron. Sustainable Dev. 30:83–107.
- Lacan, D., and J.C. Baccou. 1998. High levels of antioxidant enzymes correlate with delayed senescence in non-netted muskmelon fruits. Planta 204:377–382.
- Li, W.Y.F., F.L. Wong, S.N. Tsai, S.N. Tsai, T.H. Phang, G.H. Shao, and H.M. Lam. 2006. Tonoplast-located GmCLC1 and GmNHX1 from soybean enhance NaCl tolerance in transgenic bright yellow (by)-2 Cells. Plant Cell Environ. 29:1122–1137.
- Lin, C.C., and C.H. Kao. 2000. Effect of NaCl stress on H2O metabolism in rice leaves. Plant Growth Regul. 30:151–155.
- Lowry, O.H., N.J. Rosenberg, A.L. Farr, and R.J. Randall. 1951. Protein measurement with folin phenol reagent. J. Biol. Chem. 193:265–275.
- Mohammadi, P., and A.H. Khoshgoftarmanesh. 2014. The effectiveness of synthetic zinc (Zn)-amino chelates in supplying Zn and alleviating salt-induced damages on hydroponically grown lettuce. Sci. Hort. 172:117–123.
- Molassiotis, A., T. Sotiropoulos, G. Tanou, G. Diamantidis, and I. Therios. 2006. Boron-induced oxidative damage and antioxidant and nucleolytic responses in shoot tips culture of the apple rootstock EM 9 (Malus domestica Borkh). Environ. Exp. Bot. 56(1):54–62.
- Mondy, N.I., and C.B. Munshi. 1993. Effect of boron on enzymatic discoloration and phenolic and ascorbic acid content of potatoes. J. Agr. Food Chem. 41:554–556.
- Mukherjee, S.P., and M.A. Choudhuri. 1985. Implication of hydrogen peroxide-ascorbate system on membrane permeability of water stressed Vigna seedlings. New Phytol. 99:355–360.
- Nable, R.O., G.S. Baouelos, and J.G. Paull. 1997. Boron toxicity. Plant Soil 193:181–198.
- Nakano, Y., and K. Asada. 1981. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22:867–880.
- Noctor, G., and C. Foyer. 1998. Ascorbate and glutathione: Keeping active oxygen under control. Ann. Rev. Plant Physiol. Plant Mol. Biol. 49:249–279.
- Ortiz-Lopez, A., H.C. Chang, and D.R. Bush, 2000. Amino acid transporters in plants. Biochem. Biophys. Acta. 1465:275–280.
- Rai, V.K. 2002. Role of amino acids in plant responses to stresses. Biol. Plant. 45:481–487.
- Rice-Evans, A.C., N.J. Miller, and G. Pagaga. 1997. Antioxidant properties of phenolic compounds. New Trends Plant Sci. Rev. 2:152–159.
- Sadak, M.S., and M.T. Abdelhamid. 2015. Influence of amino acids mixture application on some biochemical aspects: Antioxidant enzymes and endogenous polyamines of Vicia faba plant grown under seawater salinity stress. Gesunde Pflanzen 67(3):119–129.
- Sairam, R.K., P.S. Deshmukb, and D.S. Shukla, 1997. Tolerance to drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. J. Agron. Crop Sci. 178:171–177.
- Sharma, P.N., N. Kumar, and S.S. Bisht. 1994. Effect of zinc deficiency on chlorophyll content, photosynthesis and water relations of cauliflower plants. Photosynthetica 30:353–359.
- Sinha, P., R. Jain, and C. Chatterjee. 2000. Interactive effect of boron and zinc on growth and metabolism of mustard. Com. Soil Sci. Plant Anal. 31(1–2):41–49.
- Tavallali, V., M. Rahemi, S. Eshghi, B. Kholdebarin, and A. Ramezanian. 2010. Zinc alleviates salt stress and increases antioxidant enzyme activity in the leaves of pistachio (Pistacia vera L. ‘Badami’) seedlings. Turk. J. Agr. For. 34:349–359.
- Thomas. J., A.K.A. Mandal, R.R. Kumar, and A. Chordia. 2009. Role of biologically active amino acid formulations on quality and crop productivity of tea (Camellia sp.). Int. J. Agr. Res. 4(7):228–236.
- Vadas, T.M., X. Zhang, A.M. Curran, and B.A. Ahner. 2007. Fate of DTPA, EDTA and EDDS in hydroponic media and effects on plant mineral nutrition. J. Plant Nutr. 30:1229–1246.
- Vallee, B.L., and K.H. Falchuk. 1993. The biochemical basis of zinc physiology. Physiol. Rev. 73:79–118.
- Velikova, V., I. Yordanov, and A. Edreva. 2000. Oxidative stress and some antioxidant system in acid rain treated bean plants: protective role of exogenous polyamines. Plant Sci. 151:59–66.
- Wang, H., and J. Jin. 2007. Effects of zinc deficiency and drought on plant growth and metabolism of reactive oxygen species in maize (Zea mays L.). Agr. Sci. China 6:988–995.
- Welch, R.M. 2002. The impact of mineral nutrient in food crops on global human health. Plant Soil 247:83–90.
- Wen, T., W. He, Y. Chong, Y. Liu, J.J. Yin, and X. Wu. 2015. Exploring environment-dependent effects of Pd nanostructures on reactive oxygen species (ROS) using electron spin resonance (ESR) technique: Implications for biomedical applications. Phys. Chemis. Chemic. Phys. 17(38):24937–24943.
- Werdin-Pfisterer, N.R., K. Kielland, and R.D. Boone. 2009. Soil amino acid composition across a boreal forest successional sequence. Soil Biol. Biochem. 41:1210–1220.