 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The present study aims to assess the nutritional quality and the antioxidant capacity of (Punica granatum L. ‘Gabsi’) and date (Phoenix dactylifera L. ‘Bouhattam’) juices and their combination at three proportions (PPD 2:1, PD 1:1, and PDD 1:2). This investigation revealed the interesting potentialities in terms of bioactive molecules, natural antioxidants, and high organoleptic characteristics of various prepared juice formulations. For all juices, the sugar contents were averaged to 16°Brix. The highest monosaccharide content was obtained at the pomegranate/date juice (PDD) (glucose: 9.52 ± 0.88 g/100 ml, fructose: 9.91 ± 0.44 g/100 ml). The comparative study of the five juice formulations based on phenolic contents showed that they are loaded with phenolic compounds. Indeed, the PD juice had the highest polyphenols content (10.77 mg GAE/ml of juice); however, the pomegranate juice had the highest flavonoids content (5.71 mg RE/ml of juice). The assessment of the antioxidant activity was conducted based on three methods: ferric reduction power, DPPH°, and ABTS+ assays. Our results confirmed that all juices were loaded with natural antioxidants. Moreover, the sensorial test of the five juice formulations showed an acceptable quality by the tasting panel. To conclude, this study reveals that the formulation of a cocktail pomegranate/date juice seems to be an interesting initiative for the processing of noncommercial varieties.
Introduction
In Tunisia, the oasis areas, mainly located in the southern, have continued to expand to go from 16,720 ha in 1973 to 41,710 ha in 2010. Gabes oasis, the only littoral of the Mediterranean oasis, covers an area of 6660 ha, or about 22% of total Tunisian oases (Ben Salah, Citation2011). It is known for its extreme wealth of palm (Phoenix dactylifera L.) and pomegranate (Punica granatum L.) varieties. Both species are recognized by their tolerance to drought and severe weather conditions. P granatum L. (Punicaceae) and P dactylifera L. (Arecaceae) fruits are widely consumed fresh and in beverage forms as juice. Both juices have excellent nutritional and healthy characteristics. The medicinal properties of pomegranate are extensively described by the major religions and folk medicine. It has been demonstrated that pomegranate juice is a rich source of antioxidants (Goula et al., Citation2014). The antioxidant proprieties of pomegranate juice would place it on the top among fruit beverages including strawberries, raspberries, blueberries, and blackcurrants. It constitutes a good source of natural compounds, polyphenols, and mineral nutrients that are important for an assessment of the quality and the nutritive value of the juice (Yang et al., Citation2016). Pomegranate juice is also an important source of anthocyanins, 3-glucosides and 3.5-diglucosides, delphinidin, cyanidin, and pelargonidin (Du et al., Citation1975). It contains 7 mg/L ascorbic acid and 0.1 g/100 ml citric acid (El-Nemr et al., Citation1990). It can be used to treat and prevent many diseases including cancer, inflammation, neurodegeneration (Goula et al., Citation2014; Hartman et al., Citation2006; Koyama et al., Citation2010), and hypertension (Asgary et al., Citation2014). Previous studies reported that consumption of pomegranate juice for 2 weeks decreases systolic blood pressure (Stowe, Citation2011), inhibits serum angiotensin converting enzyme activity (Gil et al., Citation2000), reduces oxidative stress (Elfalleh et al., Citation2009), and may improve endothelial function by decreasing serum concentrations of vascular cell adhesion molecule 1 (VCAM-1) (Asgary et al., Citation2014).
Recently, more attention has been paid to the antioxidants contained in pomegranate juice. Previous studies showed that this fruit is endowed with a strong antioxidant activity (Elfalleh et al., Citation2009; Guo et al., Citation2003). With similar importance, date juice, as a natural and nutritional additive, is traditionally used as one of the best choices for milk flavoring (Mohammed et al., Citation2014). Date juice is rich in reducing sugars (16.1%) and total sugars (18.3%) (Kulkarni et al., Citation2010). Previous studies suggested that date juice could provide the useful basis for lactic acid production by Lactobacillus casei subsp. rhamnosus (Nancib et al., Citation2005). It is used to decrease the risk of spontaneous bleeding by shorting the bleeding time, for example on hemorrhagic fever and the consequence of consuming drugs (anti-coagulant, aspirin, anti-cancer, diuretic, etc.) (Sari et al., Citation2013).
