ABSTRACT
The aim of this study was to investigate the effect of salicylic acid (SA) immersion incorporated with UV-C irradiation alleviating chilling injury (CI) of longan fruit cv. “E-Dor”. The fruit were immersed in 2 mM SA for 1 hour and then illuminated under UV-C light at the dose of 4.4 kJ·m−2 (SA + UV-C), and the untreated fruit were used as control. All treatments were held at 4°C and 90 ± 2% relative humidity for 8 days. The determined parameters were browning score of skin and electrolyte leakage (EL), lipoxygenase (LOX) activities, malondialdehyde (MDA) contents, fatty acid contents, and free radical scavenging activities of both skin and flesh fruit. SA + UV treatment apparently delayed and lowered skin browning incidence by preventing the increases in EL, LOX activity, and MDA content, induced the ratio of unsaturated fatty acids per saturated fatty acid content, and maintained free radical scavenging activity. The treatment also lowered EL and LOX activity and maintained free radical scavenging activity of the fruit flesh during cold storage. These indicated that the use of SA + UV-C is an effective alternative preventing skin browning caused by CI of longan fruit during cold storage.
Introduction
Longan (Dimocarpus longan Lour.) fruit is known as a commercial non-climacteric fruit belonged to the Sepindaceae family. The origin of the fruit is from Northern Burma and Northeast and Southern China, and it had been widely spread and commercially grown around Southeast Asia and North-eastern India. Recently, Thailand is claimed as the world’s largest producer of the fruit followed by Vietnam (Batten, Citation1986; Noichinda et al., Citation2015; Partridge, Citation1997). Most popular longan cultivars commercially grown in Thailand are E-dor, Chompoo, Haew, Beow-keow, and Phet Sakhon. Among these cultivars, E-dor has been required for export more than others and was selected as a produce champion to export (Jaroenwanit et al., Citation2001). In the year 2016, Thailand was exported longan fruit more than 500 million tons per year (Office of Agricultural Economics, Citation2016). The most problem affecting longan fruit quality is rapid peel browning after harvest. Browning of longan fruit peel is associated with desiccation, fungal attack, and chilling injury (CI) (Jiang et al., Citation2002). SO2 fumigation and sodium metabisulfite immersion have been commercially used to control the browning incidence and disease of longan fruit (Jiang et al., Citation2002; Tongdee, Citation1997). Recently, sulfur residue in fruit, especially in longan, has been concerned by consumer. Limited level of sulfur residues in fruit and dried fruits must be less than 10 µg·g−1 as specified by the EU, Australia, and Japan (Sivakumar et al., Citation2010). Therefore, alternative approaches have been studied to control discoloration in longan fruit instead of sulfur compound application. UV-C treatment at low doses is an effective means delaying ripening, senescence, CI, and decay of fresh commodities such as bell pepper (Promyou and Supapvanich, Citation2013; Vincente et al., Citation2005), peaches (González-Aguilar et al., Citation2004), strawberries (Erkan et al., Citation2008), mango (González-Aguilar et al., Citation2007), and longan (Suiubon et al., Citation2017). Postharvest UV-C treatment directly affects both vegetative and sporulated bacteria and fungi and induces resistance against diseases (Stevens et al., Citation1996). Moreover, it also accelerates bioactive compounds having the antioxidant capacity by inducing phenylpropanoid biosynthesis (Korkina, Citation2007). Similarly, exogenous salicylic acid (SA) application also induces pathogenic resistance, delays fruit ripening, and alleviates physiological disorders, especially CI in fruit and vegetables (Asghari and Aghdam, Citation2010; Supapvanich and Promyou, Citation2013). Our previous works found that SA maintained postharvest quality and alleviated CI in anthurium flower (Promyou et al., Citation2012), papaya (Promyou and Supapvanich, Citation2016), lemon basil (Supapvanich et al., Citation2015), wax apple fruit (Supapvanich et al., Citation2017a), and guava fruit (Supapvanich et al., Citation2017b). Chen et al. (Citation2006) indicated that SA induces the expression of PAL genes and enhances the synthesis of new PAL protein and activity leading to the accumulation of phenylpropanoid compounds such as phenols and flavonoids. Moreover, both SA and UV-C also maintain membrane permeability functions by stimulating heat shock protein accumulation and reducing oxidative reaction of membrane lipids (Supapvanich and Promyou, Citation2013). Thus, the aim of this recent work was to determine the use of SA incorporated with short UV-C illumination on factors related to CI of longan fruit cv. “E-Dor” during cold storage.
