ABSTRACT
The present study was aimed at assessing the effect of storage temperature, hot water, and 1-MCP treatments on postharvest quality of ‘Karaj’ persimmon. To do so, fruits were harvested at the commercial maturity stage, treated with treatments of water at 25°C for 20 min (control), hot water at 45°C for 25 min, hot water at 50°C for 15 min, and 1-MCP at 500 nL.L−1 for 24 hours, and then stored at two temperatures of 1°C for 30 days and 13°C for 20 days. Finally, fruits were subjected to an astringency removal treatment (98% CO2 at 20°C for 24 h) before being transferred to shelf-life conditions for 5 days at 20◦C. Fruits’ quality was assessed after storage time and after shelf-life conditions, and the results showed that fruit tissue gelling, ethylene production, and ethanol and acetaldehyde contents increased in control fruit after cold storage at 1°C. The results also showed that at 1°C storage, the firmness of control fruits reduced and their coloration retarded. The existence of these symptoms in persimmon, which are chilling symptoms, showed that ‘Karaj’ persimmon is sensitive to chilling, but using treatments of 1-MCP and to some extent hot water at 50°C for 15 min effectively reduced these symptoms and maintained fruit quality. The fruits stored at 13°C soften quickly during storage, but gelling of fruit tissues did not occur and coloration was not affected. At this temperature, treatments of hot water and 1-MCP did not show a significant effect on the maintenance of the fruit quality compared to control.
Introduction
Japanese persimmon fruit (Diospyros kaki, Thunb.), belonging to the family of Ebenaceae, is divided into two main groups of astringent and nonastringent based on the amount of soluble tannin during physiological maturity (Itoo and Monselise, Citation1986). The majority of Iranian persimmon cultivars are of astringent type. Persimmon is an autumn fruit that its maintenance after the harvest is of high importance. Reduction in postharvest quality of persimmon fruit during storage or transport depends on the rate of tissue softening, change in quality and appearance of physiological disorders (Promyo and Park, Citation2009). The rate of metabolism and postharvest decline are directly influenced by temperature, so that reduction of temperature in the physiological limit increases the shelf life of the climacteric fruits by delaying ripening after harvest, but tropical and subtropical fruits such as persimmon are sensitive to chilling and show symptoms of cold injury at low temperatures; these symptoms are different based on the crop (Wills et al., Citation1998).
Common temperature for storage of persimmon fruit was reported zero degree centigrade (Ozdemir et al., Citation2009), but many studies have shown that the response of persimmon fruit to low temperatures varies based on cultivar (Besada et al., 2008; Collins. and Tisdell, 1995; MacRae, Citation1987; Nakano et al., Citation2001; Salvador et al., Citation2008). Symptoms of chilling in different cultivars of persimmon include rapid reduction of firmness, development of dark color in tissue, tissue gelling, increase of respiration rate, and increase in ethylene, ethanol and acetaldehyde production, all of which usually appear after cold storage at room temperature (Arnal and DelRio, Citation2004a; MacRae, Citation1987; Woolf et al., Citation1997a).
If the persimmon fruit is sensitive to chilling, use of effective treatments for reducing chilling symptoms can lead to the increase of shelf life. In recent years, use of thermal treatments for the induction of resistance to chilling, control of ripening and maintenance of the quality of horticultural crops in postharvest has attracted high interest (Lurie and Pedreschi, Citation2014). Part of the interest is due to the increased call from consumers for reducing the use of chemicals in postharvest industry. Among temperature treatments, immersion in hot water has advantages such as easy use, treatment in short time, high performance, low cost and commercial value compared to other methods (Fallik, Citation2004; Lurie and Pedreschi, Citation2014). The positive effects of hot water treatment on the reduction of chilling symptoms and the maintenance of postharvest firmness in ‘Fuyu’ nonastringent persimmon (Burmeister et al., Citation1997; Lay-Yee et al., Citation1997) and the astringent cultivar of ‘Rojo Brillante’ (Besada et al., Citation2008; Khademi et al., Citation2014) have been reported in different studies. In a study on persimmon cv. ‘Karaj’, hot water treatment at different times and temperatures was applied on fruits, and the results showed that hot water treatments at 45°C for 20–30 min, and 50°C for 15–20 min were the most effective treatments in the maintenance of fruit quality during cold storage and showed no adverse effects on the appearance of the fruits (Khademi et al., Citation2015).
Role of ethylene in lipid peroxidation and intracellular free radical production before the development of chilling symptoms or aging of the product has been proven. 1-Methylcyclopropene (1-MCP) can reduce the production of intracellular free radicals under stress conditions by inhibiting the action of ethylene, leading to the increase of cell resistance to stress and enhancement of shelf life. In addition to having effect on ethylene, 1-MCP directly regulates intracellular antioxidant capacity, especially enzymatic types (Yuan et al., Citation2010). It was reported in ‘Rojo Brillante’ persimmon that reduction of softening (as the main manifestation of chilling injury in this cultivar) by use of 1-MCP was associated with the upregulated catalase activity, which resulted in lower H2O2 accumulation after cold storage (Novillo et al., Citation2015). In other study by Zhang et al. (Citation2010) on ‘Fuyu’ persimmon, it was shown that treatment of 1-MCP at concentration of 500 nL.L−1 led to the reduction of chilling symptoms in the fruit during cold storage at 4°C. In this study, compared with control, 1-MCP delayed increases in respiration and ethylene production and exhibited increased superoxide dismutase and catalase activities, as well as lower membrane permeability and peroxidase and polyphenol oxidase activities throughout the cold storage. In general, 1-MCP treatment is considered one of the most effective treatments reducing chilling symptoms in persimmon fruit (Khademi et al., Citation2014; Salvador et al., Citation2004b). The alleviation of chilling injury as a result of 1-MCP treatment occurs through inhibition of ethylene action and improvement of antioxidant status.
