ABSTRACT
We evaluated the potential for using preplant trunk injections of emamectin benzoate in nonbearing apple trees. Trees were evaluated for pest injury and emamectin residues throughout the planting season and into the following year. Injections into the trunk best delivered emamectin benzoate to the canopy compared with injections into the taproot, and the higher rate reduced insect pests more than the lower rate. In the following year, differences in insect control between trunk and root injections were less pronounced, but the higher rate of emamectin benzoate persisted longer and better reduced pests relative to the other treatments.
Introduction
Protecting nonbearing apple trees from insect damage is a high priority and growers cannot generally justify the same high-input pest management for nonbearing trees as is used for bearing trees. Trees in commercial apple production pass through multiple management stages, starting with a multiyear nonbearing period after nursery trees are first planted in orchards. Mature apple orchards are managed using pesticides, delivered by airblast sprayers to protect fruit from insect pests. Overspray from airblast sprayers can result in as much as 56% off-target drift and less than 0.1% of the insecticide actually reaching the target pest (Pimentel, Citation1995; Steiner, Citation1969). Airblast sprayers are also less efficient at covering young apple trees from nurseries compared with mature fruit-bearing trees because of greater open space between tree canopies (Zhu et al., Citation2006). Since young apple trees do not bear fruit, management programs for nonbearing trees focus on indirect pests.
Systemic insecticides such as imidacloprid and abamectin have been utilized for pest management on several species of nursery trees. Basal drenches of imidacloprid controlled Agrilus planipennis on young white and green ash trees (Fraxinus americana and Fraxinus pennsylvanica) (Smitley et al., Citation2010). Soil drenches of neonicotinoids controlled Scirtothrips perseae in nursery avocado trees (Persea americana) (Byrne et al., Citation2007). Trunk injections of imidacloprid and abamectin have been shown to successfully control Corythucha cydoniae on recently established green hawthorn (Crataegus viridis) nursery trees (Gill et al., Citation1999). Several indirect pests of apples could potentially be managed by trunk injections, including potato leafhopper (Empoasca fabae), spotted tentiform leafminer (Phyllonorycter blancardella), and obliquebanded leafroller (Choristoneura rosaceana). However, no studies to date have investigated trunk injection in apple nursery trees.
Trunk injection is a method of applying chemicals directly into the vascular system of a tree, first by piercing the bark to access the xylem tissue. The chemicals are then injected and distributed systemically via the xylem sap. Trunk injection is a discriminating delivery system that targets pests while reducing pesticide inputs and environmental exposure (Vandervoort C Vandervoort and VanWoerkom, Citation2014). It has recently been demonstrated for control of foliar pests and diseases in fruit-bearing apple orchards (Aćimović et al., Citation2016; VanWoerkom et al., Citation2014). Trunk injection may be an ideal management technique for nursery trees. This represents a novel area of research with potential benefits to nursery production and the apple industry.
The objective of this study was to evaluate the feasibility and efficacy of nursery tree injection, using a formulation of emamectin benzoate designed for injection (TREE-age®, Arborjet, Woburn MA) into apple (Malus domestica). We postulated that due to small canopy size, injecting formulated emamectin benzoate at a lower concentration will be as effective as a high concentration. We also postulated that injections into the root of nursery trees would perform as well as injections into the trunk. Efficacy of injection treatments was quantified with bioassays of laboratory-reared C. rosaceana and field evaluations of E. fabae and P. blancardella.
Materials and methods
Study design and setup
Nursery apple trees (“Gala” on M.9 rootstock; Adams County Nursery, Inc., Aspers, PA) approximately 1.5 m tall, with 5 cm diameter rootstock and 2.5 cm diameter scion, were acquired at the beginning of each season and stored in a cold room at 40°C until use. Injections were performed in the laboratory prior to planting. Two holes were drilled, spaced 180° apart on either side of the tree, 4.7625 mm (0.1875 in) in diameter and 6.35 mm (0.25 in) deep. The two holes were drilled below the graft union, either into the taproot below the first lateral roots or into the trunk just below and at right angles to the graft union. Injections were performed with a micropipette (Pipetman® Classic, Gilson Inc., Middleton WI). Holes were capped with nylon plugs (Widgetco, Houston TX) and sealed with grafting tape. Trees were then watered and returned to the cold room. On the same day or the day thereafter, trees were planted at the Trevor Nichols Research Center, Michigan State University, in Fennville, MI (42.595609, −86.160192). They were spaced 1.8 m apart, with rows spaced 4.9 m apart, in cultivated Blount silt loam soil (Soil Survey Staff, Citation2016). Treatments were assigned in a randomized complete block design.
