ABSTRACT
Guava is one of the most important tropical fruit crops; however, its cultivation has been affected by the prevalence of the nematode Meloidogyne enterolobii, which leads to a large-scale reduction in the production of fruits and restricts new plantations in the contaminated areas. The discovery of the resistance of Psidium cattleianum to this nematode has increased the possibility of its selective use as rootstock for guava; a few studies have worked on the graft; however, the rootstock exhibits a high incompatibility with the commercial varieties of guava. Therefore, the present study aimed to develop an in vitro-grafting technique of guava scions under rootstocks of P. cattleianum obtained using an in vitro seed germination method. The successful in vitro-grafting was performed using semisolid MS1/2 (Murashige and Skoog) culture medium with 0.5 mg L−1 of Benzyladenine. P. cattleianum with approximately 3 cm length was used as rootstocks, and apical guava stems with 0.5 cm length were used as scions. The grafting procedure used was wedge grafting. To increase the success rate of in vitro-grafting, we used a sterile paper moistened with sterilized distilled water to prevent dehydration. The use of MS1/2 and the wedge grafting resulted in the overall success rate of 30% for the graft between P. cattleianum and Psidium guajava. In conclusion, this is the first report of successful in vitro-grafting between guava and P. cattleianum.
Introduction
Guava (Psidium guajava L.) is one of the most widely cultivated tropical fruit throughout the world. The fruits are important for both in natura consumption and fruit processing. It is grown majorly in tropical regions, and Brazil is one of the largest producers of this fruit (Altoéet al., Citation2011).
The evidence indicates that the prevalence of the nematode Meloidogyne enterolobii, also known as the root-knot nematode, is the major harmful factor affecting the guava production in Brazil (Almeida et al., Citation2006; Tigano et al., Citation2010). Nevertheless, it has also been reported from other countries where this factor is also affecting various other species (Kiewnick et al., Citation2009).
This nematode destroys the entire root system of the plant, causing impairment in the nutrient absorption and therefore affecting all productive performance of the species that may eventually lead to death (Siqueira et al., Citation2009). At present, the control of this nematode, at least for other species, e.g., sweet potato, is based primarily on phytosanitary measures and the use of chemicals to reduce the population of nematodes (Quesada-Ocampo, Citation2018), however, with unsatisfactory results.
Alternatively, the characterization of Psidium cattleianum as resistant to M. enterolobii (Carneiro et al., Citation2007) and its genetic similarity with guava suggested new alternatives to resolve this problem by the development of new effective technique using this species as a rootstock for guava susceptible varieties (Martins et al., Citation2013).
Nevertheless, a majority of the previous reports have indicated unsatisfactory results on grafting, despite a large number of studies on P. cattleianum accessions as a source of resistance and with 71% genetic similarity as demonstrated by Souza et al. (Citation2014). Moreover, studies carried by de Almeida et al. (Citation2008) evaluated different grafting methods (green strip budding, whip grafting, and the top cleft grafting) have demonstrated incompatibility between the guava cv. Paluma scion and the P. cattleianum rootstock. Similar results on the incompatibility of P. cattleianum with P. guajava were also observed (Robaina et al., Citation2015).
To address these challenges, the development of an efficient in vitro grafting system, which includes younger tissues under controlled environmental conditions in the growth chamber, seems to be potential and has successfully been utilized in different types of fruit species (Cardoso et al., Citation2012; Miguelez-Sierra et al., Citation2017).
Therefore, the present study aimed to establish a novel technique for in vitro grafting of guava and P. cattleianum, an approach that has not yet been adequately reported in the literature. This method may help overcome the damages caused by the nematode, representing one of the major concerning issues, limiting the production of guava fruit in tropical region.
Material and Methods
The experiment was carried out in two steps as described follow; in the first step, the in vitro-germinated seedlings were used as rootstocks and scions that were followed by the second step of the in vitro-grafting method. In vitro grafting was performed by grafting the scion onto an in vitro germinated seedling.
To obtain plant material, seeds from the genotype of yellow-fruit-type P. cattleianum were collected from plants cultivated under field conditions in Taquaritinga, SP, Brazil being characterized by its resistance to the nematode M. enterolobii, confirmed by Almeida et al. (Citation2009). The guava seeds were collected from cultivar Século XXI cultivated under greenhouse conditions in Araras, SP, Brazil.
