 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Botryosphaeriacean fungi usually cause mango rot in the tropics. They cause quiescent infections, and symptoms are detectable only in advanced stages of fruit ripening, imposing that control strategies begin in preharvest. Biocontrol is one of the few alternatives to control postharvest decay of mango in organic or biological production. However, there is very few research specifically designed for organic mango production. The objective of this study was to evaluate four yeast strains applied in individual formulations to control postharvest decay of mango, as part of the integrated management of postharvest fruit rot decay using preharvest spraying in an organic orchard. In a first experiment, the antagonist yeast strains Saccharomyces cerevisiae ESA45, Saccharomyces sp. ESA46, Saccharomyces sp. ESA47, and Pichia kudriavzevii CMIAT171 were applied to artificial wounds in organic mango fruits inoculated with propagule suspensions of virulent strains of Lasiodiplodia theobromae and Neofusicoccum parvum. All treatments significantly increased the period until the detection of rot symptoms and reduced rot severity. Formulations containing starch+carboxymethyl celullose and the yeast strains were applied in two different production cycles (2014 and 2015/16) in a semi-commercial organic orchard. All treatments significantly reduced mango rot. Field spraying of Pichia kudriavzevii CMIAT171 reduced disease index in 69.4% in an average for the two years.
Introduction
Postharvest decay causes severe losses in the fruit and vegetable segments around the world. It can reach up to 30% in tropical and developing countries (Donnell et al., Citation2015; Mutungi, Citation2015). Primary causes are related to deficient preharvest control of pathogens, mechanical damage during harvest and transport, and inappropriate storage (Snowdon, Citation2010). Postharvest decay has been a major problem in the mango production chain, and control strategies commonly rely on the integrated use of cold storage and application of synthetic fungicides in preharvest spraying and postharvest processing (Sales Júnior et al., Citation2009). Colletotrichum spp Pens. and fungi belonging to the Botryopheriacea family as Fusicoccum aesculi Corda (1829), Lasiodiplodia theobromae (Pat.) Griffon & Maubl. (1909), and Neofusicoccum parvum (Pennycook & Samuels) Crous cause most important postharvest mango fruit rot in tropical regions (Costa et al. Citation2010; Lima et al., Citation2013). Infections can occur during fruit development, even in flowering, remaining quiescent until fruit ripening (Prusky et al., Citation2013). Stem-end rot caused by L. theobromae and Dothiorella spp., and Anthracnose (Colletotrichum spp.) causes losses commonly around 15%, reaching even up to 50% in mango production chain around the world (Singh et al., Citation2013)
Organic agriculture does not imply production without technology, but a combination of traditional and scientific knowledge in search of sustainable production systems (Konvalina, Citation2016). A set of practices aiming the adequate soil and nutrient management, reduction of physiological stress, and construction of suppressive environment for harmful organisms can reduce the occurrence of phytosanitary problems in organic production (Finckh et al., Citation2015). However, the maturation process increases fruit susceptibility to postharvest rots during transport, storage, and commercialization (Seymour et al., Citation2013).
Control of stem-end rot usually relies on the application of fungicides such as propiconazole, prochloraz, and hexaconazole, and also using hydrothermal treatment (Singh, Citation2011; Singh et al., Citation2013). However, in addition to the general concern about contamination of fresh fruit by pesticide residues, the employment of synthetic fungicides is strictly forbidden in organic production. Some alternatives as immersion in salt solutions (such as calcium chloride), application of essential oils and edible films, hydrothermal treatment, application of ionizing radiation (gamma and ultraviolet) have been evaluated with different degrees of success (Lobo and Sidhu, Citation2017).
A significant increase in the use of biological control agents (BCA) of postharvest decay was seen in recent years (Wisniewski et al., Citation2016). Among them, yeast BCAs received great attention because of their easy management, control efficiency, natural occurrence on fruit surfaces, and several biotechnological applications that allowed the accumulation of know-how on fermentative processes and large-scale production of yeast biomass (Droby et al., Citation2016). Their potential to control postharvest rot has been shown for different crops, such as pear, apple, citrus, and grapes (Dal Bello et al., Citation2008; Zhang et al., Citation2009; Robiglio et al., Citation2011; Parafati et al., Citation2015).
