ABSTRACT
Effect of 2,4-D and naphthalene acetic acid (NAA) on fruit drop reduction in pummelo cv. Thong Dee was investigated in the pummelo growing areas of Nakhon Pathom province, Thailand. Five similar sized and aged of pummelo trees were selected to set up the experiment. Ten mature branches with the same size from each pummelo tree were randomly selected around the canopy for 2,4-D (20 and 40 mg L−1), NAA (20 and 40 mg L−1) application and control. All treatments were applied to selected pummelo branches 2 times at full bloom and 2 months after fruit set. The results showed that 20 mg L−1 NAA (14.84%) and 40 mg L−1 NAA (12.26%) gave significantly higher percent of fruit retention at 6 months after fruit set. However, leaf total nonstructural carbohydrate concentration analysis showed that 40 mg L−1 2,4-D (104.86 mg g−1) and 20 mg L−1 2,4-D (96.55 mg g−1) gave significantly higher total nonstructural carbohydrate than those in control (78.44 mg g−1). For fruit quality, 40 mg L−1 2,4-D and 20 mg L−1 2,4-D gave the highest peel weight with 435.55 and 358.57 g, respectively, and 40 mg L−1 2,4-D gave the highest peel thickness with 20.25 mm, while 20 mg L−1 NAA gave statistically higher total soluble solid than those in 20 mg L−1 2,4-D and 40 mg L−1 2,4-D. Therefore, 20 mg L−1 of NAA sprayed 2 times at full bloom and 2 month after fruit set effectively reduced fruit drop and increased percentage of fruit retention in pummelo cv. Thong Dee.
KEYWORDS:
Introduction
Pummelo growers in Thailand have encountered preharvest fruit drop problems. Over the last 2–3 years, this problem has become quite serious, with growers in Thailand losing both production and income. The data from the Office of Agricultural Economics showed that the quantity of exporting pummelo has been decreased from 14,338 t in 2012 to 12,522 t in 2013. A shortage of carbohydrate, imbalances in plant nutrition and plant hormones as well as insect and pathogen have all been reported as causes of the fruit drop and fruit retention reduction in pummelo and other citrus fruit (Davies and Albrigo, Citation1994). The fruit drop problem has been found in many fruit crops. Azher Nawaz et al. (Citation2008) reported that preharvest fruit drop is the major reason of the low-yield mandarin in Pakistan; this drop of fruit at various stages of fruit development is due to malnutrition, water stress, excessive insect pest attack, and most important is the hormonal imbalance. Davies and Albrigo (Citation1994) and Modise et al. (Citation2009) also reported premature fruit drop of many fruit crops particularly of the oranges. In apples, Ward (Citation2004) reported that fruit drop in apple depended on plant hormone content. Cultural control of preharvest fruit drop has relied upon plant growth regulators (PGRs) when naphthalene acetic acid (NAA) applied 2 or 4 days after cutting delayed fruit drop. Moreover, Stern and Ben-Arie (Citation2006) reported that 3,5,6-TPA also reduced fruit drop in apple, while, in lychee, ABA promoted fruit drop but auxin reduced fruit drop. Koukourileou-Petridou (Citation2003) found that June drop in almond took place concomitance with the reduction of IAA content in almond fruit. However, the application of a PGR was able to reduce fruit drop in almonds. PGRs are known to have a great influence on fruit drop and fruit retention in fruit trees. An imbalance of auxins, cytokinins, and gibberelins for example may lead to the formation of an abscission layer at the stem point and eventual fruit drop (Lahey et al., Citation2004; Chen et al., Citation2006). The application of PGR’s can reenforce hormone balance or retard the precipitous fall and losses prior to harvest. The use of auxins prevents dropping of fruit by maintaining the cells at the zone of abscission, preventing the synthesis of hydrolytic enzymes such as cellulose, which decompose the cell wall (Modise et al., Citation2009). In citrus fruits, 2,4-DP application before mature fruit dropping reduced fruit drop in sweet orange (Agusti et al., Citation2006) and 2,4-D application also reduced preharvest fruit drop in other citrus fruits such as navel oranges (Anthony and Coggins, Citation1999; Modise et al., Citation2009). Azher Nawaz et al. (Citation2008) reported that 2,4-D and NAA reduce preharvest drop of Kinnow mandarin compared to control. It is clear that 2,4-D treatments proved better compared to NAA, while, in Satsuma mandarin, treatments 400 mg L−1 of NAA and 60 mg L−1 of 2,4-D reduced preharvest drop compared to control significantly (Akhlaghi Amiri et al., Citation2012). Moreover, the other auxin type such as 2,4,5-TPA can also be utilized to prevent fruit drop in citrus (Michael et al., Citation1999).