Materials and Methods
Raw Material Preparation and Treatment
“Dor” longan fruit were obtained from a commercial orchard in Chiang Mai Province, Northern Thailand. The fruit were harvested at full maturity stage (7 months after anthesis) and delivered to laboratory at Kasetsart University, Chalermprakiate Sakhon Nakhon province campus, within 8 h. The individual fruit was cut from bunch. The fruit with physical damages and diseases were discarded. The selected fruit were cleaned with tap water followed by immersion in 200 µL·L−1 sodium hypochlorite for 5 min and then air-dried at room temperature (RT) for 30 min. The fruit were distributed into two groups (900 fruit per group) for two treatments. In the first treatment, the fruit were stored at 4°C without treatment (control) for 8 days and, in the second treatment, the fruit were immersed in 2.0 mM SA for 1 h and then air-dried at RT for 30 min. The SA-treated longan fruit were placed in a clear plastic box containing two UV-C lamps (TUV 30 W, Sylvania, Japan) with the exposure distance of 70 cm above and below fruit. The fruit were held under UV-C light until obtained dosage was 4.4 kJ·m−2 which our preliminary work on UV-C illumination alleviating CI symptom showed that 4.4 kJ·m−2 UV-C illumination prevented skin browning of longan fruit rather than 2.2 and 6.6 kJ·m−2 UV-C illuminations (data not shown). A UV light meter model UVC-580010 (Sper Scientific, USA) was used to measure the dose of UV light. After treatment, the fruit were stored in a refrigerated room at 4°C and 90 ± 2% relative humidity for 8 days. One hundred and eighty fruit per treatment (60 fruit per replicate) were sampled in every 2 days. The fruit were left at RT for 3 h before parameter determinations.
Browning Score Evaluation
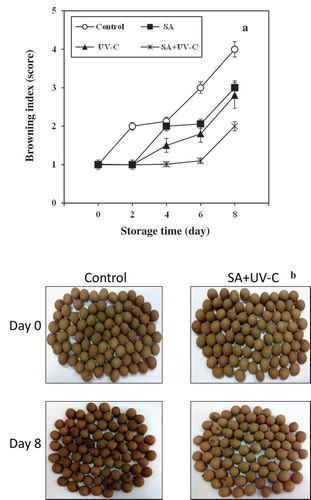
Ten trained panelists evaluated the severity of skin browning of longan fruit using four sensorial scoring tests. The severity of skin browning index (BI) was evaluated on a relative scale of 1 to 4; 1 = no occurrence; 2 = moderate (1–35% of fruit affected); 3 = severe (36–70% of fruit affected); and 4 = very severe (71–100% of fruit affected) as showed in . BI was calculated as follows: (BI scale × number of fruit at that level)/total number of fruit in each group (60 fruits in the present experiments).
Electrolyte Leakage Measurement
Longan fruit peel and pulp were punctured using a 15-mm-diameter cork borer. A 5 g of skin or pulp discs was rinsed with deionized water. After that, the surface water was removed using Whatman no. 1 filter paper and weighted. The discs of each tissue were incubated in 20 mL of 0.4 M mannitol at RT for 1 h. The conductivity of the sample was measured using a conductivity meter (sensION5, Hach Company, USA). The total conductivity of the sample was then measured after the sample was autoclaved at the temperature of 120°C for 15 min. The percentage of sample electrolyte leakage (EL) was calculated as follows: (final conductivity divided by the total conductivity) x 100.
Malondialdehyde Concentration Assay
Malondialdehyde (MDA) concentration of both peel and pulp tissues were assayed using the method of Heath and Packer (Citation1968). Five grams of sample were blended in 20 mL of 5% trichloroacetic acid (TCA) and then centrifuged at 10,000 x g for 10 min. One mL of supernatant was reacted with the solution of 2 mL of 15% TCA containing 0.5% thiobarbituric acid for 30 min at 60°C. After that, the sample was immediately placed in an ice bath for 30 min. The absorbance at 532 (OD532) and 600 (OD600) nm wavelengths of the sample was measured using an extinction coefficient of 1.55 mM.cm−1 calculated as follows: MDA content (nmol MDA·g−1) = [(OD532 – OD600) × 2 mL × (total volume of extract (mL) × 1 mL)]/(1.55 × 10–1 × 5 g).