The seeds of P. cattleianum were sterilized by immersing in 70% alcohol for 20 s, followed by immersion in a sodium hypochlorite solution (1.0–1.25% active chlorine) for 20 min with the addition of three drops of Tween-20 (Sigma-Aldrich, USA) per 100 mL solution. Then, they were washed three times with sterile distilled water and inoculated in flasks containing 30 mL of Murashige and Skoog (MS) culture medium (Murashige and Skoog, Citation1962), containing half of the macronutrients concentrations (MS1/2) and supplemented with 15 g L−1 of sucrose, 0.1 g L−1 of myo-inositol, and solidified with 7.0 g L−1 agar (Cardoso et al., Citation2017). The pH of the culture medium was adjusted to 5.8 before autoclaving at 121°C for 20 min. The cultures were grown under a 14-h photoperiod and at a temperature of 25 ± 2°C with a light intensity of 20–25 μM m−2 s−1 provided by cool-white fluorescent lamps.
The germination rate of P. cattleianum and the contamination rate of the inoculated seeds were monitored weekly through the counting of germinated seeds in each flask. Seeds were considered germinated when the roots and hypocotyls were emerged from the seeds. Around 50 seeds for each species were used for germination, containing each flask 10 seeds.
Shoot segments were obtained from previously germinated guava seeds (cv. Século XXI × self) to provide the scions for in vitro grafting, following the same procedures as described for P. cattleianum. After the germination, the epicotyls with single nodal segments of 1.0 cm length were inoculated in MS1/2 containing 0.5 mg L−1 Benzyladenine (BA), 30 g L−1 sucrose, 0.1 g L−1 myo-inositol, and solidified using 7.0 g L−1 agar. The pH of the medium was adjusted to 5.8 ± 0.1 prior to the addition of the agar and then autoclaved at 120°C and pressure of 1.0 kg f cm−2 for 20 min. This culture medium was tested and used in our laboratory conditions for multiplication of apical shoots of P. guajava and P. cattleianum aiming to obtain a high quantity of scions for in vitro-grafting procedures.
The in vitro grafting was performed using P. cattleianum as rootstock and guava as the scion. All stages of the in vitro-grafting procedures were carried out under aseptic conditions in a laminar flow hood and the plant material was handled with sterilized razor blade and forceps.
The shoot segments of guava (30 mm in length and 2 mm in diameter) were placed on a filter paper moistened with sterile distilled water (to avoid dehydration) and was excised approximately 8–10 mm of apical stem region (). Thus, the rootstocks of P. cattleianum consisted of the hypocotyl length of about 15 ± 2 mm and root length of 15 ± 2 mm.
Figure 1. The procedure of in vitro grafting between P. guajava cv. Século XXI and P. cattleianum. P. guajava cv. Século XXI micropropagated in vitro in MS1/2 containing 0.5 mg L-1 BA (a1) and isolated shoot (a2) with apical shoot excision as for use as scion (a3). In vitro germinated P. cattleianum seedlings (b1) at moment of in vitro grafting (b2) and the use of hypocotyl and root as rootstock (b3). In vitro-grafted plantlets of P. guajava/P. cattleianum (c) with details of excision of P. cattleianum apical stem to be used as rootstock with a vertical slit made at the top and stem apex to be used as scion with a “v-shape” cut made at the basal shoot (d) (i.e., in vitro grafting).
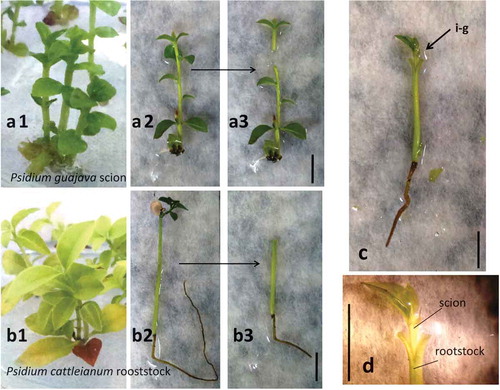
The slit method was used for in vitro grafting, in which a vertical slit (approximately 2–3 mm in length) was made at the top which is ready to receive the graft (). A cut of the terminal portion of the root system of the seedling was also performed to facilitate its inoculation in the flasks containing culture medium after the in vitro-grafting procedure ().
Apical shoots of guava were excised with about 5 ± 1 mm in length containing a part of the stem, and at least one pair of expanded leaves was used as scions. The lower leaves were removed, and the grafting procedure was performed by making a “v-shape” cut at the basal shoot segments (), exposing the vascular region of the scion. Subsequently, a vertical cut was made in the rootstock of P. cattleianum with a depth similar to that of the scion slit to allow the two parts to fit together.