In addition, there was some evidence that showed auxin promoted cell elongation by increasing vacuole enlargement and cell wall plasticity resulting in fruit retention increasing. Moreover, the spraying of auxins prevented the dropping of fruit by maintaining the cells at the zone of abscission, preventing the synthesis of hydrolytic enzymes, such as cellulase, which decomposed the cell walls (Monselise and Goren, Citation1978). The same authors reported also that hormone balance acted on the polygalacturonase activity, which, together with cellulase, was responsible for the degradation of the two important components of cell walls, cellulose, and pectin. That can result in the breakdown of abscission layer in the abscission zone where fruit abscission occurs.
However, the effect of PGR application on fruit drop reduction in pummelo has not been studied. Therefore, the objective of this study was to investigate the effect of 2,4-D and NAA on fruit drop reduction in pummelo cv. Thong Dee.
Material and methods
Plant selection and experimental design
This research was conducted in the pummelo growing areas of Nakhon Pathom province during January–August 2013. Five similar size and age of pummelo trees cv. Thong Dee were selected to set up the experiment. Ten similar size branches from each pummelo tree were selected for 2,4-D (20 and 40 mg L−1), NAA (20 and 40 mg L−1) application and control (distilled water) (two mature branches/treatment/tree), totally five replication per treatment. All of the PGRs were applied 2 times at full bloom and 2 months after fruit set which was the period of highest fruit drop in pummelo (Nartvaranant, Citation2012). NAA (the commercial preparation “PLANOFIX®” contains 4.5% 1-naphthylacetic acid, May & Baker Ltd.) and 2,4-D (the commercial preparation “2,4-D®” contains 84% 2,4-dichlorophenoxic acid, Pato chemical Ltd.) were prepared before foliar application each time. Both the 20 and 40 mg L−1 NAA solutions were prepared from the concentrated commercial solution by mixing 0.89 and 1.78 mL of concentrated solution with water until the solution volume of 2 L. The 20 and 40 mg L−1 2,4-D solution were prepared from the concentrated commercial solution by mixing 0.48 and 0.95 mL of concentrated solution with water until the solution volume of 2 L. Those solutions combined with surfactant 2 mL L−1 (Surfix®, Grow more group (Thailand) Co., Ltd.) were sprayed to the selected branches by high-pressure hand sprayer with two periods of application between 08:00 and 10:00 a.m.: (1) full bloom stage and (2) 2 months after fruit set. The solutions were sprayed to a point of run-off. It was observed that about 2 L was required to spray each treatment to a point of run off.
The randomized complete block design was used for this study and the data were analyzed by the analysis of variance (ANOVA) at 95% confidence interval.
Fruit diameter, percent of fruit retention, and fruit quality measurement
Fruit diameter measurement
Twenty pummelo fruits from each treatment were selected randomly for fruit diameter measurement using vernier caliper every 2 weeks during 1–3 months after fruit set. After 3 months, fruit diameter was measured monthly until harvesting.
Percentage of fruit retention measurement
Two similarly size branches per treatment (totally 10 branches per tree) were selected toward the edge of the tree branches, on opposite sides of the fruit trees. One was selected on the eastern orientation of the pummelo tree and the other on the western side. The number of fruits on the branches was counted using a manual counter, prior to the application of the PGRs. After application, fruit counting resumed every 2 weeks during 1–3 months after fruit set. After 3 months, fruit counting was measured monthly until harvesting. Percentage of fruit retention was calculated by formula as follow:
Fruit quality measurement
Ten pummelo retained fruits per treatment were randomly collected at the harvesting stage for fruit quality measurement as follow: fruit weight, fruit circumference, peel fresh weight, pulp fresh weight, peel thickness, total soluble solid (TSS), and titratable acidity (TA).