Gava et al. (Citation2018) selected a group of yeast strains obtained from ripe fruits of native and cultivated plant species from the Brazilian semi-arid region, showing their potential for postharvest application in controlling mango decay. However, the occurrence of quiescent infections in organic mango fruits requires that biocontrol agents should be applied during preharvest in integrated disease management (MID) program for organic production (Nunes, Citation2012; Wisniewski et al., Citation2016). The study conducted by Silimela and Korsten (Citation2007), Govender et al. (Citation2005), and Govender and Korsten (Citation2006) has shown the potential of pre- and post-harvest application of Bacillus licheniformis to control fruit rot. Preharvest application of Rhodosporidium paludigenum, for example, significantly reduced rot incidence caused by Penicillium digitatum, and P. italicum in citrus (Lu et al., Citation2013). A similar effect was obtained by preharvest application of Cryptococcus laurentii in tomato (Wang et al., Citation2008). Besides antibiosis, Spadaro and Droby (Citation2016) reported that resistance induction and competition for nutrients and physical space are common mechanisms applied by yeast BCA.
The objective of this study was to evaluate four yeast strains as part of an integrated management of postharvest decay of mango. Initially, the strains were applied in the postharvest treatment of mango fruits, and in a second study using preharvest field spraying in an organic orchard.
Materials and Methods
The experiments were carried out in the Biological Control Laboratory and the Experimental Field of the Embrapa Semi-Arid (Petrolina-PE, Brazil). The yeast strains were isolated from the epidermis of native (Jamacaru cereus) and cultivated fruit trees (mango and cashew) and selected for their in vivo antagonism to fruit rot associated fungi (Gava et al., Citation2018). The BCA strains were submitted to sequencing of ITS1/ITS4 intergenic region and identified as Saccharomyces cerevisiae ESA45 (Accession Number MF438280.1), Saccharomyces sp. ESA46 (MF438281.1), Saccharomyces sp. ESA47 (MF438282.1), and Pichia kudriavzevii CMIAT171 (MF438285.1) (Gava et al., Citation2018).
The yeast strains were kept at the Embrapa Semi-Arid Collection of Microorganisms of Agronomic Interest, frozen at −80ºC in 20% glycerol solution. The pathogen fungi Lasiodiplodia theobromae (Pat.) Griff. & Maubl. and Neofusicoccum parvum (Pennycook & Samuels) Cross, both belonging to the Botryosphaeriacea family, were isolated from symptomatic fruits acquired from local farms (Costa et al., Citation2010) and kept in the collection of the Embrapa Semi-Arid Phytopathology Laboratory.
Production of Inoculum of the Yeast and Pathogen Strains
The fungal pathogens were cultivated in a potato-dextrose-agar-PDA medium (Himedia), incubated for 15 days at 28°C and 12 h photoperiod, until maximum sporulation was obtained. Suspensions of propagules were prepared by adding 5 mL 0.05% Triton X-100 to densely colonized PDA plates and scraping with a Drigalski loop. The suspension was filtered through two layers of sterile gauze and concentration was adjusted to 106 spores mL−1 after counting in an optimized Neubauer’s Chamber.
The yeast strains were initially cultured in Sabouraud Dextrose Agar (SDA), then inoculated in flasks containing 1.0 L SD+Y liquid medium (10 g Peptone, 20 g Dextrose, and 10 g Yeast Extract), incubated for 96 h under constant agitation at 120 rpm and 28°C and 12 h photoperiod. After fermentation, cell suspensions were centrifuged at 3000 rpm for 5 min, and the supernatant was discarded. The precipitate was diluted twice in isotonic saline solution (0.8% NaCl) and centrifuged again to remove culture medium residues. Yeast suspensions were adjusted to 107 cells mL−1 using a hemocytometer.
Control of Mango Post-harvest Rots in Artificial Wounds
The experiment was carried out using mango fruits cv. ‘Tommy Atkins” collected from an organic orchard kept at the Embrapa Semi-Arid experimental station (Petrolina-PE). The fruits were harvested in maturation phase 2 according to OECD/FAO (Citation2011) (full shoulders, light green and purple peel, and yellowish pulp). They were selected for the absence of apparent damages, washed, and superficially disinfected through immersion into 70% alcohol and sodium hypochlorite (0.5% active chlorine) during 3 min, followed by a triple wash with distilled water.