In this context, our study aims to determine the nutritional and the real potentialities in terms of bioactive molecules and natural antioxidants of pomegranate (P granatum L.) and date (P dactylifera L.) juices and their combination at three different proportions (1:1, 2:1, and 1:2). Furthermore, the sensory characteristics and the acceptability of the cocktail pomegranate/date juice have been investigated.
Materials and methods
Chemicals and reagents
All chemicals and reagents were supplied by Sigma-Aldrich (Sigma, St. Louis, MO, USA), except the analytical reagent grade acetonitrile and methanol which were obtained from Lab-Scan (Labscan Ltd, Dublin, Ireland). Spectrophotometric measurements were performed on Shimadzu UV-1600 spectrometer (Shimadzu, Kyoto, Japan).
Juice preparation
Pomegranate (‘Gabsi’) and date (‘Bouhattam’) fruits were harvested in Oct. 2014. Both trees were grown in Gabes oasis (southeast of Tunisia, 33° 38′ 30″ N 10° 18′ 30″ E). Samples from fully mature fruits were included in the present study. Fresh fruits were stored at room temperature (18–20°C) until used.
For pomegranate juice (P) making, arils were manually removed from fruits. The aril sacs were ruptured by very light agitation in an electric blender for 5–10 s. The produced juice was then centrifuged at 10,000 rpm for 10 min at 4°C. The supernatant was collected into a clean tube and stored at 4°C for further analysis.
For date juice (D) making, the fruits were washed with water, and the seeds were removed, drained, and cut into small pieces. A quantity of deionized water was added in the proportions of 3:1 (dates:water). The mixture was pressed in an electric blender for 5–10 s. Then, the extract was filtered to separate the juice, forming the aqueous fraction, from the solid fraction. The filtrate was centrifuged at 10,000 rpm for 10 min at 4°C. The supernatant was collected into a clean tube and stored at 4°C for further analysis.
For the juice combination making, 500 g of date was thoroughly washed manually to remove dust and foreign materials. Pomegranate arils were manually separated from the rind and added to dates in different ratios; pomegranate/date (2:1): (PPD), pomegranate/date (1:1): (PD), pomegranate/date (1:2): (PDD). The mixture was centrifuged at 10,000 rpm for 10 min at 4°C to separate the cellulosic debris. The supernatant was collected for further analysis.
Dry matter and ash determination
Moisture removal is achieved in a two-stage process. In different juices, the liquid phase was predried over a steam bath before drying in an oven at 70°C until a stable weight is achieved. One gram of a dried sample, placed in a porcelain capsule, was calcined by the muffle furnace at 550°C to obtain the ash content.
Titratable acidity, pH, and total soluble solids of prepared juices
The juice yield was expressed as volume of juice per 100 g of fruits. The titratable acidity was determined by titrating 10 ml of juice with 0.1 M NaOH solution to pH 8.1 and expressed as gram of citric acid per 1 l of juice. The pH measurements were performed using a pH meter (PHS-3C pH-Meter) at 21°C. The total soluble solids (TSS) were measured by a digital refractometer. Results were reported as °Brix at 21°C.
Color determination of prepared juices
The color of the extracts was determined according to the method described previously by Locatelli et al. (Citation2016). The juices were 10-fold diluted with distilled water, and the absorbance (A) at 420, 520, and 620 nm was recorded by a T60 ultraviolet–visible spectrophotometer. The following colorimetric indices were calculated: color intensity (CI = A420 + A520 + A620), color tonality or nuance (N = A420/A520), % yellow (%Y = A420/CI × 100), % red (%R = A520/CI × 100), and % blue (%B = A620/CI × 100).