Nursery trees were injected on 4 June 2014. There were five treatments, consisting of (1) an untreated check (UTC), (2) emamectin benzoate into the trunk (TREE-age, Arborjet) at 1/8 the typical field rate or 0.2325 ml/tree (0.01 g a.i.), (3) the 1/8 rate into the root zone, (4) emamectin benzoate into the trunk at a 1/80 rate or 0.0233 ml/tree (0.001 g a.i.), and (5) the 1/80 rate into the root zone. Insecticides were diluted with deionized water which helped increase uptake into the tree. Treatments 2 and 3 were injected at a 1:1 dilution, and treatments 4 and 5 were injected at a 1:10 dilution. There were five replications of each treatment. Three additional replications, of treatments 4 and 5 only, were included for the purpose of collecting root, trunk, stem, and leaf residue samples from entire trees. These samples were taken during the second half of the growing season.
On 2 June 2015, nursery trees were injected with one of three treatments: (1) an UTC, (2) emamectin benzoate (TREE-age, Arborjet) at 1/8 field rate (0.2325 ml/tree, 0.01 g a.i.) in the trunk, and (3) the same dose in the root zone. Treatments with the 1/80 rate of emamectin benzoate were not included in the 2015 study. Eight replications of each treatment were included and planted in a randomized complete block design. Three of these replications were designated for whole tree residue samples of roots, trunks, stems, and leaves. The first five replications were maintained through the following year.
Insecticide residues
Leaves (20 per sample or approximately 10 g) were collected each year from replications 1–3, at 4 and 8 weeks after injection and planting. The three replications designated for whole tree sampling were dug out and sampled at approximately 8 weeks after planting each year. Roots, trunk, shoot stems, and leaves were cut from the nursery trees using a pair of garden pruners. The trunk and roots were cut into 2.54 cm sections to fit in the jars. Samples were then weighed, stored with dichloromethane in 120 ml graduated round glass bottles (Quorpak®, Berlin Packaging, Chicago IL) and held in a cold room at 40°C until sample cleanup using QuEChERS and analysis with HPLC (Anastassiades et al., Citation2003). Leaf residues were analyzed in SAS 9.4 using repeated measures mixed models (SAS Institute, Cary, NC). Residues of tree parts were analyzed using a two-way glimmix model in SAS with Tukey’s multiple comparisons for mean separations.
Bioassays
Leaves for bioassays were sampled from nursery trees injected in 2014 at 63 and 358 days after treatment (DAT), and from 2015 trees at 36 and 71 DAT. Eight leaves were collected from replicates 1–5 of each treatment and cut into disks with a cork borer, rinsed with acetone between treatments. In some instances, leaves were too small for the standard leaf disk size (15 mm) so that multiple leaves were aggregated to approximate the leaf disk area. Disks were placed into 5.5 cm polystyrene Petri dishes lined with moist filter paper. Five laboratory-reared C. rosaceana larvae (neonate, first or second instar) were placed in each Petri dish. Bioassays were kept in an incubation chamber with 16:8 day:night light cycle. After 7 days, dishes were opened and evaluated for mortality. Data analyses comparing mean numbers of dead larvae were performed in SAS using proc genmod to specify a log-linked Poisson distribution model, with total numbers of larvae as an offset. Mean separations were performed with paired t-tests at an alpha of 0.05.
Remaining leaf disk parts were removed from the completed bioassays and cleaned with a moist paper towel. They were then taped to sheets of printer paper, imaged with a flatbed scanner, and saved as JPEG files at 600 dpi. Total area and defoliation of leaf disks was quantified by number of pixels using ImageJ software (Rasband, Citationn.d.). Analyses of leaf disk area were performed with one-way ANOVA using proc glm in SAS and Fisher’s LSD with an alpha of 0.05 was used to construct mean separations.