After superficial disinfection, mangoes were punctured with a stainless-steel perforator (5 mm diameter and 2 mm deep). Afterward, they were sprayed with a patent-pending formulation containing starch and carboxymethylcellulose (CMC) mixed to the different strains. A gel formulation with 1.0% of a matrix containing food grade corn starch (Unilever Brazil, São Paulo) and analytical grade CMC (Sigma-Aldrich, St. Louis USA) was prepared in phosphate buffer 0.01M pH 6.0, autoclaved for 15 min at 121ºC and added with yeast suspensions adjusted to obtain 109 cells mL−1. The formulations containing ESA45, ESA46, ESA47, and CMIAT171 were diluted in distilled water obtaining 10% solution that was individually sprayed on the fruits until run-off. The control treatment received only the gel matrix solution at 10%.
The fruits were left to dry in a tabletop for 2 to 3 h under natural conditions. Twenty microliters of the propagule suspensions, obtained as described in item 1, were deposited in the punctures and again dried in the laboratory environmental conditions. After inoculation procedures, the fruits were transferred to humid chambers and incubated at 26 ± 2°C for 24 h. After this period, they were kept within an incubation chamber at 26 ± 2°C and 60% RH. Evaluations were performed daily for 10 days, recording the incidence and lesion diameter with a digital caliper to the estimation of disease severity. Rot incidence was registered for those fruits with necrotic lesions (tissue discoloration and dark brown spots) greater than 5 mm, exceeding the area of the perforator used in the inoculation.
This experiment was repeated twice using two independent fruit groups, and the results are the average of the experiments. The statistical design was a completely randomized, composed of eight treatments: yeast formulations (ESA45, ESA46, ESA47, and CMIAT171), pathogens, and negative control (CMC) with five repetitions and eight fruits per plot.
Preharvest Application of Yeast Formulation for Control of Postharvest Mango Decay
Two experiments were carried out in October/November 2014 and November 2015/January 2016, in an organic orchard of mango cv. “Tommy Atkins” at the Embrapa Semi-Arid experimental area, located in Petrolina (Pernambuco State, Brazil). The experiments evaluated the control efficiency of formulations containing 109 cells mL−1 of ESA45, ESA46, ESA47, or CMIAT171 in 1.0% starch+CMC. Tank mixing was previously adjusted to pH 6.5 using acetic acid and 0,1% (v/v) of esterified soybean oil as an adhesive (Agr’oleo® – Gota Ltda, PR, Brazil). One percent of the yeast formulations were finally added to the preparation.
Pest and disease management in the mango orchard was limited to micronized sulfur spraying during the flowering period, and Bordeaux mixture was sprayed in the fruit setting. The control of mango rot along fruit development followed an integrated management strategy based on the application of copper oxychloride in the recommended dosage alternated with the treatments tested, according to Silimela and Korsten (Citation2007). The treatments were four formulations prepared with 109 cells mL−1 of the yeast BCA strains and a control treatment containing only the adjuvants. The formulations were stored in a domestic refrigerator, and cell viability was evaluated weekly by cultivation on SDA medium. They were applied in the experiments only while presenting the original viability (109 CFU mL−1). Spraying was performed using a backpack sprayer equipped with a standard solid cone nozzle directed to the fruits. The treatments started after mango fruits achieved egg size (after the second physiological abortion) and were applied weekly until harvesting.
Mangoes harvesting was done picking fruits in the maturation stage 2–3 (OECD/FAO, Citation2011). One hundred fruits were harvested in the morning from each plot and selected for no apparent damage. They were packed in plastic containers, previously lined with bubble wrap and carefully transported to the laboratory where they were processed similarly to the procedures adopted in commercial packing houses. Briefly, the fruits were washed with detergent under tap water and their peduncles standardized at 20 mm; selected for the absence of mechanical lesions and uniformity (size and maturation); and dried using forced air provided by an industrial blower. After processing, the fruits were placed in a standard paper box for 6 kg of fruits (8–12 fruits), containing corrugated paper at the bottom and covered with a waxed paper sheet (hygroscopic internal surface and hydrophobic external surface). Fruit boxes were cold stored under controlled temperature (10 ± 2ºC and 90% RH) during 21 days, followed by a shelf life evaluation under natural conditions (25ºC and 70% RH) during 10 days.
Fruit rot incidence (number of fruits showing rot symptoms) were evaluated daily during storage and shelf life periods for each box, measuring the lesion dimension using a digital caliper (EC799; Starret Ltd, Brazil) . The incubation period (IP) for each repetition was the time spent until the first fruit of a box showed one necrotic lesion (dark brown spots in the fruit or in the base of the pedicel). At the end of the experiment, symptomatic fruits were selected to the identification of the etiological agent. Infected tissue fragments were deposited in potato dextrose agar (Himedia, India) and fungi colonies morphologically identified.