After grafting, in vitro-grafted plants () were inoculated in MS1/2, 30 g L−1 sucrose, 0.1 g L−1 of myo-inositol, and solidified with 7.0 g L−1 agar and pH of the medium were adjusted before addition of the agar to 5.8 ± 0.1. The flasks were then sealed with polyvinyl chloride (PVC) plastic film and maintained in the culture room under similar conditions as described above. A total of 30 in vitro-grafting were realized, with 6 flasks containing 5 in vitro-grafted plantlets each.
The most vigorous and well-positioned developed bud sprouts were selected and removed from the flasks, washed with running tap water to remove the culture medium, and placed into plastic cups filled with vermiculite substrate. Afterward, they were kept in a growth chamber at a temperature of 25 ± 2°C under the photoperiod of 16 h in white fluorescent light (35–40 µmol m−2 s−1) for 30 days before greenhouse cultivation.
The percentages of survival of in vitro-grafts and their vegetative development were evaluated during in vitro and ex vitro transfer (45 days after in vitro-grafting) and after 60 days of cultivation in greenhouse conditions to evaluate the survival capacity of guava and their development when in vitro grafted using rootstocks of P. cattleianum. Furthermore, to observe the formation of microcallus in the region of grafting, the in vitro-grafted plantlets were recorded under stereoscopy.
Results and Discussion
The aseptic procedure followed for the establishment of in vitro culture of P. cattleianum seeds was efficient and able to eliminate all possible contaminants, leading to a contamination rate of nearly 0%. Moreover, this aseptic procedure did not affect the germination potential, which was 100% after 60 days of inoculation. Similarly, Cardoso et al. (Citation2017) also observed a rapid (after 60–70 days) and a high percentage of germination (above 80%), when seeds were obtained from mature green fruits (C5 stage) and cultivated in MS1/2 with 0–15 g L−1 of sucrose. Besides for guava, which served as scion, the multiplication of in vitro nodal segments was successfully observed, with a high propagation rate and shoot development, using MS1/2 culture medium supplemented with 0.5 mg L−1 of BA.
According to Hussain et al. (Citation2014), for the development of a micrograft under in vitro conditions, the first step is the establishment of micropropagation protocol of the species.
All the preliminary experiments were conducted under aseptic conditions in a laminar flow chamber, wherein the seedlings were used as graft and rootstocks were cut directly in sterilized Petri dishes which resulted in the death of the guava scions, mainly due to dehydration and oxidation. The detachment of scions from their rootstocks limited the success of in vitro grafting and resulted in an initial success rate of 0–5% with more than 50 in vitro grafting procedures (data not presented).
Therefore, to reduce the dryness and oxidation of the plant tissue, some modifications were attempted. These included the use of Petri dishes containing filter paper moistened with autoclaved distilled water and providing a less aggressive environment during the in vitro-grafting procedure. Thus, a significant increase in the survival rate was observed, which varied during the different periods in the order they were carried out, approaching a success rate of 50% in in vitro grafting. Following nearly 100 in vitro-grafting procedures under similar conditions, the mean success in vitro-grafts rate obtained was 30%.
After 15 days of the in vitro-grafting procedure, the two main types of response in in vitro grafts observed were the absence of any connection between the rootstock and the scion, with necrosis, and death of the graft (). Furthermore, the maintenance of live in vitro grafts with green scions, in which the growth of scion was also observed, resulted from the graft connection ().
Figure 2. Plantlet development after in vitro-grafting: (a) lack of connection resulting in scion death, (b) presence of connection with scion stem and leave growth, (c) stereomicroscopy observation of microcallus (mc) developed in the guava scion at graft region, (d) 60 days after acclimatization of grafted plantlet, and (e) graft cicatrized (grc) connection of ex vitro acclimatized plantlets.