Total nonstructural carbohydrates analysis
Ten recently mature leaves per treatment (two leaves per shoot) were first collected before PGRs application and then every month after application. Those leaves from each treatment were taken to laboratory for total nonstructural carbohydrates (TNC) analysis using the Nelson’s procedure. All the leaf samples were analyzed for TNC by washing the samples in tap water then rinsing in distilled water and finally drying in a hot air oven at 65°C for 72 h. After drying, the material was ground in a Wiley Mill and stored in a desiccator. Total nonstructural carbohydrates were extracted with 0.2 N H2SO4 (Smith, Citation1969) and determined by the Nelson reducing sugar procedure method described by Hodge and Hofreiter (Citation1962) at the Central Laboratory, Kasetsart University, Kamphaeng Saen Campus.
Results
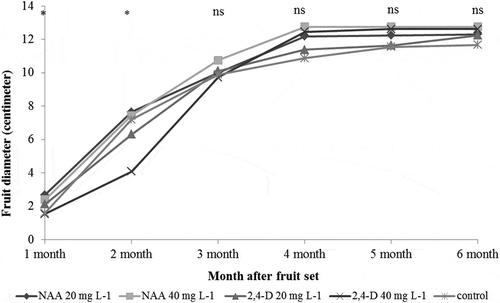
The results showed that 20 and 40 mg L−1 NAA gave significantly higher percent of fruit retention than the other treatments as 20 mg L−1 NAA, 40 mg L−1 NAA, 20 mg L−1 2,4-D, control, and 40 mg L−1 2,4-D had 14.84, 12.26, 9.57, 4.60, and 2.11 percentage of fruit retention, respectively, at 6 months after fruit set (). However, NAA and 2,4-D did not have effect on fruit diameter at 3–6 months after fruit set. Although, 20 mg L−1 NAA gave the highest fruit diameter at 2 months after fruit set ().
Table 1. Effect of 2,4-D and NAA on percent of fruit retention in pummelo cv. Thong Dee.
Figure 1. Effect of 2,4-D and NAA on fruit diameter (cm.) in pummelo cv. Thong Dee.
ns: Nonsignificant difference at 95% confidence interval analyzed by the analysis of variance; *significant difference at 95% confidence interval analyzed by the analysis of variance.
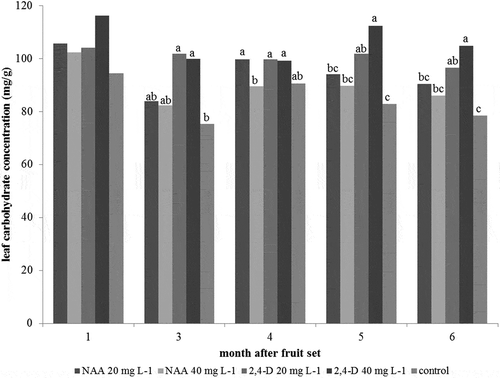
Leaf total nonstructural carbohydrate concentration analysis showed that 40 mg L−1 2,4-D (112.43 mg g−1) and 20 mg L−1 2,4-D (101.83 mg g−1) gave statistically more total nonstructural carbohydrate than those in control (82.97 mg g−1) at 5 months after fruit set, while, at 6 months after fruit set, 40 mg L−1 2,4-D (104.86 mg g−1) and 20 mg L−1 2,4-D (96.55 mg g−1) also gave statistically more total nonstructural carbohydrate than those in control (78.44 mg g−1) (). However, there was no significant difference in total nonstructural carbohydrate in pummelo leaf among 20 mg L−1 NAA (90.44 mg g−1), 40 mg L−1 NAA (85.98 mg g−1), and control (78.44 mg g−1).
