ABSTRACT
Limitations against propagation of Cola nitida include pronounced dormancy of between 1 and 8 months after sowing (MAS) leading to uneven germination and seedling growth. This experiment was conducted in a screenhouse, Federal University of Agriculture Abeokuta, Nigeria, to evaluate the effects of mini-nut and nut-color on germination and seedling growth of C. nitida nuts. The experiment was in a completely randomized design replicated 3 times. Treatments comprised two factors: nut/seed-colored biotype (white, pink, and red) and mini-nut (embryonic portion of cotyledon) [excised embryonic portion cotyledon (EEPC), 25% embryonic portion cotyledon (EPC), 50% EPC, 75% EPC, and 100% EPC (whole nut/seed)], giving 15 treatment combinations sown in plastic cups of 50 cl filled with sawdust. Germination percentage and morphological growth of plantlets (plant height (cm), number of leaves, dry matter yield (g/plant)) were monitored; data subjected to analysis of variance and mean comparisons done (p ≤ 0.05). As early as 4 WAS, the EEPC and 25% EPC had 67.78% and 54.44% germination, respectively. Within the same period, 51.33%, 48.00%, and 31.33%, respectively, of white-, pink-, and red-colored seeds germinated. At 6 WAS, the white-, pink-, and red-colored seeds had mean germination of 80.67%, 92.00%, and 72.67%, respectively. At 12 WAS, germination was observed to complete with the pink-, red-, and white-colored nuts having 97.33%, 92.61%, and 91.33%, respectively. Within the same period, the mini-nut sizes had between 92.22% and 95.56% germination. Combining the two factors, 50% EPC, 25% EPC, and EEPC of white-nut biotype germinated earliest with 50.00–76.67% at 4 WAS and 73.33–80.00% at 5 WAS. The EEPC of pink-colored biotype attained 100% at 6 WAS, while other treatments were between 56.67% and 93.33%. Thus, while earliness in germination pattern follows the order: EEPC > 25% EPC > 50% EPC > 75% EPC > whole nut, the plantlet morphological growth followed a reverse order. With mini-nut technique therefore, timely and even germination of kola nuts were obtained and 50%EPC would be recommended for conventional propagation while EEPC would require nutrient amendment for improved growth.
Introduction
Kola (Cola species) is one of the tropical commodity tree crops in the family of Sterculiaceae (Oladokun, Citation1985). Cola nitida, Schott, (Vent. & Endl.) and Cola acuminata, Beauv. & Endl. are the species of greatest economic importance (Lovejoy, Citation1980). Nut of C. nitida is not just a stimulant; it also has some nutritional values as reported by Abolude (Citation2004) that proximate composition of C. nitida nut is 2.3% moisture, 10.5% crude protein, 3.1% ash, 13.3% crude fat, 17.5% crude fiber, 5.3% total carbohydrate, abundance of Na, Mg, K, and P, low level of trace elements, 2.8% caffeine, and 0.05% theobromine. Therefore, in some industrial companies, kola nut serves as component of soft drinks and many pharmaceutical preparations (Ogutuga, Citation1975). According to FAOSTAT (Citation2015), Nigeria is the largest producer of kola nuts in the world with 132,000 metric tons (44.9% of total world production) followed by Cote D’Ivore, Cameroon, Ghana, and Sierra Leone with 82,000, 46,500, 24,000, and 8645 metric tons, respectively.