Identification and quantification of soluble sugars by HPLC method
Juice samples were analyzed using an Agilent HPLC-RID System (HP. 1100). Separation was performed on a ZORBAX column (Eclipse plus Column C18: 250 mm × 4.6 mm i.d., 5 μm). The detection was carried out by using a G1362A RI Detector. The column temperature was maintained at 35°C. The used solvent was composed of 75% water and 25% acetonitrile at a flow rate of 1.5 ml/min. The calculation of concentrations was based on the external standard method. These standards (fructose, glucose, sucrose, and maltose) were used to fit a standard curve (peak area versus concentration in mg/l) with linear regression for each individual compound. The concentrations are calculated by the following formula:
where Csample is sample concentration, Ccontrol is concentration of the standard solution,
Asample is area peak of the sample solution, and Acontrol is area peak of the standard solution.
Determination of total polyphenol (TP) and total flavonoids (TF) contents
The TP of different juices was determined by using the Folin–Ciocalteu method. A known amount of diluted juice in the ratio of 1:100 with methanol:water (6:4) was mixed with 0.5 ml Folin–Ciocalteu and 4 ml of sodium carbonate solution (1 M). The tubes were laid for 5 min in a water bath at 45°C and then put in a cold water bath. The absorbance was measured by a T60 ultraviolet–visible spectrophotometer at 765 nm. Gallic acid was used as the calibration standard. The results were expressed as mg gallic acid equivalent per ml of fruit juice (mg GAE/ml of juice).
Total flavonoids content of different juices was determined spectrophotometrically according to the method described by Djeridane et al. (Citation2006). This method is based on the formation of a complex flavonoid-aluminum having the maximum absorbance at 430 nm using a T60 ultraviolet–visible spectrophotometer. One milliliter of diluted sample was separately mixed with 1 ml of 2% aluminum chloride methanolic solution. After incubation at room temperature for 15 min, the absorbance of the reaction mixture was measured at 430 nm. The rutin was used as the calibration standard. The flavonoids content was expressed as mg rutin equivalent per ml of juice (mg RE/ml of juice).
Determination of condensed tannin (CT)
The determination of CT content in different juices was carried out as described by Price et al. (Citation1978). Diluted H2SO4 (1.5 M) mixed with 3 ml of vanillin solution (4%) was added to 50 ml of each sample. The absorbance was read at 500 nm against a blank that contained aqueous methanol instead of the sample after 15 min incubation at room temperature. Catechin was used as the calibration standard. The CT content was calculated from the standard curve and expressed as catechin equivalent per 100 ml of juice (mg CE/100 ml of juice).
Determination of DPPH radical scavenging activity
The radical scavenging activity of different juices was determined following the method reported by Okonogi et al. (Citation2007). First, 100 µl of diluted juice (1:100) was mixed with methanol:water (6:4) and added to 2 ml of 100 μM methanolic solution of 2,2-diphenyl-1-picrylhydrazyl (DPPH). The mixture was shaken vigorously and left to stand in the dark for 30 min at room temperature before the absorbance was measured at 517 nm. The control solution was prepared by mixing methanol (100 µl) and DPPH radical solution (2 ml), and the absorbance was recorded as Abscontrol. The radical scavenging activity was expressed as the percentage of inhibition by the following formula:
The radical scavenging activity of test juices was expressed as IC50, defined as the effective concentration of a sample required to decrease the absorbance at 517 nm by 50%. All measurements were performed in triplicate.
Determination of ABTS+ radical scavenging activity
The radical scavenging activity of different juices for 2,20-azinobis-3-ethylbenzothiazoline-6-sulphonate (ABTS) radical cation was determined as described by Re et al. (Citation1999) with few modifications. ABTS was generated by mixing a 7 mM ABTS solution with 2.45 mM (final concentration) potassium persulfate and allowing the mixture to stand in the dark at room temperature for 16 h before use. The ABTS+ solution (blue–green) was diluted with ethanol to an absorbance of 0.70 ± 0.02 unit at 734 nm. After adding 25 μl of test materials or ascorbic acid to 2 ml of diluted ABTS+ solution, absorbance at 734 nm was recorded up to 6 min at 1 min intervals. Results were expressed as mg ascorbic acid equivalent antioxidant capacity (AEAC) per ml of juice (mg/ml AEAC). All measurements were performed in triplicate.