Considering the different responses of different persimmon cultivars to storage temperatures, as well as the positive effects of hot water and 1-MCP treatments on the reduction of chilling symptoms and control of ripening process in different persimmon cultivars, the aim of the present study was to assess the effect of storage temperature, hot water and 1-MCP treatments on the postharvest life of persimmon fruit cv. ‘Karaj’.
Materials and methods
Fruit samples and treatments
Fruits of Japanese persimmons cv. ῾Karaj᾿ were harvested at commercial maturity (full coloring stage) from an orchard near Karaj city of Iran and transported at the same day to the experimental station in Malayer University, where fruits were sorted for uniformity of size, color and absence of defects. On the day of harvest, a sample of 21 fruits were separated from the selected fruits, and analyzed immediately after the harvest (at harvest).
Other fruits were divided into four homogeneous groups of 84 fruits which were subjected to the following treatments: water treatment at 25°C for 20 min (control), hot water treatment at 45°C for 25 min (HWT 45°C–25 min), hot water treatment at 50°C for 15 min (HWT 50°C–15 min) and 1-MCP treatment at 500 nL.L−1 for 24 hours (1-MCP).
For hot water treatments, persimmons were dipped in a recirculating hot water bath. Bath temperature was constantly monitored by thermometer and never reduced less than 1°C below the established value during each treatment. Following the treatment, fruits were dried at room temperature for about 1 hour. 1-MCP (Smart FreshTM), provided by Agro Fresh Inc. (Rohm and Hass Co., Italy), was formulated as powder (0.14% 1-MCP). Fruits were placed in a 40 l plastic container and exposed to 1-MCP at concentration of 500 nL.L−1 for 24 hours at room temperature (20°C) and 85% RH. Then, fruits of each treatment were divided into 2 subgroups, each containing 42 fruits, one subgroup was stored at 1°C and 90% RH for up to 30 days and the other one was stored at 13°C and 90% RH for up to 20 days.
At the end of the storage, the fruits of each treatment were divided into two samples of 21 fruits (as three replications); one sample was assessed directly after the storage and the other one was evaluated after deastringency treatment and during shelf-life period.
Deastringency treatment was carried out in closed polyethylene container, where desired CO2 atmosphere (≥95 CO2) was provided by replacing the air of the container by the flow from a CO2 gas cylinder and thereafter the container was sealed completely. The fruits were kept within enriched-CO2 atmosphere for 24 hours at 25°C and 80% RH (Yamada et al., Citation2002). After CO2 treatment application, the fruits were stored for 5 days at 20°C and ≥ 80% RH to simulate the shelf-life period and were finally assessed.
Fruit assessment
Extension of gelling within flesh was assessed visually by cutting fruit across the equator and rated on a scale of 0 (no gel) to 4 (firm, dark gel over the entire cut surface; mottled external appearance) according to MacRae (Citation1987). After scoring, gelling index (GI) was calculated as following formula:
Skin color was determined by measuring parameters of L*, a*, and b* by using a chroma meter (Minolta CR-400, Japan) at three points of the fruit. The hue angle was calculated by formula of hue° = tan−1 (b*/a*) (Khademi et al., Citation2013).
Fruit firmness was measured using an Effegi Penetrometer (model FT 327, Italy) fitted with an 8 mm tip. Firmness was measured at two opposite points around the equator on each fruit following the peel removal and the result was expressed as Newton (N).
The soluble tannin content was determined based on the Folin–Denis method. As such, 1 g of fruit sample was homogenized with 10 ml of 80% methanol using a mortar for 5 min. The homogenate was centrifuged at 8000 rpm at 4°C for 10 min, then 10 ml distilled water for each 5 ml of the extract was added in a test tube, followed by addition of 5 ml of the Folin–Denis reagent for color development. After 5 min, 2.5 ml saturated sodium carbonate solution was added and the absorbance was measured at 760 nm within 1 h by using a spectrophotometer. The amount of tannin content was estimated against standard tannic acid, and expressed as % of tannic acid (Taira, Citation1996).
The ethylene production rates were measured in six fruits per treatment. Fruits were sealed individually in a 1 l airtight jar for 2 h at 20°C, then 1 ml of headspace gas sample was withdrawn and injected into a gas chromatography (Varian, Model: 3800), equipped with propack QS 80/100 column and a flame ionization detector. Ethylene production was expressed as µL C2H4 kg−1h−1.
Acetaldehyde and ethanol production were measured in 6 replicates per treatment from 5 ml of juice sample of two fruits. For the analysis, juice samples were heated for 10 min at 60°C and then 1 ml of head space was withdrawn and injected in the gas chromatography (Varian, Model: 3800) fitted with flame ionization detector (FID). Ethanol and acetaldehyde were identified by comparison of retention times with those of a standard solution at different concentrations (Salvador et al., Citation2004b).