Botanically, kola fruit is a follicle of five to six pods, and each pod containing 6–10 kola nuts (Van Eijnatten, Citation1966). The nut possesses a bud at an extreme end with varying sizes of cotyledon which is fresh to touch. Propagation of plant involves the use of the nut (Oladokun, Citation1988; Olaiya and Hammed, Citation2003; Opeke, Citation2005) which occurs in three distinct colored biotypes: white, pink, and red. The predominant is red-biotype, while white-biotype is rarest (Ogutuga, Citation1975; Oladokun, Citation1988). Unfortunately, bigger kola nuts that are ≥15 g, which Oladokun (Citation1988) recommended as generative propagules, command highest market premium, especially if the nuts are white-colored followed by pink- and red-colored nuts in that order. Agronomically, the red kola nuts had been reported to have least germination rate, while the white nuts have the highest rate (Oladokun, Citation1988). Kola nut exhibits long dormancy period of between 1 and 8 months (Oladokun, Citation1988). This suspension of germination has been attributed to its high contents of caffeine and polyphenols. Polyphenols had been reported to make amino acids unavailable by binding strongly to them (Elias and Bressani, Citation1979), while caffeine is believed to delay kola nut germination until its hydrolysis begins (Hammed et al., Citation2013; Oladokun, Citation2000). Mobilization of nitrogen reserves as a source of energy, and nutrient supply to the expanding new tissues is therefore proposed to take part in the control of germination (Garciarrubio et al., Citation1997). This is because germination of seeds accompanies metabolism of food reserves in the cotyledons (Onomo et al., Citation2010). Cotyledons of C. nitida seeds, besides being a reservoir of nutrient reserves (Hammed et al., Citation2013; Oladokun, Citation1989), contain high amount of polyphenols and caffeine, and these chemicals are more in red-colored nuts (Ogutuga, Citation1975).
An expansion of kola hectarage may have resultantly effect shortfalls in the market supply of C. nitida nuts for the year. In addition, the kola farmer will have to sacrifice his/her income for such an expansion in the meantime. This situation calls for an intervention technology which ensures security of farmers’ incomes from kola farms without jeopardizing the hectarage expansion, such that a kola farmer who intends to expand his/her kola farms will not necessarily have to sacrifice all his/her incomes from kola farms for the season. Mini-nut technique involves the use of embryo portion of the nut’s cotyledons as generative propagule, while the remaining non-embryonic portion of cotyledons is available for commercial uses.
More so that, during transplanting operations of kola seedlings into the field, the attached cotyledons that constitute primary nutrient sources for the seedlings (Hammed and Adeyemi, Citation2005) are not exhausted of nutrient reserves and they become inevitably detached and thus become preyed upon by the rodents while the seedlings continue its growth on the field. This observation clearly indicates that upon the development of rooting system by the seedlings, the unexploited nutrient reserves in the cotyledons become food sources for rodents. Mini-nut technique, besides reducing the loss in food reserves in the attached cotyledons of kola seedlings, provides improved surface area for imbibition of water by the nuts/seeds for assumption of germination. The preliminary observations of this technology had been reported (Hammed et al., Citation2013), but the report was only limited to the pre-nursery and did not cover establishment of the seedlings in the nursery preparatory to transplanting into the field. This experiment was thus set up to determine germination and growth of kola seedlings as affected by mini-nut technique in the nursery.
Materials and methods
The experiment was conducted at the Federal University of Agriculture, Abeokuta, Ogun State, Nigeria (7°15′N, 3°25′E). Cured kola nuts (seeds) were sourced from farmers’ farms, Ogunmakin village, Oluyole local government area, Ibadan, Oyo State, Nigeria. Seeds of 15 g and above were selected for the experiment, while small seeds or bored seeds or seeds with visible insect and/or pathogenic attacks were discarded. It is a two-factored experiment: factor-1 is seed-colored biotype (white, pink, and red biotypes), while factor-2 is mini-nut treatments, i.e. excised embryo portion of cotyledon (EPC), 25%, 50%, 75%, and 100% EPC, giving 15 treatment combinations. These are defined in . The experiment was in completely randomized design with three replicates. The mini-nut treatments were prepared with a clean kitchen knife. Sowing was done, immediately after the mini-nuts were prepared, in plastic containers of 50 cl, perforated at the base and filled with sawdust that had been piled up for 2 weeks. The containers were arranged inside a screenhouse (pre-nursery stage). A number of 10 nuts were sown per treatment per replicate, giving a total of 450 nuts in the trial. They were observed daily for emergence of plumule and minimum of two leaves as an index of germination (ISTA, Citation2004) until no further germination observed. When germination percentage was above 70%, observations of growth of the plantlets began at the pre-nursery stage. Dry matter accumulation was determined at 16 WAS. Data were subjected to analysis of variance procedures using CoStat 6.400 software (CoStat, Citation2008), and mean performance was compared using least significant difference and Duncan multiple range test (p ≤ 0.05) where necessary.