Reducing power assay
The reducing power of juice extracts was determined according to the method of Ferreira et al. (Citation2007). First, 1 ml of various concentrations of ascorbic acid (0.05, 0.1, 0.2, 0.4, 0.8, and 1.2 mg/ml) was added to phosphate buffer (0.2 M, pH 6.6). The mixture was incubated with potassium ferricyanide (1% w/v) for 20 min at 50°C. The reaction was terminated by adding TCA solution (10% w/v) and the reaction mixture was centrifuged at 2000 g for 10 min. The supernatant was mixed with distilled water and ferric chloride (0.1% w/v) solution, and the absorbance was measured at 700 nm. The decreased absorbance of the reaction mixture indicated decreased reducing power. Results were expressed as µg ascorbic AEAC per ml of juice (µg/ml AEAC). All measurements were performed in triplicate.
Sensory analysis
In order to estimate the juice quality and acceptability, a consumer sensory testing was conducted. A total of five samples were evaluated by panelists. Color, brightness, sweetness, salinity, acidity, bitterness, odor, viscosity, and general agreement of the juice were evaluated. Fifty persons (females and males aged 19−33 years old) participated in the sensory analysis. The panelists indicate their liking for the sample on a 10-point hedonic scale from 0 (not perceivable) to 10 (perceivable at the level of saturation). The samples were prepared one day before analysis and were warmed at room temperature. The juices (around 10 ml) were presented with a five digit code on a white plastic cup (usually used for coffee). Before analysis, panelists were advised to rinse their mouths carefully with water between samples to minimize residual flavor effects.
Statistical analyses
All measurements were carried out in triplicate and the results were presented as means ± SD. All data analyses were performed using Xlstat 2016 (www.xlstat.com). An analysis of variance was used to compare juices. The difference was considered statistically significant at P < 0.05.
Results and discussions
Titratable acidity, pH, and total soluble solids of prepared juices
The diverse physicochemical characteristics of prepared juices were presented in . The pH values of pomegranate and date juices ranged from 4.30 to 5.20, respectively. Accordantly, date juice had the lowest titratable acidity (1.28 citric acid g/l of juice) followed by PDD juice (2.01 citric acid g/l of juice). The highest titratable acidity was obtained in pomegranate juice (2.13 citric acid g/l of juice). All juices have comparable TSS and they ranged from 15.87°Brix in date juice to 16.40°Brix in PD juice. The ash values ranged from 1.84% in pomegranate juice to 7.02% in date juice.
Table 1. Physicochemical properties of different juices.
Even if the result showed no significant differences between PD, PDD, and date juices, the dry matter in PDD juice was slightly higher (32.28%). It could be presumed that dry matter increased when the date/pomegranate ratio increased. It is mainly due to the high dietary fiber content in dates which ranged between 8.1% and 12.7% of the dry weight (AL-Shahib and Marshall, Citation2002). In fact, different juice ratios were prepared by adding pomegranate aril directly to the date and the water from juicy arils was used when mixing pomegranate date juices. However, when preparing date juice deionized water was used as the solvent. This fact may explain the fact that dry matter from date and arils together (PDD) was slightly higher than the date juice.
Color index
The results of color variables of tested juices were presented in . The analysis revealed that date juice had the lowest intensity value (1.07) against pomegranate juice which has 2.23. Even though the results show no significant differences between juices nuance, yellow and red colors, it is clearly seen that both the yellow and red colors were relatively high in almost all juices which confirm the research of El Kar et al. (Citation2013) reporting similar finding for the color intensity of pomegranate juice.
Table 2. Color index of different juices.
Anthocyanins are the water-soluble pigments responsible for the bright red color of pomegranate juice. According to Noda et al. (Citation2002), three major anthocyanidins were found in pomegranate juice: delphinidin, cyaniding, and pelargonidin. Both flavonoids and carotenoids cores were abundant in pomegranate and date fruits (Chaira et al., Citation2009; Seeram et al., Citation2006). Carotenoids, as carotenes or xanthophylls, were responsible for the yellow, orange, and red colors. Flavonoid compounds are yellow or cream while anthocyanins give red, pink, blue, and purple colors (Valeur, Citation2011).