LOX Activity Assay
LOX was extracted and assayed as described by Lara et al. (Citation2003). Five grams of peel and pulp longan fruit were homogenized in 5 mL of extraction solution containing 0.1 M phosphate, pH 7.5, 2 mM dithiothreitol, 1 mM EDTA, 0.1% (v/v) Triton X-100, and 1% (w/v) polyvinylpolypyrrolidone. The homogenate was centrifuged at 10,000 × g for 20 min at 4◦C and the supernatant held at 0◦C. LOX activity assayed by mixing 100 µL of the supernatant with 2.5 mL 0.1 M phosphate, pH 8, and 400 µL substrate solution (8.6 mM linoleic acid, 0.25% (v/v) Tween-20, 10 mM NaOH, in 0.1 M phosphate, pH 8). Activity was measured by following the increase in absorbance at 234 nm due to the formation of hydroperoxides from linoleic acid. One unit of enzyme activity was defined as the increase in absorbance per minute and per milliliter enzyme solution.
Fatty Acid Composition Assay
Methyl esters of fatty acids were separated and quantified by the AOAC (Citation1995) method, using a gas chromatograph (Varian, model CP 3800, Palo Alto, CA, USA) with a 60 m × 0.25 mm DB-23 capillary column and a film thickness of 0.25 mm (Agilent Technologies, Wilmington, DE, USA), coupled to a flame ionization detector. The injector and detector were maintained at 230◦C and 250◦C, respectively. Samples were injected on the column at a split rate of 1:50 with a helium carrier gas flow rate of 1 mL/min. All solutions were injected using three replications, and standards were also injected three times. Total saturated fatty acid (SFA) content is the sum of palmitic (16:0) and stearic (18:0) and total unsaturated fatty acid (UFA) is the sum of linoleic (18:2) and linolenic (18:3).
Free Radical Scavenging Activity Assay
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity was measured following the method of Nanjo et al. (Citation1996). Methanol extracts with varying concentrations were taken in different test tubes, and the volume was made up to 1 mL. DPPH (0.004%) 4 mL was added to each test tube and the contents were incubated at RT for 30 min. The absorbance (OD) of the solution was read at 517 nm and calculated as % of DPPH radical scavenging = [(OD0 min – OD30 min)/OD0 min] x 100.
Statistical Analysis
Experiment data are the average of three replications ± standard error (SE). Analysis of variance (ANOVA) was calculated, and the difference of each treatment was determined using Duncan’s multiple range tests at p≤ 0.05.
Results and Discussions
Peel Browning Index
Peel browning incidence and visual appearance of the longan fruit are shown in and b. Peel browning of longan fruit during cold storage is commonly acknowledged as CI symptom. The results show that SA + UV-C treatment delayed the fruit peel browning for 6 days, while the peel browning of control was found on day 2 and progressively increased throughout the storage. After 2 days of the storage, the peel browning score of control was higher than that of SA + UV-C-treated fruit until the end of the storage. On day 8 of the storage as shown in , the peel browning score of control was reached to 4 and that of SA + UV-C-treated fruit was lower than 2.5. This suggests SA + UV-C treatment could alleviate browning incidence of longan fruit peel during cold storage. It is widely accepted that both SA and UV-C treatments alleviate CI of fresh commodities by preventing membrane degradation and stimulating antioxidant system (Supapvanich and Promyou, Citation2013; Vincente et al., Citation2005). In a similar vein, Supapvanich et al. (Citation2017b) suggested that 2 mM SA immersion prevented skin browning of “Kimju” guava fruit during cold storage. Pongprasert et al. (Citation2011) reported that UV-C illumination inhibited skin browning and polyphenol oxidase activity of banana fruit during storage at 8°C. Moreover, our previous work also showed that SA + UV treatment maintained longan fruit peel brightness and color greater than UV treatment alone (Suiubon et al., Citation2017). The result suggests that SA + UV treatment alleviated CI severity of longan fruit during cold storage.