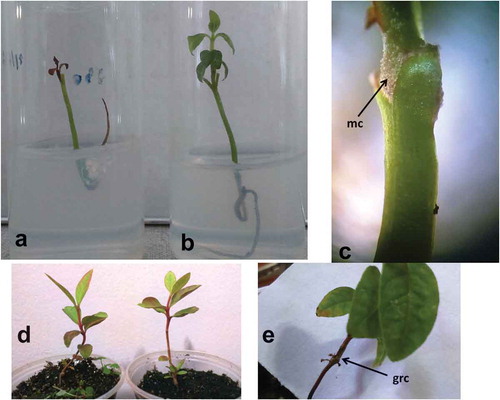
Furthermore, various factors affect the success of in vitro-grafting. Of note, the reestablishment of vascular continuity is essential for the grafting region as this union is required for the translocation and assimilation of water, minerals, and hormones to the scion. Although the in vitro environmental conditions have high air humidity, the absence of nutrients and water deficiencies may affect the success rate as the scion is not in direct contact with the culture media. Thus, necessitating some minimal connection between the scion and the rootstock to prevent the excessive dehydration and nutrient deficiencies, this may influence the efficiency of in vitro-grafting method from the day 1 of scion development under the new conditions. According to Badalamenti et al. (Citation2016), besides the growth requirements, in the micrografting technique, the rootstock also provides necessary plant hormones required by the scion.
After 2 months of in vitro grafting, an initial connection between the rootstock and the scion was observed through a stereomicroscope, revealing the development of microcallus in the region of the graft, between the scion and the rootstock (). This observation was only possible in the scions with green tissues that resulted in the production of new leaves after in vitro graft, mean of 2.8 leaves/scion compared with at moment of graft (1.0–2.0 leaves/scion). This latter characteristic of production of new leaves, together with increases in another parameters of the in vitro-grafted plantlets, as example the scion height from 5.0 to 17.4 mm and leaf expansion/elongation, from 0.4 to 10.6 mm in length, and from 0.28 to 4.6 mm in diameter (), supports the hypothesis of a vascular connection between the rootstock and the scion. Also, the rootstock presented the growth of roots from 15 to 23.6 mm in 60 days from in vitro-grafting procedures.
Table 1. Percentage of live and vegetative development of in vitro micrografts of guava (Psidium guajava) upon Psidium cattleianum rootstocks.
Vascular elements are regenerated through a complicated process, which includes the tissue differentiation during which calluses are formed from the proliferation of the cells of the parenchyma tissue of the plant completing the spaces between the two grafted tissues, providing continuity of the vascular tissue after the differentiation of its cells. Thus, low success rates of grafting and compatibility between the scion and the rootstock are directly correlated to low callus formation at the graft junction (Fragoso et al., Citation2011).
Nevertheless, callus formation is a prerequisite for successful graft union formation and therefore a close contact between the rootstock and scion is essential for tissue fusion and callus formation. According to Pathirana and Mckenzie (Citation2005), the success of grafts in grapes (Vitis sp.) depends heavily on careful handling and positioning of the scion on the rootstock cleft. All grafts that were in a correct orientation with a contact between vascular tissues of the two species were able to produce callus in the graft junction and consequently resulted in successful grafts.
In spite of the difficulties encountered during the in vitro-grafting procedure, due to the very small size and diameter of the materials used, the clefts of the rootstock that was in most of the conditions (>80% of the grafts realized) were able to hold the scion without using any other support or application of a constituent at the graft junction.
Furthermore, the elongation of the scions stems, from 17.4 to 47.5 mm, and leaves, from 10.6 to 24.8 mm (), and the formation of new leaves (2.8–8.3/plantlet) after 60-days of acclimatization (), similar to non-grafted seedlings of guava, was noticed. After the acclimatization of the successful in vitro grafts at growth chamber. (30 days) and in greenhouse conditions (after 60 days of transferring to ex vitro conditions), it further supported the hypothesis of the tissue fusion and the reestablishment of the vascular continuity.
However, there is still a research gap to be filled in with new studies that include the improvement of this technique aiming to enhance the success rate of in vitro grafting. Despite the low success rate of 30% of in vitro-grafting procedure, this is the first report of in vitro-grafting between guava and P. cattleianum.
None of the previous studies on the graft of guava in P. cattleianum rootstocks resulted in viable plants. In most cases, the death of commercial guava plantlets due to the incompatibility issues between scion and the accessions of the rootstock of P. cattleianum was noted (Carneiro et al., Citation2007; de Almeida et al., Citation2008; Robaina et al., Citation2015, Citation2012). The study realized by Robaina et al. (Citation2015) obtained viable grafts, but authors reported later incompatibility (after 1-year cultivation) between guava “Paluma” and P. cattleianum. Recently, there was reported the intraspecific mini-grafting of P. guajava and ''Psidium''/ p. guineense, with efficiency of 52% and 82%, using guava “Cortibel 1”/Psidium guineense and guava “Paluma”/P. guineense, respectively, showing the capacity of guava to graft with another species of Psidium (Campos et al., Citation2017). In our study, after 1-year of in vitro-grafting of P. guajava/P. cattleianum in greenhouse conditions, we also noted an excessive swelling of the guava graft region, compared with the P. cattleianum rootstock, also suggested a possible later incompatibility of the species.