Data Processing and Statistical Analysis
A randomized block experimental design was applied in the field, with four replicates, and the experimental plot was composed of four plants per treatment. After harvesting and processing, 6 to 9 fruit boxes became experimental subunits for each replicate.
Disease indexes for rot severity were obtained from lesions dimensions using the equation ; adapted from the McKinney index (Madden et al., Citation2007), where D is the dimension of the lesions (mm); n the number of fruits injured; N the number of fruit boxes. Data obtained as a percentage, were transformed using the equation
while incidence and incubation period (PIN) were transformed using
, in which Xij are the observed values and Xii’ are the transformed values. Data homoscedasticity and homogeneity of variance were evaluated using the Lilliefors and the Levene tests, respectively. Data were submitted to analysis of variance and treatment means were compared using Tukey’s test (p < .05) using Statistica for Windows v. 12 (StatSof Inc.). Results were presented with untransformed values (mean ± standard deviation).
Results
Control of Mango Decay in Artificial Wounds
All fruits inoculated with the postharvest pathogens developed rot symptoms in artificial wounds (). However, the F test was significant (p < .05) for the incubation period (IP) and disease index (severity). L. theobromae and N. parvum were highly virulent to the mangoes, rapidly producing large lesions in non-treated artificial wounds ().
Table 1. The incubation period (IP) and rot severity of mango fruits inoculated with postharvest pathogens on artificial wounds in mango fruits cv. “Tommy Atkins” after covering with formulations containing antagonist yeasts. Results are the average of two experiments performed in a randomized block design with four replications and 10 fruits per plot.
Treatment with ESA46, ESA47, and CMIAT171 resulted in significantly larger (p < .05) IP than the control treatment in fruits inoculated with L. theobromae by the Tukeys’ test (p < .05). ESA47 and CMIAT171 applied to the lesions also showed the lower value for fruit lesion sizes (p < .05). All yeast treatments increased mango fruit shelf life in fruits inoculated with N. parvum, showing IP values significantly larger than the control treatment by the Tukeys’ test (p < .05), except for ESA46 that showed IP values statistically similar to the control. All treatments also reduced rot dimensions, but only the yeast strains ESA45, ESA46 and CMIAT171 have injury lesions significantly lower than the control treatment.
Preharvest Application of Yeast
Rot symptoms did not develop during cold storage in both experiments. Results presented in and refer to rot incidence and severity (McKinley index) during the shelf life period. In both experiments, fungi recovered from the necrosis were predominantly L. theobromae from stem end rots, and Fusicoccum sp. and Neofusicoccum sp. from black spot. A low density of Alternaria sp. was also isolated from black spots in experiment 2.
Figure 1. Accumulated fruit rot incidence during shelf-life of organic mango fruits treated during fruit development (preharvest) with formulations containing S. cerevisiae ESA45, Saccharomyces sp. ESA46, Saccharomyces sp. ESA47 e P. kudriavzevii CMIAT171 after 20 days in cold storage. Time in the X-axis is the shelf life evaluation period at 25ºC and 70% RH. A – experiment conducted October/November 2014; B – experiment conducted from November 2015 to January 2016.
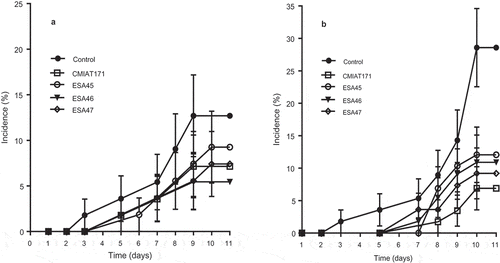
Figure 2. Incidence (%) and mango rot severity (McKinley disease index) at the end of the shelf life period (11 days) in natural infections in organic mango orchard cv. “Tommy Atkins” in two seasons with the preharvest applications of formulations containing S. cerevisiae ESA45, Saccharomyces sp. ESA46, Saccharomyces sp. ESA47 e P. kudriavzevii CMIAT171, Petrolina-PE. A – experiment conducted from October to November 2014; B – experiment conducted from November 2015 to January 2016.