EL Level
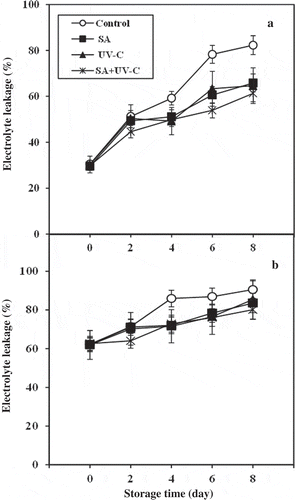
The leakage of tissue electrolyte is an acceptable indicator of CI measurement. The increases in both peel and pulp EL of the longan fruit were lowered by SA + UV-C treatment (). The electrolyte efflux of both peel and pulp tissues from control fruit was significantly higher than those of SA + UV-C-treated fruit throughout the storage (P< 0.05). This indicates that SA + UV-C treatment could maintain membrane integrity of both peel and pulp tissues. As mentioned before, the peel browning incidence, CI symptom, of longan fruit was alleviated by SA + UV treatment which accompanied by the low EL of peel tissue compared to the peel of control. Many previous works reported that the use of both SA and UV-C treatments is an effective alternative alleviating CI due to the preservation of membrane integrity by inducing heat shock proteins and bioactive compounds having antioxidant and free radical scavenging activities (Asghari and Aghdam, Citation2010; Promyou & Supapvanich, Citation2012; Alegria et al., Citation2012; Supapvanich and Promyou, Citation2013).
LOX Activity and MDA Concentration
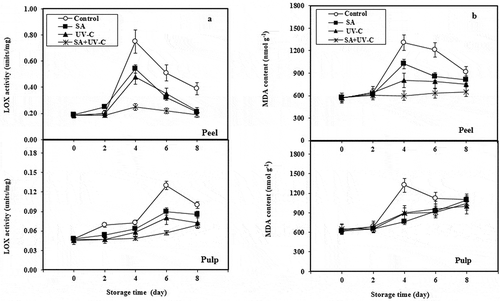
The increase in LOX activity and MDA concentration is accompanied by membrane lipid peroxidation in plant tissues. LOX activities of both peel and pulp tissues of longan fruit increased throughout the storage ( and ). The result also shows that peel LOX activity was higher than the pulp LOX activity. LOX activity of the peel tissue of SA + UV-C-treated fruit remained constant during storage for 6 days and then increased on day 8 of the storage, while that of the control increased continuously. On days 6 and 8 of the storage, the LOX of control peel tissue was significantly higher than that of the UV-C treated fruit (P < 0.05). MDA concentration of the control fruit peels was low during storage for 2 days and then massively increased and reached the peak on day 4. Afterward, the MDA concentration was decreased. However, peel MDA concentration of SA + UV-C-treated fruit was also low and similar to that of control fruit during storage for 2 days. After day 2, the MDA concentration of SA + UV-C-treated fruit also increased but was significantly lower than that of the control fruit (P < 0.05). Afterward, the MDA concentration of SA + UV-C-treated fruit remained constant over the storage (). In pulp tissue, LOX activity of control fruit also significantly increased on day 2 of the storage (P < 0.05) and then increased slightly. In a similar vein, LOX activity of SA + UV-C-treated fruit gradually increased and was significantly lower than that of control fruit over the storage. MDA content of the control and SA + UV-C-treated fruit was increased during storage, but no significant difference between both treatments was found over the storage (). The results also showed that increases in both LOX activity and MDA concentration of peel tissue of the control were related to the increases in EL and browning incidence. The recent work reveals that SA + UV-C treatment could control lipid peroxidation of longan fruit peel resulting in lower peel browning incidence and EL level during cold storage. Barka et al. (Citation2000) suggested that the primary target of UV-C irradiation was cell membrane in which the level of membrane peroxidation of UV-C-treated fruit was lowered by UV-C treatment due to the enhancement of defense mechanism and induction of antioxidant activity and the activities of antioxidant enzymes such as catalase, peroxidase, and superoxide dismutase. Similarly, our previous work found that UV-C treatment delayed lipid peroxidation increase and induced antioxidant activity and bioactive compound contents including antioxidant enzyme activities in yellow bell pepper fruit (Promyou & Supapvanich, Citation2012). However, exogenous SA treatment can also reduce lipid peroxidation during cold storage by inducing defense mechanism and bioactive compounds having antioxidant property as well as antioxidant enzymes. Many previous works reported that postharvest SA treatment inhibited the increases of LOX activity and MDA concentration in mango fruit (Ding et al., Citation2007), sugar apple fruit (Mo et al., Citation2008), peach fruit (Cao et al., Citation2010), and tomato fruit (Aghdam et al., Citation2014) during cold storage.