Table 1. Treatment definition.
Results
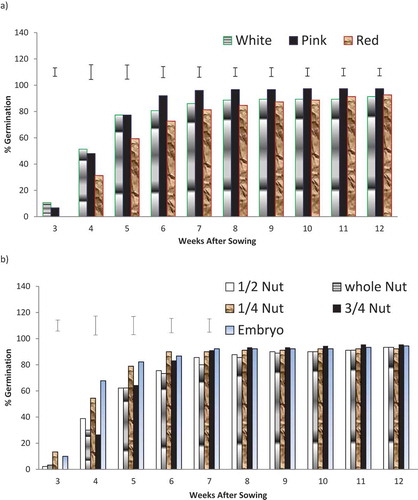
White-colored nuts germinated at early (3 WAS) with 10.67% germination followed by pink-colored nuts (6.67%), while red-colored nuts germinated at 4 WAS (31.33%). Each of the three nuts-colored biotypes had over 50% germination at 5 WAS and by 6 WAS, 80.67%, 92.00%, and 72.67%, of white-, pink-, and red-colored nut biotypes, respectively, germinated ()). With respect to the effects of the mini-nut treatment on germination, the EEPC and 25% EPC germinated early, giving 67.78% and 54.44% germination at 4 WAS. At 6 WAS, 25% EPC had highest germination of 90.00%, while all other treatments were between 73.33% and 86.67%, and the differences were significant (p < 0.05) ()). Excised embryo portion of cotyledon of pink nut biotype (EEPCP), 25% embryo portion of cotyledon of white nut biotype (25% EPCW), and excised embryo portion of cotyledon of white nut germinated early at 3 WAS with 33.3%, 16.7%, and 13.3%, respectively, while none of the red-colored biotype germinated. At 4 WAS, more than 50% germination was recorded in some treatments: 50% EPCW, 25% EPCW, EEPCW, 25 EPCP, EEPCP, and EEPCR (). At 6 WAS, EEPCP had 100% germination, while all other treatments had between 56.7% and 90.0%, and at 12 WAS, all the treatments had between 86.7% and 100% germination (p ≤ 0.05) ().
Table 2. Effects of nut-color × mini-nut treatments on germination percentage of kola (Cola nitida).
Figure 1. Percentage germination of kola nuts (Cola nitida) as affected by (a) nut-color biotype (b)mini-nut treatments, 3–12 WAS.
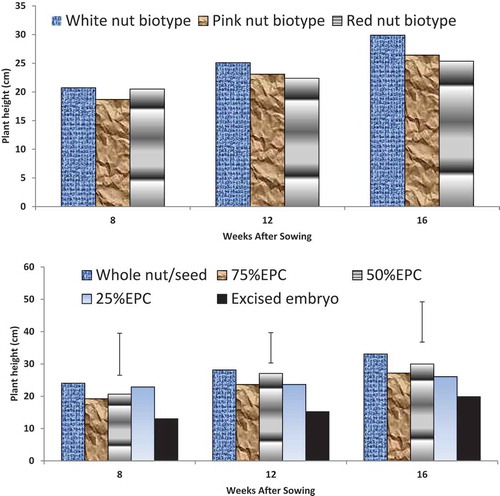
Kola nut color did not influence height of the plantlets (p ≤ 0.05). Heights of the plantlets raised from red, pink, and white nuts were between 18.68 and 20.71 cm at 8 WAS, 22.37 and 25.08 cm at 12 WAS, 25.36 and 29.88 cm at 12 WAS ()). However, mini-nut treatment influenced height of the plantlets. Plantlets raised from excised embryo had reduced height of 13.03, 15.24, and 19.86 cm at 8, 12, and 16 WAS respectively, compared to the height of plantlets raised from whole nut that were 24.04, 28.13, and 33.07 cm at the respective periods ()). Interactions between nut color and mini-nut size showed that the heights of plantlets raised from whole nuts of red-, pink-, and white-colored biotypes were higher than raised from excised embryo at 8 WAS. At 16 WAS, plantlets raised from whole nuts, 75% EPC, 50% PC, and 25% EPC of red, pink, and white nuts were tallest with height between 24.94 and 34.95 cm whereas, plantlets raised from EEPC of the respective nut colors had height of 23.99, 17.42, and 23.18 cm ().