Figure 2. Effect of 2,4-D and NAA on leaf carbohydrate concentration in pummelo cv. Thong Dee. Different letters on bars denote significant differences between the means at 95% confidence interval analyzed by Duncanʼs new multiple range test (DMRT).
For fruit quality, 2,4-D and NAA had no effect on fruit weight, fruit circumstance, pulp fresh weight, and TA but 40 mg L−1 2,4-D and 20 mg L−1 2,4-D gave highest peel fresh weight with 435.55 and 358.57 g, respectively, followed by 40 mg L−1 NAA (333.56 g), 20 mg L−1 NAA (328.43 g), and control (298.92 g) ().
Table 2. Effect of 2,4-D and NAA on fruit quality in pummelo cv. Thong Dee at harvesting stage.
An amount of 40 mg L−1 2,4-D gave the highest peel thickness with 20.25 mm, followed by 20 mg L−1 2,4-D (17.18 mm), control (16.15 mm), 20 mg L−1 NAA (14.43 mm), and 40 mg L−1 NAA (13.92 mm). However, there was no significant difference in peel thickness among 20 mg L−1 NAA, 40 mg L−1 NAA, and control (), whereas no significant difference in TSS among 20 mg L−1 NAA (13.05°Brix), 40 mg L−1 NAA (11.98°Brix), and control (11.50°Brix) was found but 20 mg L−1 NAA gave statistically higher TSS than those in 20 mg L−1 2,4-D (10.20°Brix) and 40 mg L−1 2,4-D (10.00°Brix) (). However, 20 mg L−1 NAA gave the statistically highest TSS:TA ratio (27.19) followed by control (16.73), 40 mg L−1 NAA (16.41), 40 mg L−1 2,4-D (11.76), and 20 mg L−1 2,4-D (11.33) ().
Discussion
From the results, 20 and 40 mg L−1 NAA gave the highest percentage of fruit retention, while there was no effect on pummelo fruit size as the pummelo fruit reached 4–7 months after the fruit set. However, 40 and 20 mg L−1 of 2,4-D gave statistically more leaf carbohydrate concentration than control but there was no significant difference in those among 20 mg L−1 NAA, 40 mg L−1 NAA, and control. Therefore, it was obviously found that NAA application increased percentage of fruit retention more than those in 2,4-D application as 20 and 40 mg L−1 NAA gave the highest percentage of fruit retention. However, 20 mg L−1 NAA was more suitable to be used than 40 mg L−1 NAA, as less substance concentration gave the similar percentage of fruit retention. This result may be different from the other plants or the other citrus species which there have been reported that 2,4-D increased fruit retention. Agusti et al. (Citation2006) reported that use of the 2-ethylhexyl ester of 2,4-DP sprayed before mature fruit abscission significantly reduced fruit drop in sweet orange (Citrus sinensis L. Osbeck) “Washington navel” and “Navelate.” At 15 mg L−1, the percentage of abscised fruit was reduced by 50–75% compared to untreated trees, depending on the variety and the orchard. Anthony and Coggins (Citation1999) found that 2,4-D application prevented preharvest fruit drop in citrus. Moreover, Modise et al. (Citation2009) reported that 20 mg L−1 2,4-D reduced fruit abscission in navel orange but an increase in the concentration to 30 and 40 mg L−1 of 2,4-D increased fruit abscission. Therefore, the suitable concentration of 2,4-D for navel orange was 16–20 mg L−1. From this research, using 20 and 40 mg L−1 2,4-D did not effectively reduce fruit drop in pummelo. It could be that the concentration of 2,4-D used in this study was not suitable for pummelo cv. Thong Dee. These concentrations might be toxicity to pummelo as 40 mg L−1 2,4-D gave the less percentage of fruit retention than control, although both of 2,4-D concentrations gave the higher carbohydrate concentration than those in NAA. Apart from this, the time of 2,4-D application in this research might not be suitable due to there having been reported that 2,4-D application should be applied on the middle of fruit growth stage to prevent fruit drop, while, in this research, 2,4-D was applied to pummelo trees at full bloom and 2 months after fruit set which is the beginning stage of fruit growth.