Statistical analysis
All the data were subjected to an analysis of variance (ANOVA) using SAS software (ver. 9.2), and differences among the means was compared by using the LSD test at a probability level of 5%.
Results
Gelling index
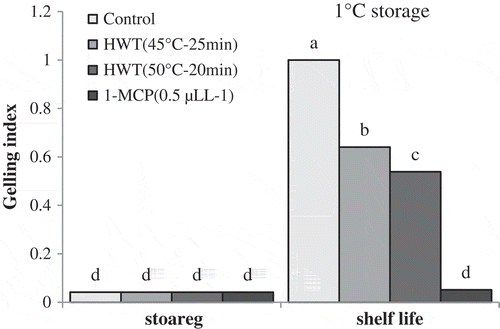
At a storage temperature of 13°C, fruit gelling was not observed in all the fruits treated with hot water and 1-MCP, as well as control treatment even after shelf-life conditions.
During the storage at 1°C, considerable gelling was not seen in all the samples, but the gelling increased significantly after shelf life in control fruits and the ones treated with HWT 45°C–25 min and HWT 50°C–15 min. Under shelf-life conditions, fruits treated with 1-MCP did not show significant gelling. The index of gelling in control fruits was significantly higher than that of fruits treated with hot water (). Fruits treated with HWT 45°C–25 min had higher gelling index compared to the fruits treated with HWT 50°C–15 min.
Color parameters
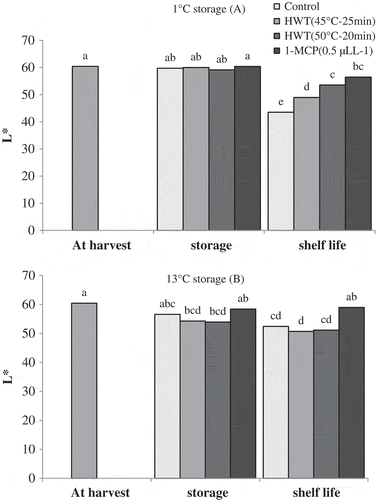
The trend of changes in color parameters showed that, during cold storage of 1°C, L* parameter of fruits did not show significant change compared to that at harvest time. Under shelf-life conditions, the L* value of all the fruits showed significant decrease as compared to the storage period. Meanwhile, the L* value of control fruit was significantly lower than that of hot water and 1-MCP treated fruits. The L* value of HWT 45°C–25 min treated fruits was lower than that of HWT 50°C–15 min and 1-MCP treated fruits (). During storage of 13°C, L* value of fruits subjected to HWT 45°C–25 min and HWT 50°C–15 min showed significant reduction when compared to the harvest time, while the L* value of control and 1-MCP treated fruits did not exhibit significant changes during this storage. During the shelf-life conditions, no significant changes were observed in L* value in all the fruits as compared to that after storage time, however after shelf-life period, the L* value of 1-MCP treated fruits was significantly higher than that of fruits treated with hot water as well as control fruits ().
Figure 2. Effect of storage temperatures, hot water (HWT) and 1-MCP treatments on L* of ‘Karaj’ persimmon after 30-day storage of 1°C (A), 20-day storage of 13°C (B), and shelf-life conditions. Means with the same letter in each fig are not significantly different at the 5% level of the LSD test.
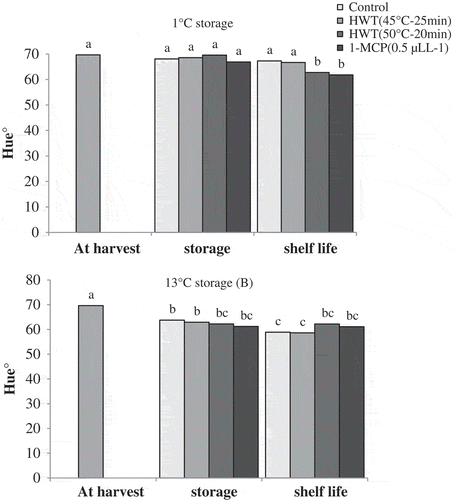
Based on the results presented in , hue° value in fruits of control and HWT 45°C–25 min did not show any significant changes during the cold storage and shelf-life conditions when compared to the harvest time. Fruits of HWT 50°C–15 min and 1-MCP treatments did not also show significant changes in hue° value during the cold storage as compared to the harvest time, however under the shelf-life conditions, the hue° value of these fruits decreased significantly. During the storage of 13°C, hue value of the fruits in all the treatments showed significant decrease as compared to the harvest time. Furthermore, under the shelf-life conditions, the hue value in fruits of control and of HWT 45°C–25 min treatments decreased significantly when compared to the storage time, while no significant changes were observed in hue value in the fruits of 1-MCP and HWT 50°C–15 treatments during the shelf-life conditions ().
Firmness
Fruit firmness at two storage temperatures of 1°C and 13°C reduced significantly in comparison with harvest time, with 13°C treatment showing higher amount of firmness reduction than 1°C treatment. After taking out the fruits from storage of 1°C, the results showed that the firmness of control fruits was significantly lower than that of other treatments, while no significant differences were found between other treatments in terms of this trait. Furthermore, the lowest rate of fruit firmness after shelf-life conditions was observed in control treatment. In this period, fruits of HWT 45°C–25 min treatment had lower firmness rate than fruits treated with HWT 50°C–15 min and 1-MCP ().