Fatty Acid Contents and Proportional
It is commonly acknowledged that lipid compositions in membrane influence on chilling sensitivity of plants. shows SFA, UFA, and UFA/SFA contents of longan fruit peel after storage for 8 days at 4°C. The result shows that there was no significant change in palmitic acid (16:0) content of both control and SA + UV-C-treated fruit over storage for 8 days, while stearic acid (18:0) content of both fruits was significantly decreased after storage for 8 days (P < 0.05). In the control fruit, linoleic acid (18:2) content remained constant and linolenic acid (18:3) content increased during storage. Significant increases in both 18:2 and 18:3 UFA contents were detected in SA + UV-C-treated fruit after storage for 8 days (P< 0.05). The contents of both 18:2 and 18:3 UFAs of SA + UV-C-treated fruit were 16.11 and 3.52 g·100 g−1 on day 0 and reached to 20.78 and 9.76 g·100 g−1 on day 8, respectively. The ratio of UFA/SFA of the control fruit did not significantly change during storage for 8 days while that of the SA + UV-C-treated fruit was increased almost two times after storage for 8 days. The same trend of fatty acid composition was found in pulp of longan fruit as shown in . These suggest that SA + UV-C treatment induced the accumulation of UFAs in longan fruit peel leading to lower EL level () and lipid peroxidation () during cold storage. In a similar vein, certain previous works reported that both SA and UV-C treatment could induce proportion of UFAs in membrane lipids leading to chilling tolerance of plant during cold storage (Asghari and Aghdam, Citation2010; Martínez-Hernández et al., Citation2013; Supapvanich and Promyou, Citation2013). The increase in the proportion of UFAs in the membrane in which the marked increases of linoleic and linolenic acids in SA + UV-C-treated longan peel were found in this work can help maintain its liquid crystalline state phase and functions resulting in CI tolerance increase (Terrados and Lopez-Jimenez, Citation1996).
Table 1. Fatty acid composition (g 100 g−1) in the peel of longan fruit treated with 2.0 mM SA, 4.4 kJ·m−2 UV-C, incorporated with SA + UV-C and untreated fruit (control) during storage at 4°C for 8 days.
Table 2. Fatty acid composition (g 100 g−1) in the pulp of longan fruit treated with 2.0 mM SA, 4.4 kJ·m−2 UV-C, incorporated with (SA + UV-C) and untreated fruit (control) during storage at 4°C for 8 days.
DPPH Radical Scavenging Capacity
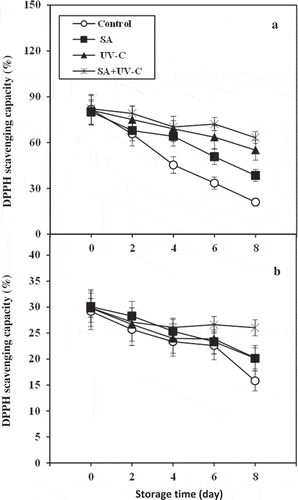
presents the change in free radical scavenging activity of longan fruit peel and pulp during cold storage. The result shows that free radical scavenging activity of the control fruit decreased and was lower than that of SA + UV-C-treated fruit throughout the storage. SA + UV-C treatment retained free radical scavenging activity of both peel and pulp which remained constant throughout the storage. The retained free radical scavenging activity by SA + UV-C treatment was associated with the lower browning incidence, EL level, MDA concentration, and LOX activity of longan fruit peel compared to the control fruit. Similarly, the low EL and LOX activity in fruit pulp were also concomitant with the retention of free radical scavenging activity by SA + UV-C treatment. Moreover, this also indicated that SA + UV-C treatment could maintain antioxidant activity in the fruit pulp which might be related to nutritional value retention. Many previous works confirmed that both SA and UV-C treatments can induce or retain antioxidant capacity of fruit and vegetables (Asghari and Aghdam, Citation2010; Liu et al., Citation2012; Promyou & Supapvanich, Citation2012; Supapvanich et al., Citation2017a; Citation2017b; Choi et al., Citation2015; Suiubon et al., Citation2017). Reactive oxygen species induced by chilling stress accelerates oxidative reactions including membrane lipid peroxidation in plant (Junmatong et al., Citation2015; Prasad, Citation1996). The enhancement of antioxidant capacity including free radical scavenging activity reduces oxidative stress and alleviates CI symptoms in fruit (Junmatong et al., Citation2015; Yang et al., Citation2012).