An amount of 20 and 40 mg L−1 NAA used in this study reduced fruit drop in pummelo cv. Thong Dee. There was possibility that 2,4-D had more auxin activity than NAA when it used in the same concentration (Nartvaranant, Citation2006). Thus, 2,4-D could give more toxicity to the plant than those in NAA resulting in fruit drop promotion in pummelo for this research. Effect of NAA on fruit drop reduction has been reported in many plants. Bankar and Prasad (Citation1990) reported that 10, 20, and 30 mg L−1 NAA prevented fruit drop in berry cv. Gola. In tomato, NAA applied at full bloom stage reduced fruit drop by increasing the auxin concentration in flower and fruit (Alam and Khan, Citation2002). An amount of 25 mg L−1 NAA sprayed at full bloom stage increased fruit retention in mango (Nkansah et al., Citation2012). Moreover, 45 mg L−1 NAA gave the highest fruit drop reduction in guava compared to 30, 60, 75, and 90 mg L−1, respectively. Agrawal and Dikshit (Citation2008) reported that NAA promoted cell elongation by increasing vacuole enlargement and cell wall plasticity resulting in fruit retention increasing. Moreover, the spraying of auxins prevented the dropping of fruit by maintaining the cells at the zone of abscission, preventing the synthesis of hydrolytic enzymes, such as cellulase, which decomposed the cell walls (Monselise and Goren, Citation1978). The same authors reported also that hormone balance acted on the polygalacturonase activity, which, together with cellulase, was responsible for the degradation of the two important components of cell walls, cellulose and pectin.
An amount of 20 and 40 mg L−1 NAA reduced fruit drop compared with the control in this study, whereas these solutions gave the higher TNC in leaf than control. It could be that NAA may induce carbohydrate accumulation in leaves and more carbohydrate supply for fruit retention than control. This was according to Agusti et al. (Citation2001) who reported that 3,5,6-TPA induced the carbohydrate accumulation with its stimulatory effect on fruit growth which might operate via promotion of sink strength. In addition, there is some evidence which indicate that carbohydrate depletion is responsible for diminishing fruit set and the early fruitlet abscission during the initial phase of cell division correlates negatively with carbohydrate content (Agusti et al., Citation2001; Mehouachi et al., Citation2000, Citation1995) and the higher rates of fruit set from leafy inflorescences can be explained through the role of leaves supporting the young fruitlet by supplying carbohydrates (Mehouachi et al., Citation1995). For citrus, Iglesias et al. (Citation2002) also reported that leaf photosynthesis appears to be crucial in determining fruit set in citrus. Several studies on source–sink imbalances in citrus species support the hypothesis that competition for photoassimilates among developing fruitlets regulates fruit abscission. Increased carbohydrate availability to growing citrus fruitlets was associated with a decreased probability of abscission during fruit set, resulting in a greater number of fruits at the end of the growing period. Iglesias et al. (Citation2006) revealed that the carbohydrate content may be a biochemical signal involved in the mechanism controlling abscission through abscission zone A in citrus.
Moreover, it has also been demonstrated that carbon shortage induces fruit abscission through a characteristic hormonal sequence of which the final product is ethylene (Gomez-Cadenas et al., Citation2000). Gomez-Cadenas et al. (Citation2000) also reported that nutritional factors are limiting factors, whereas hormonal compounds are effectors of the regulation of the abscission process in citrus. Thus, from this study, leaf carbohydrate availability and hormonal compound could be a factor controlling fruit drop in pummelo.
From these results, 20 mg L−1 NAA gave the highest TSS in pummelo cv. Thong Dee compared to those in the other treatment resulting in the highest TSS:TA ratio. This was according to Nkansah et al. (Citation2012) who reported that 25 and 50 mg L−1 NAA increased sugar content in mango fruit, whereas 40 and 20 mg L−1 2,4-D gave the highest peel weight and 40 mg L−1 2,4-D gave the highest peel thickness compared with the other treatment. It could be that 2,4-D, which has high activity of auxin, can promote cell division and cell enlargement more than NAA resulting in more cell division and cell enlargement than those in the other treatment. Moreover, it was possible that the peel of pummelo fruit has been the first part receiving substance or more absorb the substance than those in the other parts. Thus, 40 mg L−1 2,4-D could give higher peel weight and peel thickness than those in the other treatments.