Figure 4. Effect of storage temperatures, hot water (HWT) and 1-MCP treatments on firmness of ‘Karaj’ persimmon after 30-day storage of 1°C (A), 20-day storage of 13°C (B), and shelf-life conditions. Means with the same letter in each figure are not significantly different at 5% level of the LSD test.
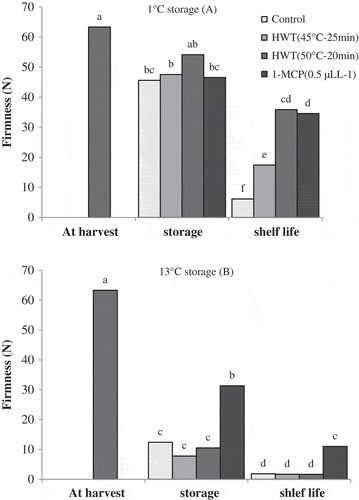
Compared to other treatments, 1-MCP treated fruits showed higher rate of firmness after storage of 13°C and the related shelf life, while no significant difference was observed in the firmness rate between the fruits of control and hot water treatments ().
Ethylene
After storage of 1°C, all the samples contained higher amount of ethylene when compared to the harvest time. The highest amount of ethylene production was related to the samples of control treatment. Fruits of HWT 45°C–25 min treatment produced higher amount of ethylene compared to the fruits of HWT 50°C–15 min and 1-MCP treatments. After shelf life, production of ethylene in all the samples showed significant reduction and reached the same amount of ethylene production as harvest time. No significant differences in ethylene production were observed among all the treatments after shelf life, as can be seen in .
Figure 5. Effect of storage temperatures, hot water (HWT) and 1-MCP treatments on ethylene production of ‘Karaj’ persimmon after 30-day storage of 1°C (A), 20-day storage of 13°C (B) and shelf-life conditions. Means with the same letter in each figure are not significantly different at 5% level of the LSD test.
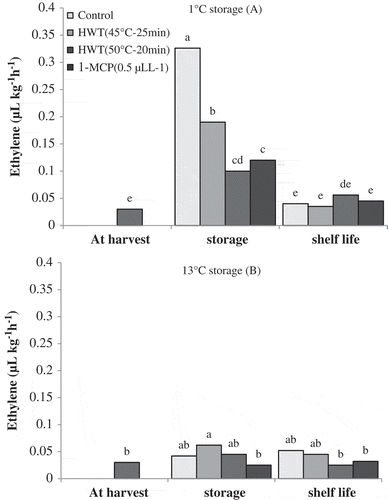
In general, ethylene production at storage of 13°C was lower than that at storage of 1°C. No significant changes were observed in ethylene production after storage of 13°C when compared with harvest time. There was not significant difference in ethylene production between fruits taken after storage and after shelf life ().
Ethanol
Ethanol production of all the treatments during two storages of 1 and 13°C did not show significant difference compared with harvest time. Under shelf-life conditions, ethanol production in all the treatments increased significantly following the storages at 1 and 13°C. After shelf life of 1°C storage, control fruits showed the highest amount of ethanol production.
At this time, fruits treated with HWT 45°C–25 min and HWT 50°C–15 min produced greater amount of ethanol compared with the fruits treated with 1-MCP, as shown in .
Figure 6. Effect of storage temperatures, hot water (HWT) and 1-MCP treatments on ethanol production of ‘Karaj’ persimmon after 30-day storage of 1°C (A), 20-day storage of 13°C (B), and shelf life conditions. Means with the same letter in each figure are not significantly different at 5% level of the LSD test.
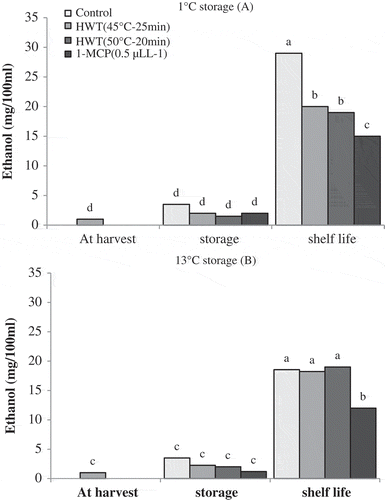
After shelf life of 13°C storage, fruits of 1-MCP treatment produced lower amount of ethanol compared with the fruits obtained from other treatments, but no significant differences were found in ethanol production between control fruits and fruits of hot water treatments ().
Acetaldehyde
The results of the present study showed that after cold storage of 1°C, control fruits produced higher amount of acetaldehyde when compared with fruits at harvest time; however the amount of acetaldehyde was not significantly different between harvest time and other treatments. The amount of acetaldehyde in all the treatments was increased by maintaining the fruits under shelf-life conditions. After the shelf life, the highest amount of acetaldehyde production was observed in control fruits. Fruits exposed to HWT 45°C–25 min and HWT 50°C–15 min contained higher amount of acetaldehyde compared to the fruits treated with 1-MCP treatment ().
Figure 7. Effect of storage temperatures, hot water (HWT) and 1-MCP treatments on acetaldehyde production of ‘Karaj’ persimmon after 30-day storage of 1°C (A), 20-day storage of 13°C (B), and shelf-life conditions. Means with the same letter in each figure are not significantly different at 5% level of the LSD test.