Field evaluations
Numbers of E. fabae nymphs infesting the apple trees were counted by checking the underside of leaves for 30 s per tree. Generally, fully expanded leaves were targeted in the timed samples. However, all leaves were often checked because there were not many leaves on each tree at the time of sampling. In 2014, nursery trees were evaluated at 36 DAT, and at 31 DAT for nursery trees injected in 2015. Data analyses comparing mean numbers of nymphs per tree were performed in SAS using proc genmod, specifying a log-linked Poisson distribution model for count data. Mean separations of the treatments were performed with individual t-tests at an alpha of 0.05.
Damage due to E. fabae feeding was evaluated by counting numbers of chlorotic and curled leaves as well as total numbers of leaves per shoot, for 20 shoots per tree. Damage was recorded for 2014 injections at 432 DAT. Nursery trees injected in 2015 were also evaluated, at 69 DAT. Data were analyzed to compare numbers of damaged leaves between treatments using proc genmod in SAS to specify a log-linked Poisson distribution model, with total numbers of leaves per shoot as an offset. Mean separations were performed with paired t-tests at an alpha of 0.05.
Infestations of nursery trees by P. blancardella were evaluated for 2014 injections 434 DAT, and for 2015 injections at 71 DAT, by counting the number of leaf mines in leaves as well as total numbers of leaves on shoots and spurs. For 2015 injections at 505 DAT, a timed count of 30 s was performed and numbers of leaves with mines were recorded for that time window.
Results
Insecticide residues
In 2014, leaf residue concentrations between treatments were affected more by injection site than injection rate overall. Leaves of trunk-injected trees had higher concentrations of emamectin benzoate than root-injected trees for both insecticide rates (F-value = 5.51, df = 1, p-value = 0.0469) (). Residues of emamectin benzoate for root injections at both 1/8 and 1/80 the field rate were low, and insecticide rate was not statistically significant (F-value = 1.95, df = 1, p-value = 0.2002). There were concentration differences between whole tree parts for trees injected at the 1/80 rate, with stems containing the highest overall concentration (F-value = 12.75, df = 3, p-value = 0.0003) (). No significant differences between trunk and root injections were found in emamectin benzoate concentration at the 1/80 rate in 2014.
Figure 1. Mean concentrations of emamectin benzoate recovered from nursery tree leaf samples. (A) Asterisks indicate significant treatment level of trunk versus root (alpha = 0.05) in 2014, at 34 and 63 DAT. (B) Treatment means at a given sample date with the same letter are not significantly different (alpha = 0.05) in 2015, at 43 and 71 DAT. Error bars are ±SEM.
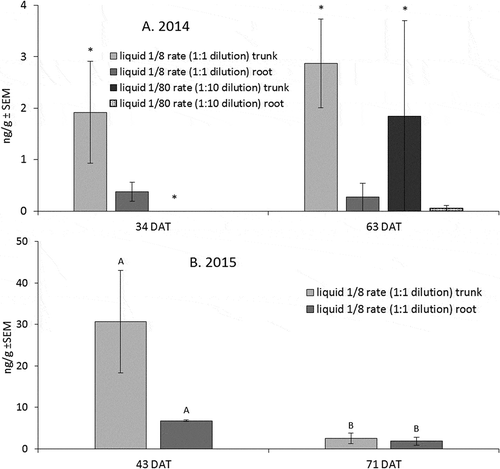
Figure 2. Mean concentrations of emamectin benzoate recovered from nursery trees in (A) 2014 treated at the 1/80 rate and in (B) 2015 at the 1/8 rate. Samples were taken from roots, trunk, stems, and leaves at 58 DAT in 2014 and 72 DAT in 2015. Error bars are ±SEM. Means followed by the same letter are not significantly different based on alpha = 0.05.