Stability and color variation of natural colorant anthocyanin could be influenced by many factors and the pH is the most significant one. In general, anthocyanins are more stable in acidic media at low pH values than in alkaline solutions (Rein, Citation2005). The pomegranate juice with the lowest pH (4.30) had the lowest percentage (%) of blue value (29.80). These results were confirmed by Aishah et al. (Citation2013) showing that increasing pH values caused rapid loss of the proton producing blue or violet quinonoidal base forms. Under acidic conditions, the anthocyanins existed primarily in the form of deep-red flavylium cation (Aishah et al., Citation2013).
Identification and quantification of soluble sugars
Individual soluble sugars of different juices were presented in . A notable difference was observed in the total sugar content of the five juices. The highest content was observed in date juice (37.77 ± 4.69 g/100 ml) while the lowest content was obtained in pomegranate juice (12.62 ± 2.19 g/100 ml). The sugars identified and quantified, in all samples, were fructose and glucose. However, the sucrose was exclusively detected in date juice (21.34 ± 2.34 g/100 ml) and the maltose was absent in all the juices (Supplemental Figure 1). Thus, it could be presumed that the mixture of these two juices enhanced the individual soluble sugar contents.
Table 3. Soluble sugars (g/100 ml) of different juices analyzed by HPLC.
Total polyphenol, total flavonoids, and condensed tannins contents
shows the TP, TF, and CT contents of the different juice preparations. The maximum content of TP was found among the PD juice (1:1) (10.77 ± 4.01 GAE mg/ml). A significant variation in TF concentration was observed among the five juices and the values ranged from 2.64 to 5.71 RE mg/ml. Our results were higher than the values reported by Chaira et al. (Citation2009) (15.73–54.66 mg/100 g fresh weight for the phenolic content and 54.46 quercetin equivalents/100 g fresh weight for the TF) while similar finding was reported by Elfalleh et al. (Citation2009). The content of CT ranged from 5.79 to 7.18 mg/100 ml (). Based on CT contents, there is no significant difference between juices except the pomegranate juice which had the lowest value (5.79 ± 0.18 mg/100 ml). These results bring attention to the high amount of TP, flavonoids, and CT in PD juice (1:1).
Table 4. Total phenols, flavonoids, and condensed tannins contents of the different juices.
Antioxidant activities
The antioxidant activity was evaluated by three different methods (DPPH, ABTS+, and reducing power) as shown in and and . The antioxidant activity of phenolic compounds was mainly due to their redox properties and chemical structure. The DPPH radical scavenging assay was commonly used to evaluate the ability of an antioxidant to scavenge-free radicals. Antioxidant activities of different juices were expressed as IC50, defined as the inhibitory concentration at which 50% of DPPH radicals were scavenged. The DPPH radical scavenging activity of five juices was significantly different and the values ranged from 7.14 to 11.22 µg/ml. It is reported that pomegranate juice had a significant variation of the antioxidant activity among juices. Tezcan et al. (Citation2009) reported a radical scavenging activity ranging from 10.37% to 67.46% on seven commercial pomegranate juices from Turkey. Tehranifar et al. (Citation2010) reported a radical scavenging activity ranging from 15.59% to 40.72% on 20 pomegranate juices from Iranian cultivars.
Table 5. DPPH/ABTS+ radical scavenging activities and reducing power of different juices.
Figure 1. A. Inhibition of DPPH radical by different juices. B. Exponential fitting of the DPPH radical inhibition by pomegranate/date (1:1) PD juice.
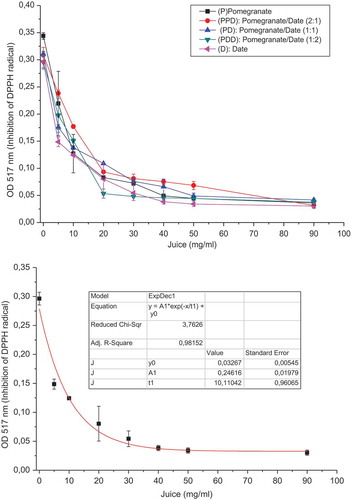
All juices showed an important antioxidant potential. It can be presumed that the mixture of date and pomegranate juices with the ratio (1:1) could enhance the radical scavenging activity.