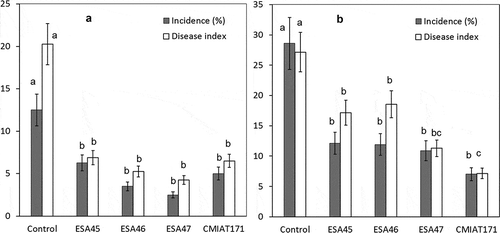
In general, fruits harvested from the control plots presented rot incidence between 8.1% and 15.7% (average of 12.4%) and a McKinley disease severity index of 20.4% and 26.8% during the shelf life for experiment 1 and 2, respectively. shows the evolution of disease incidence in the shelf life period for the two seasons. In experiment 1, preharvest application of all yeast strains significantly increased IP and first symptomatic fruits were recorded only five days after removal from refrigeration (). Spraying yeast BCAs significantly reduced final rot incidence and severity when compared to the control by the Tukeys’ test (p < .05), however, there was no difference among strains ()).
Table 2. Incubation period (IP) during shelf life in mango fruits cv. “Tommy Atkins” treated during fruit development (preharvest) with formulations containing S. cerevisiae ESA45, Saccharomyces sp. ESA46, Saccharomyces sp. ESA47 e P. kudriavzevii CMIAT171.
Incidence and severity were significantly lower than control for all treatments sprayed with yeast BCA formulations (Tukeys’ test; p < .05) in the final of the shelf life period of the second experiment ()). The application of formulations containing ESA46 and ESA47 resulted in a reduction of 68.3% and 56.0% of rot incidence, respectively. However, there were no significant differences between yeast strains. All strains also increased IP and significantly reduced rot incidence and severity in fruits by the Tukeys’ test (p < .05) (). While mangoes in control developed rot lesions after 3 days in shelf-life, BCA applications showed IP of 7 to 8 days ()). Incidence and severity of postharvest decay in the control treatment were highest in the second experiment when incidence reached values between 23.5% and 34.8% in control treatment () and lesion dimensions were larger than 35 mm in diameter and disease index was 26.7% ().
Preharvest application of all yeast formulations promoted a significant reduction of natural incidence and severity of mango rot when compared to the control in both field experiments. There was no significant difference among yeast strains, but ESA46 and ESA47 presented a greater control efficiency than the others in the first experiment. In the second experiment, the application of the formulation containing CMIAT171 resulted in the large reduction of rot severity, followed by ESA47 (73.7% and 58.4%, respectively). P. kudriavzevii CMIAT171 presented more consistent results between the experiments, with an average reduction of the 69.4% of the McKinley’s disease index, and also the largest shelf life period ().
Discussion
All treatments resulted in a significant increase of IP and reduced rot severity in the artificial wounds inoculated with L. theobromae and N. parvum. The treatment with P. kudriavzevii CMIAT171 resulted in the lowest severity and significant increase in IP and was followed by S. cerevisiae ESA45 and Sacharomyces sp. ESA47. Similar results were obtained in an analogous study of Bautista-Rosales et al. (Citation2014) using Cryptococcus laurentii for the control of C. gloeosporioides in mango. It is an important indication that these yeast formulations can increase the shelf life of mango fruits infected with Botryosphaeriacean pathogens. Although these pathogens have been causing severe losses of mango fruits in semi-arid tropical regions (Costa et al., Citation2010; Ismail et al., Citation2012), few studies showed effective biological control of stem-end rot and black spots caused by L. theobromae and N. parvum. In the studies conducted by Govender and Korsten (Citation2006), for example, there was no effective control of stem-end rot using Bacillus licheniformis in cold-stored mango fruits.
Most experiments selecting antagonists to be applied in the postharvest disease of fruits use inoculation of the pathogen artificial wounds. However, this procedure favors the colonization of host tissue by the pathogen, mainly because it reduces the efficiency of chemical and physical barriers in fruit epidermis (Lara et al., Citation2014). Co-inoculation in artificial wounds could also affect induced resistance (IR) response on fruit epidermis, an important mechanism of action of BCA (Lin et al., Citation2011; Zhang et al., Citation2013). However, this methodology allows the selection of BCA able to rapidly colonize exposed tissue and compete with the pathogen for space and nutrients, and also those able to quickly alter physicochemical characteristics of the niche or produce antibiotics. All these mechanisms are usually applied by yeast strains (Spadaro and Droby, Citation2016). The increase in IP and lowest severity obtained with the application of ESA47 and CMIAT171 probably is a result of their efficiency in tissue colonization, competition for space and nutrients, physical-chemical alteration of the substrate (acidification, perhaps) as well as the production of antibiotics (Gava et al., Citation2018).