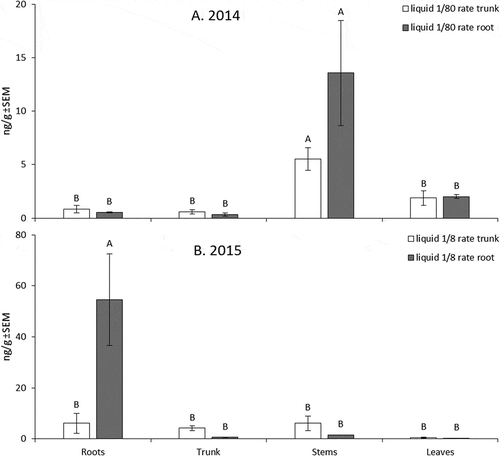
In 2015, the effect of injection site was less pronounced than in 2014. No differences were found between the 1/8 rate injected into the trunk versus into the root (F-value = 3.24, df = 1, p-value = 0.1462), though there were higher emamectin benzoate concentrations in the leaves at 43 DAT compared to root injections (). Instead, sample date had a significant effect on leaf residues for both trunk and root injections (F-value = 8.97, df = 1, p-value = 0.0401). Emamectin benzoate residues injected at the 1/8 rate had a different distribution pattern within nursery trees compared to the 1/80 rate in 2014. Trees which received root-injected 1/8 rate of emamectin benzoate had the highest concentrations of active ingredient, found in the root tissue, versus trunk injection (F-value = 7.63, df = 3, p-value = 0.0029) (). Trunk and stem tissues had higher concentrations of emamectin benzoate in trees receiving trunk injections compared with root injections, though this was not a statistically significant effect.
Bioassays
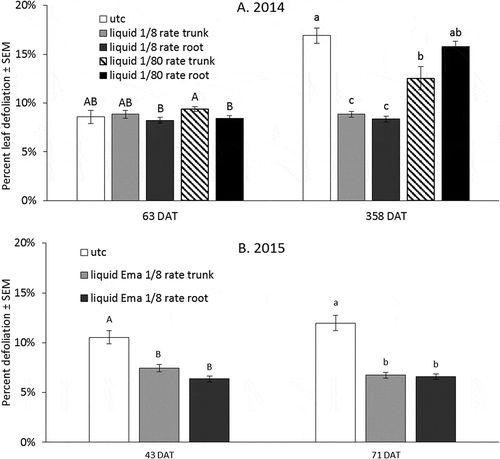
In 2014, larval mortality in the 63 DAT C. rosaceana bioassay did not differ between insecticide treatments and the UTC, and mortality was high across all treatments. There were no differences in larval feeding between injection treatments and the UTC at 63 DAT, although the model was significant overall (F-value = 2.38, df = 8, p-value = 0.0183) (). Larval feeding was slightly lower on leaves which received root injections at both rates, compared to trees with 1/80 rate trunk-injections (F-value = 2.22, df = 4, p-value = 0.0684). However, no treatments were different from the UTC.
Figure 3. Percent defoliation of leaf discs in C. rosaceana bioassays performed in (A) 2014 at 63 and 358 DAT and in (B) 2015 at 43 and 71 DAT. Percent defoliation was calculated to show relative degree of feeding by using unfed leaf discs within each sample date as reference. Error bars are ±SEM. Treatment means within a given date with the same letter are not significantly different based on alpha = 0.05.
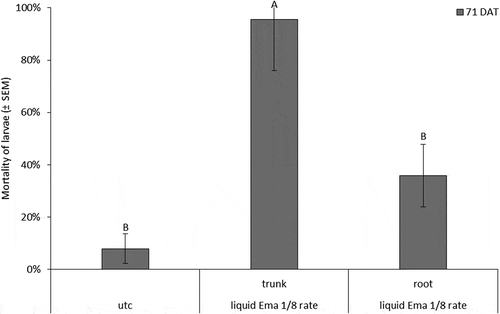
For the 2014 injection bioassay conducted at 358 DAT, mortality was relatively high in the two 1/8 rate emamectin benzoate injections (). Zero mortality across all replicates in the 1/80 rate treatments and the UTC precluded statistical analysis as there was no within-treatment variance from which to draw comparisons. Larval feeding was also lowest for the two 1/8 rate treatments compared to other treatments (F-value = 8.53, df = 4, p-value < 0.0001) (). Larval feeding on leaves from 1/80 rate trunk injected trees was also lower than the UTC.