The ABTS radical scavenging capacities of different juices were expressed as mg/ml AEAC. A high antioxidant activity was presented by the pomegranate juice (2.80 ± 0.05 AEAC mg/ml), followed by the PDD juice (2.18 ± 0.08 AEAC mg/ml) (). Even then, the lowest antioxidant activity was obtained in date juice (1.36 ± 0.11 AEAC mg/ml) and no significant differences were observed between PPD, PD, and date juices. The reducing powers of the different juices were distinct and the differences were confirmed statistically. The values ranged from 690.90 ± 7.42 (D juice) to 748.89 ± 35.75 AEAC µg/ml (PDD juice). The hierarchy of different juices for their reducing power was PDD > PD > P > PPD > D.
The differences in antioxidant potencies were often associated with the genetic variability that led to the variation in the biosynthesis of phenolic secondary metabolites in pomegranate or date cultivars. Different studied juices showed significant differences based on three methods. These differences were mainly due to the distinguishing characteristics and the composition of each juice.
Sensory analysis
The sensory profile was shown in . The color, brightness, and viscosity were selected as appearance attributes. Significant differences (P < 0.05) were revealed among the juices for the three attributes. The date juice color intensity was relatively higher than those of pomegranate juice while the result of showed the inverse. This is due to the fast oxidation of date juice which is more unstable than pomegranate juice. Brightness also was affected by the oxidation. Referring to juice taste, the panel distinguished four different attributes that were sweetness, salinity, acidity, and bitterness. The date juice was the sweetest one because of the high level of fructose, glucose, and sucrose while pomegranate juice was the most acid because of the high level of citric acid.
Table 6. Means of sensory attributes and acceptability scores of different juices.
Finally, the panel added another attribute which is the acceptability. The mean value of consumer acceptance is more than 5, so this result confirms the acceptability of all juices by the panelist. This indicated that the formulation of a cocktail pomegranate/date juice seems appreciated by the consumers and it was concluded that the mixture juice (PD juice) was well satisfying.
Conclusion
In conclusion, physicochemical characteristics, total phenolic, flavonoids, and antioxidant capacity of the five juices were studied. Statistically, significant differences were observed between juices. It can be presumed that a cocktail pomegranate/date juice (1:1) is a good source of antioxidant. The results provide important information on the composition of soluble sugars of the different juices. The pomegranate juice is loaded with glucose and fructose that are absorbed directly into the bloodstream during digestion. In addition, a hedonic test advocates that the formulation of a cocktail pomegranate/date could produce a novel beverage acceptable by most consumers. Noncommercial varieties from pomegranate and date fruits may be used in this context to enhance their consumption and commercialization after processing into an appropriate juice combination.
Supplemental Material
Download MS Word (258 KB)Acknowledgments
The authors are grateful to N. Moussa from the National Engineering School of Gabes for HPLC analysis. The authors are also grateful to anonymous referees for helpful comments on an earlier draft.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Supplemental data
Supplemental data for this article can be accessed at publisher’s website.
Additional information
Funding
References
- Aishah, B., M. Nursabrina, A. Noriham, A.R. Norizzah, and H.M. Shahrimi. 2013. Anthocyanins from Hibiscus sabdariffa, Melastoma malabathricum and Ipomoea batatas and its color properties. Int. Food Res. J. 20:2.
- Al‐Shahib, W., and R.J. Marshall. 2002. Dietary fibre content of dates from 13 varieties of date palm Phoenix dactylifera L. Int. J. Food Sci. Tech. 37(6):719–721. doi: 10.1046/j.1365-2621.2002.00615.x.