From all of these results taken into consideration, 20 mg L−1 NAA was the most suitable used to reduce fruit drop and to increase percentage of fruit retention in pummelo cv. Thong Dee.
Conclusion
An amount of 20 mg L−1 of NAA sprayed 2 times at full bloom stage and 1–2 months after fruit set effectively reduced fruit drop and increased the percent of fruit retention in pummelo cv. Thong Dee and gave the highest TSS and TSS:TA ratio. However, 40 and 20 mg L−1 2,4-D gave the highest peel weight and 40 mg L−1 2,4-D gave the highest peel thickness compared with the other treatments.
Acknowledgments
The authors would like to thank the Thailand Research Fund (TRF) for the funding to complete this study and also to the individual pummelo growers who have kindly provided material for this research.
Additional information
Funding
References
- Agrawal, S., and S.N. Dikshit. 2008. Studies on the effect of plant growth regulators on growth and yield of sapota (Achras sapota L.) cv. cricket ball. Indian J. Agric. Res. 42(3):207–211.
- Agustí, M., M. Juan, A. Martínez-Fuentes, C. Mesejo, C. Reig, and V. Almela. 2006. Application of 2,4-dichlorophenoxypropionic acid 2-ethylhexyl reduces mature fruit abscission in citrus navel cultivars. J. Hortic. Sci. Biotechnol. 81(3):532–536. doi: 10.1080/14620316.2006.11512099.
- Agusti, M., S. Zaragoza, D. Jose Iglesias, V. Almela, E. Primo-Millo, and M. Talon. 2001. The synthetic auxin 3,5,6-TPA stimulates carbohydrate accumulation and growth in citrus fruit. Plant Growth Regul. 66:1–7.
- Akhlaghi Amiri, N., A. A. Kangarshahi, and K. Arzani. 2012. Reducing of citrus losses by spraying of synthetic auxins. Intl J Agri Crop Sci. Vol.,4(22), 1720–1724.
- Alam, S.M., and M.A. Khan. 2002. Fruit yield of tomato as affected by NAA spray. Asian J. Plant Sci. 1(1):24–28. doi: 10.3923/ajps.2002.24.24.
- Anthony, M.F., and C.W. Coggin. 1999. The efficacy of five forms of 2,4-D in controlling preharvest fruit drop in citrus. Sci. Hortic. 81(3):267–277. doi: 10.1016/S0304-4238(99)00015-1.
- Azher Nawaz. M., W. Ahmad, S. Ahmad, and M. Mumtaz Khan. 2008. Role of growth regulators on preharvest fruit drop, yield and quality in kinnow mandarin. Pak. J. Bot. 40(5):1971–1981.
- Bankar, G.J., and R.N. Prasad. 1990. Effect of gibberellic acid and NAA on fruit set and quality of fruits in ber cv. Gola. Progressive Hortic. 22(1–4):60–62.
- Chen, H.Q., K.L. Dekkers, L. Cao, J. K. Burns, L. W. Timmer, and K.R. Chung. 2006. Evaluation of growth regulator inhibitors for controlling postbloom fruit drop of citrus induced by the fungus Colletotrichum acutatum. Hortscience. 41(5):1317–1321.
- Davies, F.S., and L.G. Albrigo. 1994. Citrus; Crop production science in Horticulture. UK: CAB International.
- Gomez-Cadenas, A., J. Mehouachi, F.R. Tadeo, E. Primo-Millo, and M. Talon. 2000. Hormonal regulation of fruitlet abscission induced by carbohydrate shortage in citrus. Planta 210:636–643. doi: 10.1007/s004250050054.