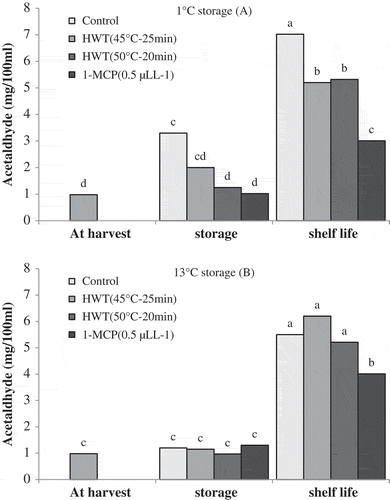
At storage conditions of 13°C, acetaldehyde production in all the treatments was low and was similar to the values obtained at harvest time. Maintenance of fruits at shelf life resulted in the increase in acetaldehyde production in all the treatments. After shelf-life conditions, there was not significant difference in acetaldehyde production between fruits of hot water and control treatments; however fruits of these treatments produced higher amount of acetaldehyde than those of 1-MCP treatment, as can be seen in .
Soluble tannin
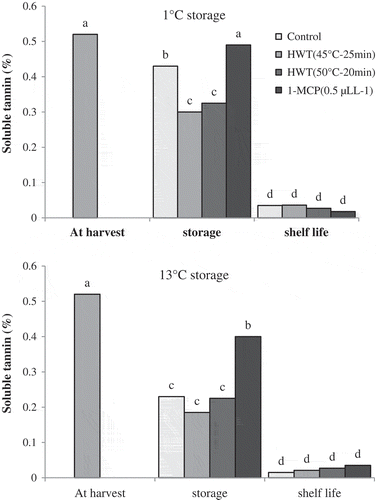
The amount of fruit soluble tannin at harvest time was higher than 0.5% fresh weight. The results showed that soluble tannin content reduced during two times of storage. Reduction of fruit soluble tannin during storage of 13°C was higher than that of 1°C storage. After both storage durations, the amount of soluble tannin in fruits treated with 1-MCP was higher than that in fruits of control and hot water treatments. After storage of 1°C, fruits related to hot water treatment contained lower content of soluble tannin than those of control treatment. Following the use of astringency removal treatment and maintenance of fruits under shelf-life conditions, the amount of soluble tannin in all the samples reduced and reached less than 0.1% fresh weight ( and ).
Figure 8. Effect of storage temperatures, hot water (HWT) and 1-MCP treatments on soluble tannin content of ‘Karaj’ persimmon after 30-day storage of 1°C (A), 20-day storage of 13°C (B), and shelf-life conditions. Means with the same letter in each figure are not significantly different at 5% level of the LSD test.
Discussion
Tissue gelling in ‘Fuyu’ persimmon is considered the most apparent symptom of chilling, but with the exception of cases that intensity of chilling injury is high, the symptoms of chilling appear after the storage and at room temperature (Lay-Yee et al., Citation1997; MacRae, Citation1987). Based on the results of the present study, tissue gelling was just observed in fruits stored at 1°C and after shelf-life conditions, while no tissue gelling was observed in fruits stored at 13°C. Therefore, ‘Karaj’ persimmon is sensitive to chilling but its symptoms do not appear during cold storage. According to the results obtained on gelling, hot water treatments especially HWT 50°C–15 min resulted in the reduction of chilling injury severity compared to the control, but 1-MCP treatment prevented chilling occurrence. In a study conducted on ‘Rojo Brillante’ persimmon, 1-MCP treatment was reported to be more effective in the reduction of chilling symptoms than hot water treatments (Khademi et al., Citation2014).
One of the main chilling injury symptoms in sensitive persimmon cultivars is increase in the production of acetaldehyde, so that there is a direct relationship between the accumulation of acetaldehyde and intensity of chilling damage (Arnal and Del Río, 2004a). In the present study, control fruits after the cold storage of 1°C showed higher amount of acetaldehyde than hot water and 1-MCP treated fruits, which indicated the higher intensity of chilling injury in these fruits. Increase in the production of acetaldehyde and ethanol as a result of applying astringency removal treatment in persimmon fruit is an inevitable issue (Salvador et al., Citation2007). In this experiment, application of CO2 deastringency treatment led to a considerable increase in acetaldehyde and ethanol production in all the samples following the shelf-life conditions. However, chilled control fruits contained higher amount of acetaldehyde and ethanol than other fruits. In similar results on ‘Rojo Brillante’ persimmon, it was shown that after CO2 treatment and shelf-life conditions following cold storage, the highest amount of acetaldehyde and ethanol was detected in control fruits, which was concomitant with higher intensity of chilling injury in these fruits (Khademi et al., Citation2014). In the present study, the higher acetaldehyde and ethanol production found in hot water treated fruits, as compared to 1-MCP, was also concomitant with higher rate of chilling injury.
In fruits stored at 13°C that showed no chilling symptoms based on tissue gelling, there was not significant difference between treatments of hot water and control in terms of acetaldehyde and ethanol production, but lower amount of these compounds in fruits treated with 1-MCP is due to the effect of 1-MCP on the inhibition of ethylene action (Blankenship and Dole, Citation2003; Watkins, Citation2006).