Table 3. Height (cm) of kola plantlets as influenced by nut color and mini-nut technique.
Figure 2. (a) Height (cm) of kola plantlets as affected by bio-type colour of nut sown in the pre-nursery. (b) Height of kola plantlets as affected by mini-nut treatment in the pre-nursery.
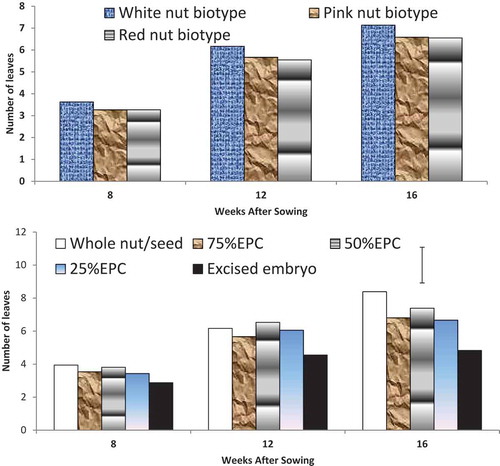
Kola color biotype did not have significant influence (p < 0.05) on leaf production, though plantlets raised from white-colored nuts produced more leaves than other treatments ()). At 8 and 12 WAS, leaf production was not significantly affected by mini-nut treatments, but at 12 WAS, plants raised from whole nut, 75% EPC, 50% EPC, and 25% EPC produced more leaves of between seven and eight, while plantlet raised from excised embryo had reduced leaf production of five ()). At 16 WAS, plantlets raised from whole nuts of the three nut-color as well as 75% EPC and 50% EPC of white nuts produced more leaves (p < 0.05) compared to other treatments ().
Table 4. Number of leaves of kola plantlets as influenced by nut-color and mini-nut technique.
Figure 3. (a) Number of leaves of kola plantlets as influenced by bio-type colour of nut sown in the pre-nursery. (b) Number of leaves of kola plantlets as influenced by mini-nut treatment sown in the pre-nursery.
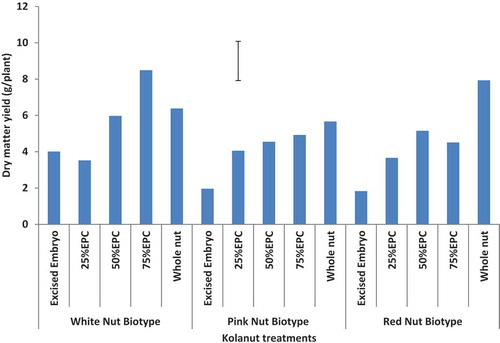
The dry matter yields of plantlets raised from whole nuts, 75% EPC, and 50% EPC of white, pink, and red nut biotypes were highest followed by plantlets raised from 25% EPC and EEPCs of white, pink, and red color nut biotypes. The differences were significant at p < 0.05 ().