Figure 4. Mortality of C. rosaceana larvae in bioassays conducted in 2014 at 358 DAT. Error bars are ±SEM. Statistical analyses and mean separations were not calculated due to zero variance within treatments.
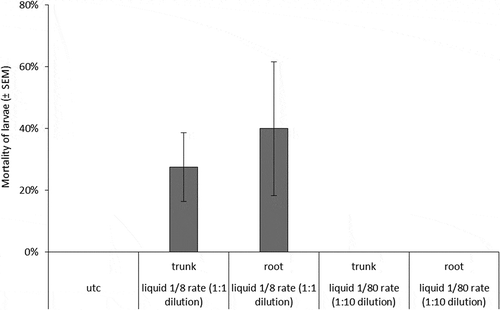
The 2015 C. rosaceana bioassay conducted at 43 DAT experienced high larval mortality in the UTC, and there were no differences between treatments. However, larval feeding was greater on the UTC leaves compared to trunk and root-injected leaves (F-value = 24.41, df = 2, p-value < 0.0001) (). At 71 DAT, larval mortality on leaves from 1/8 rate trunk-injected nursery trees was higher than on leaves from the UTC or root-injected trees (UTC: χ2 = 4.36, p-value = 0.0314; trunk-injected: χ2 = 3.70, p-value = 0.0544; root-injected: χ2 = 6.30, p-value = 0.0121) (). Similarly, larval feeding was greatest on leaves from the UTC compared with trunk and root-injected emamectin benzoate treatments (F-value = 43.95, df = 2, p-value < 0.0001) ().
Field evaluations
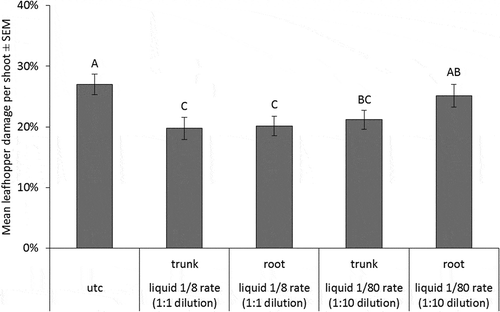
Counts of E. fabae nymphs at 36 DAT in 2014 injections were lowest on UTC trees, while 1/8 rate root-injected trees had relatively higher numbers of nymphs (p-value < 0.0001) (). The other three injection treatments were not different from either the root-injected 1/8 rate or the UTC. At 394 DAT, there were not enough leafhopper nymphs present on the nursery trees to conduct an analysis. Leaf damage due to leafhopper feeding was evaluated for the 2014 injections at 432 DAT (). The two 1/8 rate treatments and the 1/80 rate trunk-injected treatment had less leafhopper damage than the UTC (p-value < 0.0001). Counts of E. fabae nymphs at 31 DAT in the 2015 injections were not different between the trunk and root injections or the UTC. At 69 DAT, there were not enough leaves on the nursery trees to conduct a leafhopper damage analysis.
Figure 6. Mean numbers of E. fabae nymphs counted in 2014 at 36 DAT. Error bars are ±SEM. Means followed by the same letter within each sample date are not significantly different based on alpha = 0.05. Data are back-transformed from Poisson log-linked distribution used for analysis.
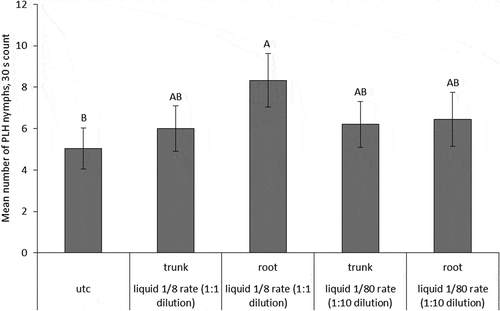
Figure 7. Mean percent of leaves damaged E. fabae nymphs on nursery trees planted in 2014, counted at 432 DAT. Error bars are ±SEM. Means followed by the same letter within each sample date are not significantly different based on alpha = 0.05. Data are back-transformed from Poisson log-linked distribution used for analysis.