Nevertheless, another alternative strategy to control this nematode exists including the hybridization of guava with resistant Psidium species (Cardoso et al., Citation2017). However, only one genotype called “‘BRS Guaraçá” (Embrapa), a hybrid between P. guajava × P. guineense, has been registered (Cultivarweb, Citation2017). The hybrids between P. guajava and P. cattleianum were not reported; nonetheless, the germination of pollen tubes and the obtaining fruits from this crossing were recently reported (Cardoso et al., Citation2017).
In conclusion, the use of resistant varieties/species is the most efficient technique for control this nematode, and this is the first report of success of in vitro-grafting between guava and P. cattleianum. The use of seedlings proves the viability of in vitro-grafting of juvenile tissues of guava with P. cattleianum rootstock, also requiring tests with micrografting using adult tissues of commercial guava upon P. cattleianum and tests with nematode infection to confirm the resistance to the nematode of grafted plantlets.
Conflict of interest
Authors declares no conflict of interests
Acknowledgments
The authors thanks to São Paulo Research Foundation (FAPESP) for the processes number 2013/07094-0 and 2016/07571-1. The authors also thanks Eduardo José de Almeida (UEMG), Evandro Henrique Schinor (DBPVA, CCA/UFSCar), and José Mauro da Silva (Mauro Mudas, Taquaritinga-SP) for intellectual contributions about the occurrence of M. enterolobii in guava and the plant material source.
Additional information
Funding
References
- Almeida, E.J., A.B.G. Martins, and J.M. Santos. 2009. Resistência de goiabeiras e araçazeiros a Meloidogyne mayaguensis e estudo da compatibilidade na enxertia. Pesq. Agropec. Bras. 44(4):421–423. doi: 10.1590/S0100-204X2009000400014.
- Almeida, E.J., P.L.M. Soares, J.M. Santos, and A.B.G. Martins. 2006. Ocorrência de Meloidogyne mayaguensis na cultura da goiaba (Psidium guajava) no estado de São Paulo. Nemat. Bras. 30:112–113. in portuguese with abstract in english.
- Altoé, J.A., C.S. Marinho, M.I.C. Terra, and D.G. Barroso. 2011. Propagação de araçazeiro e goiabeira via miniestaquia de material juvenil. Bragantia. 70(2):312–318. in portuguese with abstract in english doi:10.1590/S0006-87052011000200009.
- Badalamenti, O., A. Carra, E. Oddo, F. Carimi, and M. Sajeva. 2016. Is in vitro micrografting a possible valid alternative to traditional micropropagation in Cactaceae? Pelecyphora aselliformis as a case of study. Springerplus. 5:201. doi: 10.1186/s40064-016-1901-6.
- Campos, G.S., C.S. Marinho, C.R. Portella, B.D. Amaral, and W.S.G. Carvalho. 2017. Production of guava mini-grafted on intra and interespecific rootstock. Rev. Bras. Frut. 39(1):e635. doi: 10.1590/0100-29452016357.
- Cardoso, J.C., B.T. Costa, and E.J. Almeida. 2017. Pollination and in vitro germination of seeds for interspecific hybridization of Psidium guajava and Psidium cattleianum. Euphytica. 213:146. doi: 10.1007/s10681-017-1929-x.
- Cardoso, J.C., A.P. Martinelli, and R.R. Latado. 2012. Somatic embryogenesis from ovaries of sweet orange cv.Tobias. Plant Cell, Tiss. Organ. Cult. 109:171–177. doi: 10.1007/s11240-011-0073-x.
- Carneiro, R.M.D.G., P.A. Cirotto, A. Quintanilha, D.B. Silva, and R.G. Carneiro. 2007. Resistance to Meloidogyne mayaguensis in Psidium spp. accessions and their grafting compatibility with P.guajava cv. Paluma. Fitopat. Bras. 32:281–284. doi: 10.1590/S0100-41582007000400001.
- CULTIVARWEB. 2017. Ministério da Agricultura, Pecuária e Abastecimento - RNC. 17 Jul. 2018. http://extranet.agricultura.gov.br/php/snpc/cultivarweb/cultivares_registradas.php.