Based on the results of the current study, reduction of firmness in storage of 13°C was quicker than 1°C. Reduction of firmness in persimmon is controlled by ethylene (Burmeister et al., Citation1997; Nakano et al., Citation2001), but since a significant increase in the production of ethylene was not observed in all the treatments after storage of 13°C, it seems that enhancement of ethylene production and softening of fruits occurred during the storage. Persimmon with the firmness rate lower than 10 newtons is commercially considered unfavorable (Arnal and Del Río, 2004b), therefore just persimmon treated with 1-MCP had a good rate of firmness at the end of shelf life. The fruit firmness at storage of 1°C was better maintained compared with storage of 13°C, so that fruits during storage of 1°C showed high rate of firmness in all the treatments but their ethylene production increased after storage, leading to the reduction of firmness under shelf-life conditions. After shelf-life conditions following the storage of 1°C, just control fruits had firmness rate below 10 newtons. Since reduction of firmness in ‘Rojo Brillante’ persimmon is considered the most apparent symptom of chilling (Arnal and Del Rio, Citation2004b; Besada et al., Citation2008; Salvador et al., Citation2004a), it can be concluded that control fruits suffered from chilling injury, and treatments of hot water and 1-MCP caused a reduction in chilling intensity in ‘Karaj’ persimmon. On the other hand, the results indicated that fruits stored at 13°C showed higher reduction in firmness compared to the fruits stored at 1°C. It was shown in nonastringent persimmon of ‘Fuyu’ that softening resulting from chilling is different from natural softening, so that in softening caused by chilling, degradation of cell wall leads to the formation of tissue gelling in fruit (Grant et al., Citation1992; Woolf et al., Citation1997b). In line with the results reported, no tissue gelling was observed in fruits stored at 13°C in the present study, and therefore the nature of softening at the two storage temperatures was different. With respect to this issue, hot water treatments effectively reduced the symptoms of chilling in ‘Karaj’ persimmon but it did not show significant effect on the delay of natural softening. Treatment of 1-MCP showed higher effectiveness in the maintenance of firmness at the storage of 1°C compared to the storage of 13°C. Harima et al. (Citation2003) stated that 1-MCP delayed persimmon softening but softening occurred more over time.
Decrease in hue° value is the characteristic of advanced stage of ripening and change of color from yellow–orange to red–orange that was displayed in ‘Karaj’ persimmon when its ripening took place (Khademi et al., Citation2013). Accordingly, none of the samples showed color advance during the storage at 1°C, but fruits of HWT 50°C–15 min and 1-MCP treatments, in contrast with control fruits and the ones treated with HWT 45°C–25 min, showed color advance during the shelf-life period. One of the symptoms of chilling in some persimmon cultivars is suppressed coloration which has been shown in many reports (Collins and Tisdell, Citation1995; Ozdemir et al., Citation2009; Woolf et al., Citation1997a). Therefore, based on the results obtained from color values, the fruits related to control and HWT 45°C–25 min treatments were affected by chilling and did not show coloration despite high ethylene production. Excessive production of ethylene is a symptom of chilling in persimmon (Burmeister et al., Citation1997; Lay-Yee et al., Citation1997). Treatments of HWT 50°C–15 min and 1-MCP prevented retardation of coloration caused by chilling.
Based on the results of hue° value, fruit color of all the treatments increased during storage at 13°C, indicating natural coloration of ‘Karaj’ persimmon at higher temperature. In similar results on ‘Rojo Brillante’ persimmon, Arnal and DelRio (2004a) showed that fruits stored at 8, 11, and 15°C had higher color index than fruits stored at 1°C. After shelf-life period, treatments of HWT 50°C–15 min and 1-MCP delayed fruit coloration compared to the control. The effects of hot water and 1-MCP treatments on the delay of persimmon coloration at ambient temperature have been reported by other studies (Luo, Citation2006, Citation2007).
Visual quality of persimmon fruit decreased along with the reduction in L* value. L* value decreased when skin blackening or browning increased (Promyo and Park, Citation2009). Woolf et al. (Citation1997b) in ‘Fuyu’ persimmon and Arnal and DelRio (2004a) in ‘Rojo Brillante’ persimmon reported that increased chilling injury resulted in a darker fruit with lower L* value. In the present study based on the L* value, fruits of control and HWT 45°C–25 min treatments were darker in color than fruits of HWT 50°C–15 min and 1-MCP treatments, representing higher severity of chilling injury in these fruits after the cold storage.
After the storage of 13°C, the fruits of HWT 45°C–25 min and HWT 50°C–15 min had lower L* values than those of control and 1-MCP treatments. Fruit browning was reported as the main disorder associated with hot water treatment in ‘Fuyu’ persimmon (Lay-Yee et al., Citation1997). It seems that lower L* value in hot water treated fruits is due to the slight browning under the treatments, but enzymatic browning reaction which usually occurs at high temperature did not occur in hot water treated fruits that were stored at zero centigrade storage.