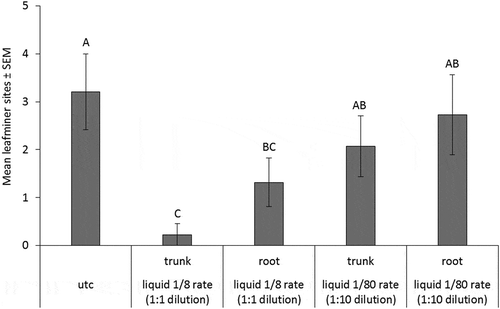
At 434 DAT, counts of P. blancardella leaf mines on trees injected in 2014 were lowest for trees which received trunk-injected 1/8 rate emamectin benzoate, followed by root-injected 1/8 rate emamectin benzoate (p-value < 0.0001) (). Numbers of leaf mines on the two 1/80 rate injection treatments were not different from the UTC. In 2015, leaf mines at 71 DAT were too few to conduct a complete analysis. In the year following the 2015 injections, at 505 DAT, there were no differences in numbers of P. blancardella leaf mines between treatments or the UTC.
Figure 8. Mean number of leaves with P. blancardella mines on nursery trees planted in 2014, counted at 434 DAT. Error bars are ±SEM. Means followed by the same letter within each sample date are not significantly different based on alpha = 0.05. Data are back-transformed from Poisson log-linked distribution used for analysis.
Discussion
This study demonstrated that preplant injections of emamectin benzoate into nursery trees move into the canopy and can reduce injury from foliar feeding insect pests. Injections into the trunks of nursery trees were generally the most effective, with higher doses of emamectin benzoate into the trunk providing the most rapid delivery into the canopy. Over a longer period of time, however, high doses injected into trunk and root injections showed similar results, especially for pest control and product persistence in the following growing season.
While higher dose of injection consistently provided superior pest control and canopy concentration, the location of injection (trunk vs. root) resulted in differing patterns of vascular delivery. The vascular system in the roots differs from the trunk and stems, in that roots primarily serve as a sugar sink (Lemoine et al., Citation2013). While trunk injections resulted in relatively uniform distribution among plant parts (), root injections show higher residue levels in stems (low dose) and roots (high dose). These differences between low- and high-dose injections into roots suggest a limiting effect of the transition zone between the root and trunk vascular systems. At the transition zone between root and trunk, bundles of xylem and phloem are divided, rotated, and fused back together (Basconsuelo et al., Citation2002). Furthermore, the transition zone between root and shoot in apple trees is physiologically distinct from other tree parts and can modulate xylem and phloem transport. Grochowska et al. (Citation1994) demonstrated active participation of the transition zone in the transport of chemicals following hormone treatments.
Using the highest rate injection into the trunk of nursery trees provides the most rapid uptake into the canopy (). This is likely a function of injection into trunk tissue, which allows for greatest exposure to xylem tissues, thus maximizing transpiration-based mobility to the tree canopy. The fact that the higher rate results in higher short-term concentrations in foliage than the low dose suggests that injections into the trunk do not have the same capacity-limiting factor as was seen in the roots. Similarly, Acimovic et al. showed higher doses in a single injection port resulted in significantly higher canopy-quadrant concentrations than lower doses injected in multiple ports per tree (Citation2014).
If apple growers consider planting pre-injected nursery trees, they will surely prefer to see multiple years of plant protection, given the investment. In our study, dose was the most important factor for achieving multiyear control. When injecting emamectin benzoate into ash trees, multiple-year activity was attributed to the “reservoir effect” in the trunk (Tanis et al., Citation2012). Whereas transpiration-driven xylem mobility is responsible for the most immediate delivery of product to the tree canopy, portions of emamectin benzoate diffuse into trunk tissues and remain until following growing seasons. This “reservoir effect” depends on the relative affinity of the injected chemical and tree species, but a similar phenomenon has been documented in apples (VanWoerkom et al., Citation2014; Wise et al., Citation2014). Thus, higher doses are likely critical for multiyear plant protection in nursery apple trees.