- de Almeida, E.J., P.L.M. Soares, A.R. Da Silva, and J.M. Dos Santos. 2008. Novos registros sobre Meloidogyne mayaguensis no Brasil e estudo morfológico comparativo com M. incognita. Nemat. Bras. 32:236–241.
- Fragoso, V., H. Goddard, I.T. Baldwin, and S.G. Kim. 2011. A simple and efficient micrografting method for stably transformed Nicotiana attenuata plants to examine shoot-root signaling. Plant Methods. 7:34. doi: 10.1186/1746-4811-7-34.
- Hussain, G., M.S. Wani, M.A. Mir, Z.A. Rather, and K.M. Bhat. 2014. Micrografting for fruit crop improvement. Afr. J. Biotech. 13:2474–2483. doi: 10.5897/AJB2013.13602.
- Kiewnick, S., M. Dessimoz, and L. Franck. 2009. Effects of the Mi-1 and the Nroot-knot nematode-resistance gene on infection and reproduction of Meloidogyne enterolobii on tomato and pepper cultivars. J. Nemat. 41(2):134–139.
- Martins, L.S.S., R.S. Musser, A.G. Souza, A.G. Resende, L. Vilela, and W.R. Maluf. 2013. Parasitismo de Meloidogyne enterolobii em espécies de myrtaceae. Ver. Bras. Frutic.. 35(2):477–484. in portuguese with abstract in english doi:10.1590/S0100-29452013000200017.
- Miguelez-Sierra, Y., A. Hernández-Rodríguez, Y. Acebo-Guerrero, M. Baucher, and M. El Jaziri. 2017. In vitro micrografting of apical and axillary buds of cacao. J. Hort. Sci. Biotechnol. 92(1):25–30. doi: 10.1080/14620316.2016.1215231.
- Murashige, T., and F. Skoog. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473–497. doi: 10.1111/ppl.1962.15.issue-3.
- Pathirana, R., and J. Mckenzie. 2005. Early detection of grapevine leafroll virus in Vitis vinifera using in vitro micrografting. Plant Cell Tiss. Organ Cult.. 81:11–18. doi: 10.1007/s11240-004-2498-y.
- Quesada-Ocampo, L. 2018. Management of the guava root knot nematode in sweetpotato. NC State Extension. 21 Dec. 2018 go.ncsu.edu/readext?557429.
- Robaina, R.R., G.S. Campos, C.S. Marinho, R.M. Souza, R. Moreira, and C.A. Bremenkamp. 2015. Grafting guava on cattley guava resistant to Meloidogyne enterolobii. Cienc. Rural. 45(9):1579–1584. in portuguese with abstract in english. doi:10.1590/0103-8478cr20131412.
- Robaina, R.R., C.S. Marinho, R.M. Souza, and G.S. Campos. 2012. Subenxertia da goiabeira ‘Paluma‘ com araçazeiros resistentes a Meloidogyne enterolobii (syn. M. mayaguensis). Rev. Bras.Frut. 34:951–955. in portuguese with abstract in english. doi: 10.1590/S0100-29452012000300041.
- Siqueira, K.M.D., V.M. Freitas, M.R.A. Almeida, M.F.A. Santos, J.A. Cares, M.S. Tigano, and R.M.D.G. Carneiro. 2009. Detecção de Meloidogyne mayaguensis em goiabeira e mamoeiro no estado de Goiás, usando marcadores moleculares. Trop. Plant Pathol. 34(4):256–260. in portuguese with abstract in english doi:10.1590/S1982-56762009000400009.
- Souza, A.G., L.V. Resende, I.P. Lima, R.M. Santos, and N.N.J. Chalfun. 2014. Variabilidade genética de acessos de araçazeiro e goiabeira suscetíveis e resistentes a Meloidogyne enterolobii. Cienc. Rural. 44(5):822–829. in portuguese with abstract in english doi:10.1590/S0103-84782014000500010.
- Tigano, M., K. Siqueira, P. Castagnone-Sereno, K. Mulet, P. Queiroz, M. Dos Santos, C. Teixeira, M. Almeida, J. Silva, and R. Carneiro. 2010. Genetic diversity of the root-knot nematode Meloidogyne enterolobii and development of a SCAR marker for this guava-damaging species. Plant Pathol. 59:1054–1061. doi: 10.1111/j.1365-3059.2010.02350.x.