Conclusions
Peel browning of the longan fruit during cold storage could be retarded by the SA + UV-C treatment. The prevented peel browning by SA + UV-C treatment was associated with membrane integrity preservation due to lower EL level, MDA concentration, and LOX activity, induce proportion of UFAs, and maintain free radical scavenging activity. SA + UV-C treatment also reduced the increase in EL level and LOX activity and maintained free radical scavenging activity of the longan fruit pulp. These suggest that SA + UV-C treatment is an effective alternative alleviating CI symptom and maintaining nutritional quality of “Dor” longan fruit during cold storage.
Acknowledgments
The authors would like to acknowledge Postharvest Technology Innovation Center, Commission on Higher Education, Bangkok, and Kasetsart University Graduate School, Thailand, for providing grants.
References
- Aghdam, M.S., M. Asghari, O. Khorsandi, and M. Mohayeji. 2014. Alleviation of postharvest chilling injury of tomato fruit by salicylic acid treatment. J. Food Sci. Technol. 51:2815–2820. doi: 10.1007/s13197-012-0757-1.
- Alegria, C., J. Pinheiro, M. Duthoit, and E.M. Gonçalves. 2012. Fresh-cut carrot (cv. Nantes) quality as affected by abiotic stress (heat shock and UV-C irradiation) pre-treatments. LWT - Food Sci. Technol. 48:197–203. doi: 10.1016/j.lwt.2012.03.013.
- AOAC. 1995. Official methods of analysis. 17th ed. Association of Analytical Chemists International, Washington, DC.
- Asghari, M., and M.S. Aghdam. 2010. Impact of salicylic acid on post-harvest physiology of horticultural crops. Trends Food Sci. Technol. 21:502–509. doi: 10.1016/j.tifs.2010.07.009.
- Barka, E.A., S. Kalantari, J. Makhlouf, and J. Arul. 2000. Effects of UV-C irradiation on lipid peroxidation markers during ripening of tomato (Lycopersicon esculentum L.) fruits. Aust. J. Plant Physiol. 27:147–152.
- Batten, D.J. 1986. The longan. Aust. Hortic. 87:14–22.
- Cao, S., Z. Hu, Y. Zheng, and B. Lu. 2010. Synergistic effect of heat treatment and salicylic acid on alleviating internal browning in cold-stored peach fruit. Postharvest Biol. Technol. 58:93–97. doi: 10.1016/j.postharvbio.2010.05.010.
- Chen, J., P. Wen, W. Kong, Q. Pan, J. Zhan, J. Li, S. Wan, and W. Huang. 2006. Effect of salicylic acid on phenylpropanoids and phenylalanine ammonia-lyase in harvested grape berries. Postharvest Biol. Technol. 40:64–72. doi: 10.1016/j.postharvbio.2005.12.017.
- Choi, I., T.J. Yoo, and H. Kang. 2015. UV-C treatments enhance antioxidant activity, retain quality and microbial safety of fresh-cut paprika in MA storage. Hortic. Environ. Biotechnol. 56:324–329. doi: 10.1007/s13580-015-0141-y.
- Ding, Z., S. Tian, X. Zheng, Z. Zhou, and Y. Xu. 2007. Responses of reactive oxygen metabolism and quality in mango fruit to exogenous oxalic acid or salicylic acid under chilling temperature stress. Physiol. Plant. 130:112–121. doi: 10.1111/ppl.2007.130.issue-1.
- Erkan, M., S.Y. Wang, and C.Y. Wang. 2008. Effect of UV treatment on antioxidant capacity, antioxidant enzyme activity and decay in strawberry fruit. Postharvest Biol. Technol. 48:163–171. doi: 10.1016/j.postharvbio.2007.09.028.
- González-Aguilar, G.A., C.Y. Wang, and G.J. Buta. 2004. UV-C irradiation reduces breakdown and chilling injury of peaches during cold storage. J. Sci. Food Agric. 84:415–422. doi: 10.1002/jsfa.1675.