After treatment of deastringency and maintenance of fruits under shelf life following the storages of 1 and 13°C, soluble tannin content in all the fruits reduced below 0.1%, showing that fruits of ‘Karaj’ persimmon were nonastringent (Khademi et al., Citation2010; Taira, Citation1996). Treatments of hot water and 1-MCP did not have any effects on the response of ‘Karaj’ persimmon to deastringency treatment. After the storage of 1°C, fruits treated with hot water contained lower soluble tannin than other fruits, which can be due to this fact that during hot water treatment, accumulation of acetaldehyde in persimmon flesh led to the induction of pyruvate decarboxylase enzyme activity and thus caused a reduction in amount of soluble tannin (Pesis and Ben-Arie, Citation1986). On the other hand, concentration of soluble tannin in fruits stored at 13°C was less than that of fruits stored at 1°C, which is due to the higher reduction of fruit firmness during 13-°C storage. Cell wall polysaccharides are degraded during the softening of persimmon fruit, and the pectin released from their degradation can be combined with soluble tannins and reduce their quantity (Cutillas-Iturralde et al., Citation1993).
In conclusion, ‘Karaj’ persimmon fruit is sensitive to chilling, and in the case of storage at low temperature of 1°C, chilling symptoms that include tissue gelling, suppress in coloration, increase in ethylene and acetaldehyde production and rapid reduction in firmness, appear after the shelf life following the cold storage. On the other hand, storage of fruit at nonchilling temperature of 13°C is accompanied with the rapid softening of fruit and reduction of quality. Use of 1-MCP was the most effective treatment in reducing symptoms of chilling injury in ‘Karaj’ persimmon. Furthermore, treatment of 50°C hot water for 15 min controlled chilling symptoms and preserved the quality of the fruit, but its effect was less than that of 1-MCP treatment, while both hot water treatments as well as 1-MCP treatment did not significantly affect the preservation of firmness and quality of fruits at 13°C storage.
Therefore, based on the results of the present study, the best way to extend the shelf life of persimmon fruit is combined use of cold storage and chilling-reducing treatments such as 1-MCP.
References
- Arnal, L., and M.A. Del Rio. 2004a. Quality of persimmon fruit (Diospyros kaki L.) cv. ʻRojoBrillanteʼ during storage at different temperatures. Span. J. Agri. Res. 2:243–247.
- Arnal, L., and M.A. Del Rio. 2004b. Effect of cold storage and removal astringency on quality of persimmon fruit (Diospyros kaki L.) cv. ʻRojo Brillanteʼ. Food Sci. Technol. Int. 10:179–185.
- Besada, C., A. Salvador, L. Arnal, and J.M. Martinez-Javega. 2008. Hot water treatment for chilling injury reduction of astringent ‘RojoBrillante’ persimmon at different maturity stages. HortScience. 43:2120–2123.
- Blankenship, S.M., and J.M. Dole. 2003. 1-Methylcyclopropene: A review. Postharvest Biol. Technol. 28:1–25.
- Burmeister, D.M., S. Sarah, S. Green, and A.B. Woolf. 1997. Interaction of hot water treatments and controlled atmosphere storage on quality of ʻFuyuʼ persimmon. Postharvest Biol. Technol. 12:71–81.
- Collins, R.J., and J.S. Tisdell. 1995. The influence of storage time and temperature on chilling injury in ‘Fuyu’ and ‘Suruga’ persimmon (Diospyros kaki L.) grown in subtropical Australia. Postharvest Biol. Technol. 6:149–157.
- Cutillas-Iturralde, A., I. Zarra, and E.P. Lorences. 1993. Metabolism of cell wall polysaccharides from persimmon fruit. Pectin solubilization during fruit ripening occurs in apparent absence of polygalacturonase activity. Physiologia Plantar. 89:369–375.
- Fallik, E. 2004. Prestorage hot water treatments (immersion, rinsing and brushing). Postharvest Biol. Technol. 32:125–134.
- Grant, T.M., E.A. MacRae, and Redgwell. 1992. RJ: effect of chilling injury on physicochemical properties of persimmon cell walls. Phytochemistry. 31:3739–3744.
- Harima, S., R. Nakano, S. Yamauchi, Y. Kitano, A. Inaba, and Kubo. 2003. Y: extending shelf - life of astringent persimmon (Diospyros kaki T.) fruit by 1-MCP. Postharvest Biol. Technol. 29:318–323.
- Itoo, S., and S.P. Monselise. 1986. Handbook of fruit set and development. CRC Press, Inc, Boca Raton, Florida.
- Khademi, O., Y. Mostofi, Z. Zamani, and R. Fatahi. 2010. The effect of deastringency treatments on increasing the marketability of persimmon fruit. Acta Hortic. 877:687–691.
- Khademi, O., Z. Zamani, E. Poor Ahmadi, and S. Kalantari. 2013. Effect of UV-C radiation on postharvest physiology of persimmon fruit (Diospyros kaki Thunb.) cv.'Karaj'during storage at cold temperature. International Food Research Journal. 20:247–253.
- Khademi, O., C. Besada, Y. Mostofi, and A. Salvador. 2014. Changes in pectin methylesterase, polygalacturonase, catalase and peroxidase activities associated with alleviation of chilling injury in persimmon by hot water and 1-MCP treatments. Sci. Hortic. 179:191–197.
- Khademi, O., Z. Zamani, Y. Mostofi, S. Kalantari, and M. Rasouli. 2015. The behavior of persimmon fruit cv. ‘Karaj’ in response to postharvest hot water treatments and storage temperature. In Persian with English abstract. J. Food Sci. Technol. 48:13–26.