Considering that insecticide residues were found in woody tissue, trunk injection of nursery trees may be valuable for control of the black stem borer, Xylosandrus germanus (Ranger et al., Citation2016). Young fruit trees are at the greatest risk of injury, although because the beetles feed on ambrosia fungi rather than plant tissue, systemic insecticides are not recommended for control of X. germanus (Haas et al., Citation2016). However, the systemic fungicide propiconazole has been demonstrated to control disease-causing fungi associated with ambrosia beetles in redbay trees (Persea borbonia) (Mayfield et al., Citation2008). Injections of systemic fungicides into young apple trees may potentially help prevent establishment of X. germanus and control other common fungal pathogens such as apple scab.
In this study, we demonstrated that trunk injections can be successfully performed on nursery trees. This represents a novel approach to pest management in nursery tree plantings. Injections of emamectin benzoate at reduced doses can reduce numbers of insect pests for multiple seasons. In future studies, the long-term survivability of trunk injected nursery trees should be investigated. Injections of systemic fungicides should also be tested to expand the utility of trunk injections in young trees. Comparisons with conventional nursery tree management should be conducted to refine injection methods and adapt current and future technologies for use in nursery systems.
Acknowledgments
We would like to thank Bert Cregg, Larry Gut, and Dave Smitley for their guidance on this research. We would also like to thank the graduate students Knute Gundersen, Daniel Hulbert, and Logan Rowe for reviewing this manuscript and Dana Acimovic, Christie Bahlai, and Abdhi Sarkar for their statistical advice. Apart from them, we would like to give a special thanks to Jennifer Coslor, Ashley Hafer, Christopher Hafer, Laura Lamb, Christopher Meredith, Nicole Raak, and Anthony VanWoerkom for their help with data collection in the field and in the laboratory.
Additional information
Funding
References
- Aćimović, S.G., A.H. VanWoerkom, T. Garavaglia, C. Vandervoort, G.W. Sundin, and J.C. Wise. 2016. Seasonal and cross-seasonal timing of fungicide trunk injections in apple trees to optimize management of apple scab. Plant Dis. 100(8):1606–1616. doi:10.1094/PDIS-09-15-1061-RE.
- Aćimović, S.G., A.H. VanWoerkom, P.D. Reeb, C. Vandervoort, T. Garavaglia, B.M. Cregg, and J.C. Wise. 2014. Spatial and temporal distribution of trunk-injected imidacloprid in apple tree canopies. Pest Manag. Sci. 70(11):1751–1760. doi:10.1002/ps.3747.
- Anastassiades, M., S.J. Lehotay, D. Stajnbaher, and F.J. Schenck. 2003. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and ‘dispersive solid-phase extraction’ for the determination of pesticide residues in produce. J AOAC Int. 86(2):412–431. http://www.ncbi.nlm.nih.gov/pubmed/12723926.
- Basconsuelo, S., F. Weberling, and T. Kraus. 2002. Transition zone between root and stem vascular system in seedlings of members of the tribe Phaseoleae (Fabaceae). Feddes Repertorium. 113(3–4):224–230. AID-FEDR224>3.0.CO;2-V doi:10.1002/1522-239X(200208)113:3/4<224.
- Byrne, F.J., N.C. Toscano, A.A. Urena, and J.G. Morse. 2007. Toxicity of systemic neonicotinoid insecticides to avocado thrips in nursery avocado trees. Pest Manag. Sci. 63(9):860–866. doi:10.1002/ps.1413.
- Gill, S., D.K. Jefferson, R.M. Reeser, and M.J. Raupp. 1999. Use of soil and trunk injection of systemic insecticides to control lace bug on hawthorn. J. Arboriculture. 25(1):38–42.
- Grochowska, M.J., U. Dziæciol, and A. Miszczak. 1994. Active participation of the root/shoot transition region treated with triiodobenzoic acid in basipetal transport of 14C-Indole-3-acetic acid and ethylene evolution in the stem of apple seedlings. Sci. Hortic. 59(3–4):207–215. doi:10.1016/0304-4238(94)90014-0.