- González-Aguilar, G.A., R. Zavaleta-Gatica, and M.E. Tiznado-Hernández. 2007. Improving postharvest quality of mango ‘Haden’ by UV-C treatment. Postharvest Biol. Technol. 45:108–116. doi: 10.1016/j.postharvbio.2007.01.012.
- Heath, R.L., and L. Packer. 1968. Photoperoxidation in isolated chloroplasts: II. Role of electron transfer. Arch. Biochem. Biophys. 125:850–857. doi: 10.1016/0003-9861(68)90523-7.
- Jaroenwanit, P., V. Srijesdaruk, and T. Ngarmsak. 2001. A preliminary study for longan variety and export markets identification. Khon Kean Univ. Res. J. 6:87–95.
- Jiang, Y., Z. Zhang, D.C. Joyce, and S. Ketsa. 2002. Review Postharvest biology and handling of longan fruit (Dimocarpus longan Lour.). Postharvest Biol. Technol. 26:241–252. doi: 10.1016/S0925-5214(02)00047-9.
- Junmatong, C., W. Chomkitichai, J. Uthaibutra, K. Saengnil, and D. Boonyakiat. 2015. Reduction of free radical content and chilling injury in ‘Nam Dok Mai No. 4ʹ mango fruit with methyl jasnonate during low temperature storage. Acta Hortic. 1088:107–112. doi: 10.17660/ActaHortic.2015.1088.13.
- Korkina, L.G. 2007. Phenylpropanoids as naturally occurring antioxidants: From plant defense to human health. Cell. Mol. Biol. 53:15–25.
- Lara, I., R.M. Miro, T. Fuentes, G. Sayez, J. Graell, and M.L. Lopez. 2003. Biosynthesis of volatile aroma compounds in pear fruit stored under long-term controlled-atmosphere conditions. Postharvest Biol. Technol. 29:29–39. doi: 10.1016/S0925-5214(02)00230-2.
- Liu, C., L. Cai, X. Lu, X. Han, and T. Ying. 2012. Effect of postharvest UV-C irradiation on phenolic compound content and antioxidant activity of tomato fruit during storage. J. Integr. Agric. 11:159–165. doi: 10.1016/S1671-2927(12)60794-9.
- Martínez-Hernández, G.B., F. Artés-Hernández, P.A. Gómez, and F. Artés. 2013. Combination of electrolysed water, UV-C and super atmospheric O2 packaging for improving fresh-cut broccoli quality. Postharvest Biol. Technol. 76:125–134. doi: 10.1016/j.postharvbio.2012.09.013.
- Mo, Y., D. Gong, G. Liang, R. Han, J. Xie, and W. Li. 2008. Enhanced preservation effects of sugar apple fruits by salicylic acid treatment during post-harvest storage. J. Sci. Food Agric. 88:2693–2699. doi: 10.1002/jsfa.v88:15.
- Nanjo, F., K. Goto, R. Seto, M. Suzuki, and M. Sakai. 1996. Scavenging effects of tea catechins and their derivatives on 1, 1-diphenyl-2-picrylhydrazyl radical. Free Radical Biol. Med. 21(6):895–902.
- Noichinda, S., K. Bodhipadma, P. Tusvil, U. Sathitwiangthong, T. Sangudom, and S. Ketsa. 2015. The Physiology of chilling injured longan fruit. J. Appl. Sci. 14:1–8.
- Office of Agricultural Economics. (2016). Total and export values of agricultural goods. 6 Dec. 2016. http://www.oae.go.th/oae_report/export_import/export.php.
- Partridge, I. 1997. Tropical fruit becomes major industry. Rural Res. 74:9–12.
- Pongprasert, N., Y. Sekozawa, S. Sugaya, and H. Gemma. 2011. A novel postharvest UV-C treatment to reduce chilling injury (membrane damage, browning and chlorophyll degradation) in banana peel. Sci. Hortic. 130:73–77. doi: 10.1016/j.scienta.2011.06.006.
- Prasad, T.K. 1996. Mechanisms of chilling-induced oxidative stress injury and tolerance in developing maize seedlings: Changes in antioxidant system, oxidation of proteins and lipids, and protease activities. Plant J. 10:1017–1026. doi: 10.1046/j.1365-313X.1996.10061017.x.
- Promyou, S., S. Ketsa, and W.G. van Doorn. 2012. Salicylic acid alleviates chilling injury in anthurium (Anthurium andraeanum L.) flowers. Postharvest Biol. Technol. 64:104–110. doi: 10.1016/j.postharvbio.2011.10.002.