- Asgary, S., A. Sahebkar, M.R. Afshani, M. Keshvari, S. Haghjooyjavanmard, and M. Rafieian-Kopaei. 2014. Clinical evaluation of blood pressure lowering, endothelial function improving, hypolipidemic and anti-inflammatory effects of pomegranate juice in hypertensive subjects. Phytother. Res. 28:193–199. doi: 10.1002/ptr.4977.
- Ben Salah, M. 2011. The palm of Gabes. Issued by Phoenix Project, France-Italy 2011. Retrieved from http://www.listephoenix.com/wp-content/uploads/2011/12/BENSALAH-oasis-Gabes-fr.pdf
- Chaira, N., M.I. Smaali, M. Martinez-Tome, A. Mrabet, M.A. Murcia, and A. Ferchichi. 2009. Simple phenolic composition, flavonoid contents and antioxidant capacities in water-methanol extracts of Tunisian common date cultivars (Phoenix dactylifera L.). Inter. J. Food Sci. Nutri 60(7):316–329. doi: 10.1080/09637480903124333.
- Djeridane, A., M. Yousfi, B. Nadjemi, D. Boutassouna, P. Stocker, and N. Vidal. 2006. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 97:654–660. doi: 10.1016/j.foodchem.2005.04.028.
- Du, C.T., P.L. Wang, and F.J. Francis. 1975. Anthocyanins of pomegranate, Punica granatum. J. Food Sci. 40:417–418. doi: 10.1111/j.1365-2621.1975.tb02217.x.
- El Kar, C.H., N. Mtimet, A. Ferchichi, and J. Bouajila. 2013. Relationships between fruit acceptability and health-case of seven pomegranate (Punica granatum L.) juices. J. Food Nutr. Sci. 4:119–130.
- Elfalleh, W., N. Nasri, N. Marzougui, I. Thabti, A. M’rabet, Y. Yahya, B. Lachiheb, F. Guasmi, and A. Ferchichi. 2009. Physico-chemical properties and DPPH-ABTS scavenging activity of some local pomegranate (Punica granatum) ecotypes. Inter. J. Food Sci. Nutr. 60:197–210. doi: 10.1080/09637480903067037.
- El‐Nemr, S.E., I.A. Ismail, and M. Ragab. 1990. Chemical composition of juice and seeds of pomegranate fruit. Mol. Nutr. Food. Res. 34(7):601–606.
- Ferreira, I.C., P. Baptista, M. Vilas-Boas, and L. Barros. 2007. Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: Individual cap and stipe activity. J. Food Chem. 100(4):1511–1516. doi: 10.1016/j.foodchem.2005.11.043.
- Gil, M.I., F.A. Tomás-Barberán, B. Hess-Pierce, D.M. Holcroft, and A.A. Kader. 2000. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 48(10):4581–4589.
- Goula, A.M., A. Tzika, and K.G. Adamopoulos. 2014. Kinetic models of evaporation and total phenolics degradation during pomegranate juice concentration. Inter. J. Food Eng 10(3):383–392.
- Guo, C., J. Yang, J. Wei, Y. Li, J., & Xu, and Y. Jiang. 2003. Antioxidant activities of peel. Pulp and seed fractions of common fruits as determined by FRAP assay. Nutr. Res. 23(12):1719–1726.
- Hartman, R.E., A. Shah, A.M. Fagan, K.E. Schwetye, M. Parsadanian, R.N. Schulman, M.B. Finn, and D.M. Holtzman. 2006. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2006(24):506–515. doi: 10.1016/j.nbd.2006.08.006.
- Koyama, S., L.J. Cobb, H.H. Mehta, N.P. Seeram, D. Heber, A.J. Pantuck, and P. Cohen. 2010. Pomegranate extract induces apoptosis in human prostate cancer cells by modulation of the IGF-IGFBP axis. Growth Horm. IGF Res. 2010(20):55–62. doi: 10.1016/j.ghir.2009.09.003.
- KulkarniS., K.G., G.P. Kulkarni, P. Vijayanand, and L.L. Shubha. 2010. Effect of processing of dates into date juice concentrate and appraisal of its quality characteristics. J. Food Sci. Technol. 2:157–161. doi: 10.1007/s13197-010-0028-y.