- Hodge, J.E., and B.T. Hofreiter. 1962. Determination of reducing sugars and carbohydrates, p. 380–394. In: Whistler R.L. and Wolfron M.L.(editors). Method in Carbohydrate Chemistry. Vol. 1, Academic Press, New York.
- Iglesias, D.J., I. Lliso, F.R. Tadeo, and M. Talon. 2002. Regulation of photosynthesis through source: sinkimbalance in citrus is mediated by carbohydrate content in leaves. Physiol Plant 116:563–572. doi: 10.1034/j.1399-3054.2002.1160416.x.
- Iglesias, D.J., F.R. Tadeo, E. Primo-Millo, and M. Talon. 2006. Carbohydrate and ethylene levels related to fruitlet drop through abscission zone A in citrus. Trees 20:348–355. doi: 10.1007/s00468-005-0047-x.
- Koukourileou-Petridou, M.A. 2003. The relation between the levels of extractable and diffusible IAA in almond fruits and their ‘June drop’. Plant Growth Regul. 39:107–112. doi: 10.1023/A:1022567714593.
- Lahey, K.A., R. Yuan, J. K. Burns, P. P. Ueng, L. W. Timmer, and K.R. Chung. 2004. Induction of phytohormones and differential gene expression in citrus flowers infected by the fungus Colletotrichum acutatum. MPMI. 17(12):1394–1401.
- Mehouachi, J., D.J. Iglesias, F.R. Tadeo, M. Agusti, E. Primo-Millo, and M. Talon. 2000. The role of leaves in citrus fruitlet abscission: effectson endogenous gibberellin levels and carbohydrate content. J. Hortic. Sci. Biotechnol. 75:79–85. doi: 10.1080/14620316.2000.11511204.
- Mehouachi, J., D. Serna, S. Zaragoza, M. Agusti, M. Talon, and E. Primo-Millo. 1995. Defoliation increases fruit abscission and reduces carbohydrate levels in developing fruits and woody tissues of Citrus unshiu. Plant Sci. 107:189–197. doi: 10.1016/0168-9452(95)04111-7.
- Michael, F.A., W. Charles, and C.W. Jr. Coggins. 1999. The efficacy of five forms of 2,4-D in controlling pre-harvest fruit drops in citrus. Hort. Science, 81: 266–277.
- Modise, D.M., A.S. Likuku, M. Thuma, and R. Phuti. 2009. The influence of exogenously applied 2,4- dichlorophenoxyacetic acid on fruit drop and quality of navel oranges (Citrus sinensis L.). Afr. J. Biotechnol. 8(10):2131–2137.
- Monselise, S.P., and R. Goren. 1978. The role of internal factors and exogenous control in flowering, peel growth, and abscission in citrus. HortScience 13:134–139.
- Nartvaranant, P. 2006. Plant growth regulator chemical. Nakhon Pathom Rajabaht University, Nakhon Prathom.
- Nartvaranant, P. 2012. Physiological changes in pre-harvest dropped fruits in the pummelo cultivars ‘Thong Dee’ and ‘Khao Nam Phueng’. Songklanakarin J. Sci. Technol. 34(4):367–374.
- Nkansah, G.O., J. Ofosu-Anim, and A. Mawuli. 2012. Gibberellic acid and naphthalene acetic acid affect fruit retention, yield and quality of Keitt mangoes in the coastal savanna ecological zone of Ghana. Am. J. Plant Physiol. 7(6):243–251. doi: 10.3923/ajpp.2012.243.251.
- Smith, D. 1969. Removing and analyzing total non-structural carbohydrates from plant tissue. Wisconsin Univ., Wisconsin: Leaflet, Vol. 14.
- Stern, R.A., and R. Ben-Arie. 2006. Effect of the synthetic auxin 3,5,6-TPA on pre-harvest drop, fruit quality and maturation of ‘Delicious’ and ‘Jonathan’ apples. Acta Hortic. 774:356–362.
- Ward, D.L. 2004. Factors affecting pre-harvest fruit drop of apple. Ph.D thesis,Virginia Polytechnic Institute and State University. p. 143.