- Promyou, S., and S. Supapvanich. 2012. Effect of ultraviolet-C (UV-C) illumination on postharvest quality and bioactive compounds in yellow bell pepper fruit (Capsicum annuum L.). during storage. Afr. J. Agric. Res. 7:4084–4096. doi: 10.5897/AJAR12.242.
- Promyou, S., and S. Supapvanich. 2013. Chilling injury alleviation in ‘Golden’ bell sweet pepper caused by UV-C treatment. Acta Hortic. 1011:357–362. doi: 10.17660/ActaHortic.2013.1011.45.
- Promyou, S., and S. Supapvanich. 2016. Effects of salicylic acid immersion on physicochemical quality of Thai papaya fruit ‘Kaek Dam’ during storage. Acta Hortic. 1111:105–112. doi: 10.17660/ActaHortic.2016.1111.16.
- Sivakumar, D., L.A. Terry, and L. Korsten. 2010. An overview on litchi fruit quality and alternative postharvest treatments to replace sulphur dioxide fumigation. Food Rev. Int. 26:162–188. doi: 10.1080/87559121003590516.
- Stevens, J.L., C.L. Wilson, J.Y. Lu, V.A. Khan, E. Chalutz, S. Droby, M.K. Kabwe, Z. Haung, O. Adeyeye, P.L. Pusey, et al. 1996. Plant hormesis induced by ultraviolet light-C for controlling postharvest diseases of tree fruits. Crop Prot. 15:129–134. doi: 10.1016/0261-2194(95)00082-8.
- Suiubon, S., S. Supapvanich, and S. Promyou. 2017. Postharvest quality maintenance of longan fruit by ultra violet-C incorporated with salicylic acid application. Emir. J. Food Agric. 29(3):179–187. doi: 10.9755/ejfa.
- Supapvanich, S., and S. Promyou. 2013. Efficiency of salicylic acid application on postharvest perishable crops, p. 339–355. In: S. Hayat and A.A.M. Alyemei (eds.). Salicylic acid: Plant growth and development. Springer, New York, USA.
- Supapvanich, S., B. Mahasap, P. Boonyaritthongchai, C. Techavuthiporn, R. Tepsorn, and P. Youryon. 2017b. Salicylic acid immersion maintains physiochemical quality and enhances bioactive compounds in ‘Kimju’ guava fruit during cold storage. Emir. J. Food Agric. 29:620–628. doi: 10.9755/ejfa.2017-07-1551.
- Supapvanich, S., P. Mitsang, and P. Youryon. 2017a. Preharvest salicylic acid application maintains physicochemical quality of ‘Taaptimjaan’ wax apple fruit (Syzygium samarangenese) during short-term storage. Sci. Hortic. 215:178–183. doi: 10.1016/j.scienta.2016.11.046.
- Supapvanich, S., R. Phonpakdee, and P. Wongsuwan. 2015. Chilling injury alleviation and quality maintenance of lemon basil by preharvest salicylic acid treatment. Emir. J. Food Agric. 27:801–807. doi: 10.9755/ejfa.2015-08-572.
- Terrados, J., and J.A. Lopez-Jimenez. 1996. Fatty acid composition and chilling resistance in the Caulerpa prolifera (Forrskal), Lamouroux (Chlorophyta, Caulerpales). Biochem. Mol. Biol. Int. 39:863–869.
- Tongdee, S.C. 1997. Longan, p. 335–345. In: S.K. Mitra (ed.). Postharvest physiology and storage of tropical and subtropical fruits. CAB International, Wallingford, UK.
- Vincente, A.R., C. Pineda, L. Lemoine, P.M. Civello, G.A. Martinez, and A.R. Chaves. 2005. UV-C treatments reduce decay, retain quality and alleviate chilling injury in pepper. Postharvest Biol. Technol. 35:69–78. doi: 10.1016/j.postharvbio.2004.06.001.
- Yang, Q., J. Rao, S. Yi, K. Meng, J. Wu, and Y. Hou. 2012. Antioxidant enzyme activity and chilling injury during low-temperature storage of kiwifruit cv. Hongyang exposed to gradual postharvest cooling. Hortic. Environ. Biotechnol. 53:505–512. doi: 10.1007/s13580-012-0101-8.