Discussion
Non-germination of seeds or nuts is generally an undesirable characteristic in agricultural crops. Rapid and even germination as well as vigorous growth are characteristically required in agriculture, more so, that a rapid germination is a measure of good seed vigour. This was in the findings of Bewley (Citation1997), Hilhorst and Toorop (Citation1997), and Finch-Savage and Leubner-Metzger (Citation2006) that untimely and uneven germination affects rates of germination, especially under suboptimal conditions. In the reports of Oladokun (Citation2000) and Opeke (Citation2005), the major factors against germination of C. nitida nuts are untimely and uneven germination. In this trial therefore, EEPC, 25% EPC, and 50% EPC exhibit good seed vigor for propagation of C. nitida. Furthermore, this report confirms the beliefs of Oladokun (Citation2000) that the untimely and uneven germination of C. nitida is more of non-conducive intrinsic causes rather than seed coat effect. The poor response of the embryo to germinate was probably a result of the high contents of caffeine and polyphenol in its immediate environment which make the required amino acids needed for germination nonavailable (Elias and Bressani, Citation1979; Ogutuga, Citation1975; Oladokun, Citation1988; Onomo et al., Citation2010). Mini-nut as a form of seed treatments exposes the internal tissues of the kola nut cotyledons to rapid water imbibition which in effects aids hydrolysis of food reserves and caffeine and polyphenol contents into simple sugars which instigates rapid and even germination within a period of 3–5 weeks after sowing. This is a major breakthrough in kola research and is of agricultural importance. This was similarly observed in earlier reports by Hammed et al. (Citation2013). Water imbibition not only aids the breakdown of food reserves, Finch-Savage and Leubner-Metzger (Citation2006) observed that, it equally aids the expansion of the cotyledons, thus aiding in germination. The small size of EEPC further aided the breakdown of food reserves in the nuts and thus the embryo became instigated to evenly germinate at a short period of 4 weeks compared to other treatments. However, the pronounced germination advantage of EEPC could not translate into growth simply because of the low food reserves occasioned by reduced cotyledon size. The germination advantage could only be carried into the growth phase only if adequate nutrients are made available for the germinates in the right proportion as reported in the seedlings of oil palm (Lucas, Citation1977) and cashew (Hammed et al., Citation2015). This explains why improved growth was recorded in seedlings raised from whole nuts, 75% EPC, and 50% EPC. It took red-colored biotype longer period to attain reasonably even germination compared to the white- or pink-colored biotypes. According to Ogutuga (Citation1975), red-nut biotype has three times polyphenol contents compared to the contents in pink- and white-nut biotypes of C. nitida. This enables red-nut biotype to exhibit slow and uneven germination. Likewise, in the reports of Prohp et al. (Citation2009), the delayed germination in red-colored biotype of C. nitida is attributable to its higher polyphenol content. Mini-nut treatments of C. nitida possibly have reduced concentrations of germination-inhibiting chemicals (caffeine and polyphenol) in two ways. First, its effect on germination earliness infers that varying amount of polyphenol is removed by the variations in the mini-nut treatments, and thus, the greater the amount removed, the better the germination. Second, the cut surface exposes the polyphenol and other germination-inhibiting phytochemicals to leaching, thus relieving germination. These two observations favor the following order of germination: EEPC > 25% EPC > 50% EPC > 75% EPC > whole nut. It is, therefore, concluded that under similar conditions, white- and pink-colored biotypes of C. nitida nuts treated to 50% EPC, 25% EPC, and EEPC gave reasonably timely and even germination of nuts of C. nitida at 5 weeks of sowing. Therefore, for a nonliterate tropical C. nitida farmer with his/her small holdings, 50% EPC is recommended, while in kola plantations with literate manager, 25% EPC is adoptable for production of seedlings of C. nitida. Producing the seedlings using EEPC would require some nutritional amendments to improve on the growth vigor.
References
- Abolude, F.O. 2004. Composition and properties of Cola nitida and Cola acuminata flour in Nigeria. Global J. Pure Appl. Sci 10(1):11–16.
- Bewley, J.D. 1997. Seed germination and dormancy. Plant Cell. 9:1055–1066.
- CoStat. 2008. CoStat, cohort statistical software. Montery, CA, 93940, USA. http.//www.cohort.com.
- Elias, I.L.G., and R. Bressani. 1979. The nutritional role of polyphenols in beans: Polyphenols in cereals and legumes. Proceedings of a symposium held during the 36th annual meeting of the Institute of Food Technologist, St. Louis, Mo. P. 10–13.
- FAOSTAT. 2015. Kola production statistics. Food and Agriculture Organization of the United Nations. FAOSTAT. www.fao.org.