Preharvest application of all yeast formulations promoted a significant reduction of natural incidence and severity of mango rot when compared to the control in both field experiments. The results showed that the treatments were able to extend the shelf life period, meaning the period until the first fruit showed rot symptoms. Other studies also demonstrated that the efficiency of BCA would be more significant when preventively applied, especially for pathogens with quiescent stages, such as in this study (Cañamás et al. Citation2008; Sarwar, Citation2015). Results reported by Mohamed and Saad (Citation2009), for example, showed that preharvest application of Pichia anomala for the control of L. theobromae in guava also reduced rot incidence in 64.4%, while postharvest and curative applications had lower efficiency. Besides, there are no efficient phytosanitary products to control postharvest rot in organic production systems.
The usage of BCA exclusively after harvesting and processing is an interesting approach for controlling postharvest fruit rot, with several advantages in the mango production chain (Droby et al., Citation2009). Mango fruits undergo a surface disinfection process through washing and application of chemical disinfectants (sodium hypochlorite) or physical methods (ionizing radiation or thermotherapy), followed by application of waxes or edible films. Finally, they are stored at controlled temperature and high humidity (Singh and Singh, Citation2012). Under such conditions, the elimination of microbial competition on fruit surface by postharvest processing and environmental stability facilitate maintenance of high BCA populations and increase control efficiency. Besides, postharvest fruit handling also eliminates beneficial microbiota from the fruit surface. Thus, application of BCA promotes re-colonization of lenticels and fissures by a beneficial population that can reduce tissue infection. However, most pathogens that cause mango rot produces quiescent infections requesting effective preharvest measures of control.
In this study, postharvest application of yeast formulations significantly reduced the development of mango rot. However, preharvest spraying of Saccharomyces sp. ESA47 and P. kudriavzevii CMIAT171 increased shelf life of mango fruits and reduced the incidence and severity of fruit rot in organic mangoes in conditions similar to those of commercial production, indicating a potential use of these microorganisms into the integrated management of mango fruit rot.
Efficient control of postharvest decay of mangoes in organic farms requires the application of a set of strategies into an integrated disease management program. These strategies should include cultural practices such as appropriate irrigation system and water amount, reducing moisture accumulation under the canopy, consequently the multiplication of the pathogen in plant residues; removal of inoculum sources as residual panicles and fruits, usually mummified; and composting pruning residues. Alternative fungicides should be applied in the orchard in periods of high favorability defined by the climate and plant development stage; preharvest application of copper, alternated with a competitive BCA (P. kudriavzevii CMIAT171); application of antagonistic microorganisms in postharvest processing.
Acknowledgment
The authors wish to acknowledge and thank the technical support granted by Mr. Herbert Targino in the conduction of the experiments.
Additional information
Funding
References
- Bautista-Rosales, P.U., M. Calderon-Santoyo, R. Servín-Villegas, N.A. Ochoa-Álvarez, R. Vázquez-Juárez, and J.A. Ragazzo-Sánchez. 2014. Biocontrol action mechanisms of cryptococcus laurentii on colletotrichum gloeosporioides of mango. Crop Prot. 65:194–201. doi: 10.1016/j.cropro.2014.07.019.
- Cañamás, T.P., I. Viñas, J. Usall, R. Torres, M. Anguera, and N. Teixidó. 2008. Control of postharvest diseases on citrus fruit by preharvest applications of biocontrol agent Pantoea agglomerans CPA-2. Part II. Effectiveness of different cell formulations. Postharvest Biol. Technol. 49:96–106. doi: 10.1016/j.postharvbio.2007.12.005.
- Dal Bello, G., C. Mónaco, M.C. Rollan, G. Lampugnani, N. Arteta, C. Abramoff, L. Ronco, and M. Stocco. 2008. Biocontrol of postharvest grey mold on tomato by yeasts. J. Phytopathol. 156:257–263. doi: 10.1111/j.1439-0434.2007.01351.x.
- de Oliveira Costa, V.S., S.J. Michereff, R.B. Martins, C.A.T. Gava, E.S.G. Mizubuti, and M.P.S. Câmara. 2010. Species of Botryosphaeriaceae associated on mango in Brazil. Eur. J. Plant Pathol. 127:509–519. doi: 10.1007/s10658-010-9616-y.
- Droby, S., M. Wisniewski, D. Macarisin, and C. Wilson. 2009. Twenty years of postharvest biocontrol research: Is it time for a new paradigm? Postharvest Biol. Technol. 52:137–145. doi: 10.1016/j.postharvbio.2008.11.009.