- Lay-Yee, M., B. Sarah, S.K. Forbes, and Woolf. 1997. AB: hot-water treatments for insect disinfestations and reduction of chilling injury of ʻFuyuʼ persimmon. Postharvest. Biol. Technol. 10:81–87.
- Luo, Z. 2006. Extending shelf-life of persimmon (Diospyros kaki L.) fruit by hot air treatment. Europ. Food Res. Technol. 222:149–154.
- Luo, Z. 2007. Effect of 1-methylcyclopropene on ripening of postharvest persimmon (Diospyros kaki L.) fruit. LWT-Food Sci. Technol. 40:285–291.
- Lurie, S., and R. Pedreschi. 2014. Fundamental aspects of postharvest heat treatments. Hortic. Res. 1:14030.
- MacRae, E.A.. 1987. Development of chilling injury in New Zealand grown ʻFuyuʼ persimmon during storage. New Z. J. Exp. Agri. 15:333–344.
- Nakano, R., S. Harima, E. Ogura, S. Inoue, Y. Kubo, and A. Inaba. 2001. Involvement of stress-induced ethylene biosynthesis in fruit softening of ‘Sajio’ persimmon. J. Jpn. Soc. Hort. Sci. 70:581–585.
- Novillo, P., A. Salvador, P. Navarro, and C. Besada. 2015. Involvement of the redox system in chilling injury and its alleviation by 1-Methylcyclopropene in ‘Rojo Brillante’ persimmon. HortScience. 50:570–576.
- Ozdemir, A.E., E.E. Çandır, C. Toplu, M. Kaplankıran, E. Yıldız, and C. Inan. 2009. The effects of hot water treatments on chilling injury and cold storage of ʻFuyuʼ persimmons. Afric. J. Agri. Res. 4:1058–1063.
- Pesis, E., and R. Ben-Arie. 1986. Carbon dioxide assimilation during postharvest removal of astringency from persimmon fruit. Physiol. Plant. 67:644–648.
- Promyo, K., and Y. Park. 2009. Shelf life of non-astringent ‘Fuyu’ persimmon by preheating and hot water dipping with antibrowning agent following cold storage. Horticulture, Environ. Biotechnol. 50:437–445.
- Salvador, A., L. Arnal, C. Besada, V. Larrea, I. Hernando, and I. Pérez-Munuera. 2008. Reduced effectiveness of the treatment for removing astringency in persimmon fruit when stored at 15°C: physiological and microstructural study. Postharvest Biol. Technol. 49:340–347.
- Salvador, A., L. Arnal, C. Besada, V. Larrea, A. Quiles, and I. Perez-Munuera. 2007. Physiological and structural changes during ripening and deastringency treatment of persimmon fruit cv.ʻRojo Brillanteʼ. Postharvest Biol. Technol. 46:181–188.
- Salvador, A., L. Arnal, A. Monterde, and J. Cuquerella. 2004a. Reduction of chilling injury symptoms in persimmon fruit cv. ‘Rojo Brillante’ by 1-MCP. Postharvest Biol. Technol. 33:285–291.
- Salvador, A., J. Cuquerella, J.M. Martinez-Javega, A. Monterde, and P. Navarro. 2004b. 1-MCP preserves the firmness of stored persimmon ʻRojo Brillanteʼ. J. Food Sci. 69:69–73.
- Taira, S. 1996. Astringency in persimmon, p. 97–110. In: Linskens H.F. and Jackson J.F., Eds. Modern methods of plant analysis, fruit analysis. Vol. 18. Springer-Verlag, Berlin.
- Watkins, C.B. 2006. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol. Advan. 24:389–409.
- Wills, R., B. Mcglasson, D. Graham, and D. Joyce. 1998. Effect of tempereature, p. 60–76. In: Postharvest. 4th. Unsw Press, Australia.
- Woolf, A.B., S. Ball, K.J. Spooner, M. Lay-Yee, I.B. Ferguson, C.B. Watkins, A. Gunson, and S.K. Forbes. 1997a. Reduction of chilling injury in the sweet persimmon ʻFuyuʼ during storage by dry air heat treatments. Postharvest Biol. Technol. 11:155–164.
- Woolf, A.B., E.A. MacRae, K.J. Spooner, and R.J. Redgwell. 1997b. Changes to physical properties of the cell wall and polyuronides in response to heat treatment of ʻFuyuʼ persimmon that alleviate chilling injury. J. Amer. Soc. Hort. Sci. 122:698–702.
- Yamada, M., S. Taira, M. Ohtsuki, A. Sato, H. Iwanami, H. Yakushiji, R. Wang, Y. Yang, and G. Li. 2002. Varietal difference in the ease of astringency removal by carbon dioxide gas and ethanol vapor treatments among oriental astringent persimmon of Japanese and Chinese origin. Scientia Hort. 94:63–72.
- Yuan, G., B. Sun, J. Yuan, and Q. Wang. 2010. Effects of 1-methylcyclopropene on shelf life, visual quality, antioxidant enzymes and health-promoting compounds in broccoli florets. Food Chem. 118:774–781.
- Zhang, Z., Y. Zhang, D.J. Huber, J. Rao, Y. Sun, and S. Li. 2010. Changes in prooxidant and antioxidant enzymes and reduction of chilling injury symptoms during low-temperature storage of ‘Fuyu’ persimmon treated with 1-MCP. HortScience. 45:1713–1718.