- Finch-Savage, W.E., and G. Leubner-Metzger. 2006. Seed dormancy and the control of germination. New Physiologist 171:501–523. doi: 10.1111/j.1469-8137.2006.01787.x.
- Garciarrubio, A., J.P. Legaria, and A. Covarrubias. 1997. Abscisic acid inhibits germination of mature Arabidopsis seeds by limiting the availability of energy and nutrients. Planta 203:182–187. doi: 10.1007/s004250050180.
- Hammed, L.A., and E.A. Adeyemi. 2005. Germination and seedling performance of cashew (Anacardium occidentale, L.) as affected by nut-sowing orientations and cotyledon removal. Niger. J. Hortic. Sci. (NJHS) 10:59–64.
- Hammed, L.A., A.B. Olaniyan, A.O. Olaiya, and J.G. Bodunde. 2013. Germination and growth performance of Kola (Cola nitida) seeds in the nursery as influenced by cotyledon reduction. Seed Sci. Technol. 41:292–297. doi: 10.15258/sst.
- Hammed, L.A., A.B. Olaniyan, A.O. Olaiya, B.A. Lawal, and E.O. Lucas. 2015. Growth, dry matter partitioning and nutrient uptake of cashew (Anacardium occidentale L.) seedlings as affected by nut-size under an extended nursery period. J. Org. Agric. Environ. 3:65–83.
- Hilhorst, H.W.M., and P.E. Toorop. 1997. Review on dormancy, germinability, and germination in crop and weed seeds. Adv. Agron. 61:112–165.
- ISTA. 2004. International rules for seed testing. Edition 2004 (Amendments). International Seed Testing Association, Bassersdorf, Switzerland.
- Lovejoy, P.E. 1980. Kola in the history of West Africa. Cahier d’Etudes Africaines 77–78:97–134. doi: 10.3406/cea.1980.2353.
- Lucas, E.O. (1977). Growth analysis of polybag nursery oil palm seedlings. Technical Consultation on Oil Crops for West and Central Africa. A Technical paper OC/77/15, FAO-UN, 13.
- Oguntuga, D.B.A. 1975. Chemical composition of kola nuts. Ghana J. Agric. Sci. 8:121–125.
- Oladokun, M.A.O. (1985). Objectives and achievements in Kola propagation research. Proceedings of the symposium marking the 21st anniversary of CRIN, Ibadan. 2–4 Dec. 1985.
- Oladokun, M.A.O. 1988. Physiological aspects of kola improvement. Càfé Cacao & Thé, (Paris) xxxii(4):311–318.
- Oladokun, M.A.O. 1989. Nut weight and nutrient contents of Cola acuminate and Cola nitida, Sterculiaceae. Econ. Bot. 43(1):17–22. doi: 10.1007/BF02859320.
- Oladokun, M.A.O. 2000. Kola: The tree of life. The inaugural LECTURE, 37. Federal University of Agriculture Abeokuta, Nigeria. 3 May. 2000.
- Olaiya, A.O., and L.A. Hammed. 2003. Vegetative propagation in kola. Niger. J. Sci. 37(1):37–42.
- Onomo, P.E., N. Nicolas, O.N. Denis, and L. Reinhard. 2010. Change in amino acids content during germination and seedling growth of Cola sp. Afr. J. Biotechnol. 9(35):5632–5642.
- Opeke, L.K. 2005. Tropical tree crops, p. 327. Spectrum Books Limited, Ibadan, Nigeria.
- Prohp, T.P., K.E. Ekpo, E.V. Osagie, A. Osagie, and H. Obi. 2009. Polyphenol contents and polyphenol oxidase activities of some Nigerian kolanuts. Pakistan J. Nutr. 8(7):1030–1031. ISSN 1680-5194. Asian Network for Scientific Information. doi:10.3923/pjn.2009.1030.1031.
- Van Eijnatten, C.L.M. 1966. Variation in Cola nitida, size of the nut in relation to length of the embryo and its position. In Memorandum, Cocoa Research Institute of Nigeria, 13:1–8.