- Droby, S., M. Wisniewski, N. Teixidó, D. Spadaro, and M.H. Jijakli. 2016. The science, development, and commercialization of postharvest biocontrol products. Postharvest Biol. Technol. 122:22–29. doi: 10.1016/j.postharvbio.2016.04.006.
- Finckh, M., A. van Bruggen, and L. Tamm. 2015. Plant diseases and their management in organic agriculture. APS Press, ST. Paul, Minesota.
- Gava, C.A.T., A.P.C. de Castro, C.A. Pereira, and P.I. Fernandes-Júnior. 2018. Isolation of fruit colonizer yeasts and screening against mango decay caused by multiple pathogens. Biol. Control 117:137–146. doi: 10.1016/j.biocontrol.2017.11.005.
- Govender, V., and L. Korsten. 2006. Evaluation of different formulations of Bacillus licheniformis in mango pack house trials. Biol. Control 37:237–242. doi: 10.1016/j.biocontrol.2005.11.012.
- Govender, V., L. Korsten, and D. Sivakumar. 2005. Semi-commercial evaluation of Bacillus licheniformis to control mango postharvest diseases in South Africa. Postharvest Biol. Technol. 38:57–65. doi: 10.1016/j.postharvbio.2005.04.005.
- Ismail, A.M., G. Cirvilleri, G. Polizzi, P.W. Crous, J.Z. Groenewald, and L. Lombard. 2012. Lasiodiplodia species associated with dieback disease of mango (Mangifera indica) in Egypt. Australas Plant Pathol. 41:649–660. doi: 10.1007/s13313-012-0163-1.
- Konvalina, P. 2016. Organic Farming - A Promising Way of Food Production. doi: 10.5772/60459
- Lara, I., B. Belge, and L.F. Goulao. 2014. The fruit cuticle as a modulator of postharvest quality. Postharvest Biol. Technol. 87:103–112. doi: 10.1016/j.postharvbio.2013.08.012.
- Lima, N.B., M.V. Marcus, M.A. De Morais, M.A.G. Barbosa, S.J. Michereff, K.D. Hyde, and M.P.S. Câmara. 2013. Five Colletotrichum species are responsible for mango anthracnose in northeastern Brazil. Fungal Divers. 61:75–88. doi: 10.1007/s13225-013-0237-6.
- Lin, J., D. Gong, S. Zhu, L. Zhang, and L. Zhang. 2011. Expression of PPO and POD genes and contents of polyphenolic compounds in harvested mango fruits in relation to Benzothiadiazole-induced defense against anthracnose. Sci. Hortic. 130:85–89. doi: 10.1016/j.scienta.2011.06.014.
- Lobo, M., and J. Sidhu. 2017. Biology, postharvest physiology, and biochemistry of mango, p. 37–60. In: M. Siddigq, J.K. Brecht, and J.S. Sidhu (eds.). Handbook of mango fruit: Production, postharvest science, processing technology and nutrition. Wiley Blackwel, Oxford, UK.
- Lu, L., C. Ye, S. Guo, K. Sheng, L. Shao, T. Zhou, T. Yu, and X. Zheng. 2013. Preharvest application of antagonistic yeast Rhodosporidium paludigenum induced resistance against postharvest diseases in mandarin orange. Biol. Control 67:130–136. doi: 10.1016/j.biocontrol.2013.07.016.
- Madden, L. V., G. Hughes, and F van den Bosch. 2007. The study of plant disease epidemics. APS Press, St. Paul, MN.
- Mohamed, H., and A. Saad. 2009. The biocontrol of postharvest disease (Botryodiplodia theobromae) of guava (Psidium guajava L.) by the application of yeast strains. Postharvest Biol. Technol. 53:123–130. doi: 10.1016/j.postharvbio.2009.04.001.
- Mutungi, C. 2015. Unpacking postharvest losses in Sub-Saharan Africa : A meta-analysis. World Dev. 66:49–68. doi: 10.1016/j.worlddev.2014.08.002.
- Nunes, C.A. 2012. Biological control of postharvest diseases of fruit. Eur. J. Plant Pathol. 133:181–196. doi: 10.1007/s10658-011-9919-7.
- O’Donnell, T. H., J. Deutsch, C. Yungmann, A. Zeitz, and S. H. Katz. 2015. New sustainable market opportunities for surplus food: a food system-sensitive methodology (fssm). Food and Nutrition Sciences 6:883-892. doi:10.4236/fns.2015.610093.