- Haas, M., J. Wilson, and L. Gut. 2016. managing black stem borer in michigan tree fruits. Lansing, MI. http://msue.anr.msu.edu/uploads/files/AABI/Michigan_BSB_Management_Guide_4-12-16.pdf
- Lemoine, Remi, Sylvain La Camera, Rossitza Atanassova, Fabienne Dédaldéchamp, Thierry Allario, Nathalie Pourtau, Jean-Louis Bonnemain, Maryse Laloi, Pierre Coutos-Thévenot, et al. 2013. Source-to-sink transport of sugar and regulation by environmental factors. Front Plant Sci. 4(July):1–21. doi:10.3389/fpls.2013.00272.
- Mayfield, A.E., E.L. Bamard, J.A. Smith, S.C. Bernick, J.M. Eickwort, and T.J. Dreaden. 2008. Effect of propiconazole on laurel wilt disease development in redbay trees and on the pathogen in vitro. Arboriculture and Urban Forestry. 34(5):317–324.
- Pimentel, D. 1995. Amounts of pesticides reaching target pests: environmental impacts and ethics. J. Agric. Environ. Ethics. 8(1):17–29. doi:10.1007/BF02286399.
- Ranger, Christopher M, Michael E Reding, Peter B Schultz, Jason B Oliver, Steve D Frank, Karla M Addesso, Juang Hong Chong, Blair Sampson, Christopher Werle, et al. 2016. Biology, ecology, and management of nonnative ambrosia beetles (Coleoptera: Curculionidae: Scolytinae) in ornamental plant nurseries. J. Integr. Pest Manag. 7(1):9. doi:10.1093/jipm/pmw005.
- Rasband, W.S. n.d. “ImageJ - NIH.” Accessed April 17, 2017. http://imagej.nih.gov/ij/.
- Smitley, D.R., E.J. Rebek, R.N. Royalty, T.W. Davis, and K.F. Newhouse. 2010. Protection of individual ash trees from emerald ash borer (Coleoptera: Buprestidae) with basal soil applications of imidacloprid. J. Econ. Entomol. 103(1):119–126. doi:10.1603/EC09137.
- Staff, S.S. 2016. “Web Soil Survey.”Natural Resources Conservation Service, United States Department of Agriculture. 2016. https://websoilsurvey.sc.egov.usda.gov/.
- Steiner, P.W. 1969. “The distribution of spray material between target and non-target areas of a mature apple orchard by airblast equipment.” Cornell University.
- Tanis, S.R., B.M. Cregg, D. Mota-Sanchez, D.G. McCullough, and T.M. Poland. 2012. Spatial and temporal distribution of trunk-injected 14C-Imidacloprid in Fraxinus trees. Pest Manag. Sci. 68(4):529–536. doi:10.1002/ps.2281.
- Vandervoort, C., and A.H. VanWoerkom. 2014. Trunk injection: a discriminating delivering system for horticulture crop IPM. Entomology, Ornithology & Herpetology: Current Research 03(02):3–9. doi:10.4172/2161-0983.1000126.
- VanWoerkom, A.H., S.G. Aćimović, G.W. Sundin, B.M. Cregg, D. Mota-Sanchez, C. Vandervoort, and J.C. Wise. 2014. Trunk injection: an alternative technique for pesticide delivery in apples. Crop Prot. 65:173–185. doi: 10.1016/j.cropro.2014.05.017.
- Wise, J.C., A.H. VanWoerkom, S.G. Aćimović, G.W. Sundin, B.M. Cregg, and C. Vandervoort. 2014. Trunk injection: a discriminating delivering system for horticulture crop IPM. Entomology, Ornithology & Herpetology: Current Research 03(02):3–9. doi:10.4172/2161-0983.1000126.
- Zhu, H., R.C. Derksen, H. Guler, C.R. Krause, and H.E. Ozkan. 2006. Foliar deposition and off-target loss with different spray techniques in nursery applications. Trans. ASABE 49(2):325–334. doi:10.13031/2013.20400.