- Locatelli, M., F. Travaglia, J.D. Coïsson, M. Bordiga, and M. Arlorio. 2016. Phenolic composition of Nebbiolo grape (Vitis vinifera L.) from Piedmont: Characterization during ripening of grapes selected in different geographic areas and comparison with Uva Rara and Vespolina cv. Eur. Food Res. Technol. 242(7):1057–1068. doi: 10.1007/s00217-015-2610-z.
- Mohammed, H.A.E., S.H. Omer, S.M. Hussen, and T.A. Eissa. 2014. The effect of dates juice on the quality of flavor milk. Bachelor dissertation, Sudan University of Science and Technology, Khartoum, Sudan Retrieved from http://repository.sustech.edu/handle/123456789/18708.
- Nancib, A., N. Nancib, D. Meziane-Cherif, A. Boubendir, M. Fick, and J. Boudrant. 2005. Joint effect of nitrogen sources and B vitamin supplementation of date juice on lactic acid production by Lactobacillus casei subsp. rhamnosus. Bioresour. Technol. 96(1):63–67. doi: 10.1016/j.biortech.2003.09.018.
- Noda, Y., T. Kaneyuki, A. Mori, and L. Packer. 2002. Antioxidant activities of pomegranate fruit extract and its anthocyanidins: Delphinidin, cyanidin, and pelargonidin. J. Agric. Food Chem. 50(1):166–171. doi: 10.1021/jf0108765.
- Okonogi, S., C. Duangrat, S. Anuchpreeda, S. Tachakittirungrod, and S. Chowwanapoonpohn. 2007. Comparison of antioxidant capacities and cytotoxicities of certain fruit peels. Food Chem. 103(3):839–846. doi: 10.1016/j.foodchem.2006.09.034.
- Price, M.L., S.V. Scoyoc, and L.G. Butler. 1978. A critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain. J. Agr Food Chem. 26(5):1214–1218. doi: 10.1021/jf60219a031.
- Rachma Purnama Sari, R.P., S. Sampurna, and D. Pertiwi. 2013. The effect of dates juice on the bleeding time an experimental study of male Wistar strain rats that induced by aspirin. J. Sains Medi 5(1):20–22.
- Re, R., N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, and C. Rice-Evans. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. J. Free Radic. Biol. Med. 26(9–10):1231–1237. doi: 10.1016/S0891-5849(98)00315-3.
- Rein, M. 2005. Co-pigmentation reactions and color stability of berry anthocyanins. University of Helsinki: Helsinki, Russia. PhD thesis.
- Seeram, N.P., S.M. Henning, Y. Zhang, M. Suchard, Z. Li, and D. Heber. 2006. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J. Nutr. 136:2481–2485. doi: 10.1093/jn/136.10.2481.
- Stowe, C.B. 2011. The effects of pomegranate juice consumption on blood pressure and cardiovascular health. Complement. Ther. Clin. Pract. 17(2):113–115. doi: 10.1016/j.ctcp.2010.09.004.
- Tehranifar, A., M. Zarei, Z. Nemati, B. Esfandiyari, and M.R. Vazifeshenas. 2010. Investigation of physico-chemical properties and antioxidant capacity of twenty Iranian pomegranate (Punica granatum L.) cultivars. J. Sci. Hortic 126(2):180–185. doi: 10.1016/j.scienta.2010.07.001.
- Tezcan, F., M. Gultekin-Ozguven, T. Diken, B. Ozcelik, and F.B. Erim. 2009. Antioxidant capacity and total phenolic. Organic Acid and Sugar Content in Commercial Pomegranate Juices. J. Food Chem. 115(3):873–877.
- Valeur, B. 2011. La couleur dans tous ses éclats. Paris, France: Belin.
- Yang, J., R. Lee, S.M. Henning, G. Thames, M. Hsu, H. ManLam, D. Heber, and Z. Li. 2016. Soy protein isolate does not affect ellagitannin bioavailability and urolithin formation when mixed with pomegranate juice in humans. Food Chem. 194:1300–1303. doi: 10.1016/j.foodchem.2015.08.099.