- OECD/FAO. 2011. International standards for fruit and vegetables: Mangoes. FAO, Paris.
- Parafati, L., A. Vitale, C. Restuccia, and G. Cirvilleri. 2015. Biocontrol ability and action mechanism of food-isolated yeast strains against botrytis cinerea causing post-harvest bunch rot of table grape. Food Microbiology 47:85–92. doi:10.1016/j.fm.2014.11.013.
- Prusky, D., N. Alkan, T. Mengiste, and R. Fluhr. 2013. Quiescent and necrotrophic lifestyle choice during postharvest disease development. Annu. Rev. Phytopathol. 51:155–176. doi: 10.1146/annurev-phyto-082712-102349.
- Robiglio, A., M.C. Sosa, M.C. Lutz, C.A. Lopes, and M.P. Sangorrín. 2011. Yeast biocontrol of fungal spoilage of pears stored at low temperature. Int. J. Food Microbiol. 147:211–216. doi: 10.1016/j.ijfoodmicro.2011.04.007.
- Sales Júnior, R., G.H.S. Nunes, L.L. de Lima, I.M. Guimarães, and P.L.D.D. Morais. 2009. Chemical control of stem rot caused by Lasiodiplodia theobromae in mango fruits. Rev. Bras. Frutic. 31:907–910. doi: 10.1590/S0100-29452009000300039.
- Sarwar, M. 2015. Practices for integrated control of mango (Mangifera indica L .) Diseases to protect in preharvest as well as postharvest phases. Biosci an Bioeng 1:57–62.
- Seymour, G.B., L. Ostergaard, N.H. Chapman, S. Knapp, and C. Martin. 2013. Fruit development and ripening. Annu. Rev. Plant Biol. 64(64):219–241. doi: 10.1146/annurev-arplant-050312-120057.
- Silimela, M., and L. Korsten. 2007. Evaluation of pre-harvest Bacillus licheniformis sprays to control mango fruit diseases. Crop Prot. 26:1474–1481. doi: 10.1016/j.cropro.2006.12.011.
- Singh, P. 2011. Integrated management of storage anthracnose of mango. J. Mycol. Plant Pathol. 41:63.
- Singh, Z., and S.P. Singh. 2012. Mango, p. 108–142. In: D. Rees, G. Farrell, and J. Orchard (eds.). Crop post-harvest: Science and technology. Wiley-Blackwell, Oxford, UK.
- Singh, Z., R.K. Singh, V.A. Sane, and P. Nath. 2013. Mango - Postharvest biology and biotechnology. CRC Crit. Rev. Plant Sci. 32:217–236. doi: 10.1080/07352689.2012.743399.
- Snowdon, A.L. 2010. A Colour Atlas of Post-harvest Diseases and Disorders of Fruit and Vegetables: General introduction and fruits. Manson
- Spadaro, D., and S. Droby. 2016. Unraveling the mechanisms used by antagonistic yeast To control postharvest pathogens on fruit. In: Acta Horticulturae. pp 63–70
- Wang, Y., Y. Bao, D. Shen, W. Feng, T. Yu, J. Zhang, and X.D. Zheng. 2008. Biocontrol of Alternaria alternata on cherry tomato fruit by use of marine yeast Rhodosporidium paludigenum Fell & Tallman. Int. J. Food Microbiol. 123:234–239. doi: 10.1016/j.ijfoodmicro.2008.02.002.
- Wisniewski, M., S. Droby, J. Norelli, J. Liu, and L. Schena. 2016. Alternative management technologies for postharvest disease control: The journey from simplicity to complexity. Postharvest Biol. Technol. 122:3–10. doi: 10.1016/j.postharvbio.2016.05.012.
- Zhang, C., K. Chen, and G. Wang. 2013. Combination of the biocontrol yeast Crypotococcus laurentii with UV-C treatment for control of postharvest diseases of tomato fruit. BioControl 58:269–281. doi: 10.1007/s10526-012-9477-8.
- Zhang, H., L. Wang, L. Ma, Y. Dong, S. Jiang, B. Xu, and X. Zheng. 2009. Biocontrol of major postharvest pathogens on apple using Rhodotorula glutinis and its effects on postharvest quality parameters. Biol. Control 48:79–83. doi: 10.1016/j.biocontrol.2008.09.004.
