?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The objective of this work was to develop a methodology for standardizing the Accelerated Aging test (AA) for J. nigra seeds. After different aging treatments, seeds were subjected to the standard germination test the results of which were expressed as the percentage of normal seedlings. Germination velocity index and mean time of maximum germination were also determined. Our results showed that storage at high temperature and relative humidity deteriorates black walnut seeds to the extent that their germination capacity is lost in only seven days. Bigger seeds had a higher percentage of normal seedlings and vigor, both under favorable storage conditions and when subjected to accelerated aging treatments. This work has clearly shown that vigor of J. nigra seeds should be assessed using AA periods no longer than 72 h. The most frequent abnormality observed in AA testing was seedlings with stubby roots and no secondary roots.
Introduction
Juglans nigra L. (eastern black walnut) belongs to the order Juglandales, family Juglandaceae, section Rhysocaryon (Woodworth, Citation1930). Also called Eastern black walnut, its hardwood is the highest priced of all walnut varieties in North America.
In the USA, it is used for confectionery, and for making bread, ice creams, salads, candies and chocolate making it a species of great economic importance with a high level of industrial and technological development, as shown by the Center of Agroforestry at the University of Missouri (Hammons, Citation1998; Reid et al., Citation2007). In addition to being grown as a wood source, it is also propagated from seed to be used as a rootstock for J. regia (Flores et al., Citation2017).
Physiological seed quality is directly related to the capacity of seeds to emerge under different field conditions. Although the Germination test is the most widely used method to evaluate physiological seed quality, it does not always account for seedling establishment in the field. For this reason, seed vigor has been suggested as the best parameter to determine seedling performance in field conditions (Delouche and Caldwell, Citation1962).
Seed vigor is defined as the sum of the properties which determine the potential level of activity and performance of a seed lot during germination and seedling emergence (AOSA, Citation2002; Bonner et al., Citation1994).
The highest seed vigor level occurs at physiological maturity; after this point, however, seeds begin to deteriorate due to damages in the systems and vital functions of their cells (Alizaga et al., Citation1994; Delouche, Citation2002). This vigor loss along time, also called aging, results in decreased emergence and slower seedling growth in the field (Delouche and Baskin, Citation1973), smaller seedling size and higher percentage of abnormal seedlings (Romano et al., Citation2008).
One of the tests to determine vigor is the Accelerated Aging test (AA), developed by James Delouche in 1965 to assess the longevity of seeds in commercial storage (AOSA (Association of Official Seed Analysts), Citation2002). Another procedure to assess seed vigor is the Controlled Deterioration test. Both tests assume that the deterioration process under artificial aging is similar to the deterioration occurring under natural conditions (Ayala-Garay et al., Citation2018).
The AA test, which measures vigor by simulating germination under stress conditions, is used in Pisum sativum (Hampton et al., Citation2004), Allium cepa (Rodo and Marcos Filho, Citation2003) and Lactuca sativa (Contreras and Barros, Citation2005), among other species. In this test, aging is induced by storing seeds in an accelerated aging chamber at high temperature (41–45ºC) and high relative humidity (95–100%) for a specified time (48–144 h) depending on the species (Delouche and Baskin, Citation1973; TeKrony, Citation2005). Exposure to high temperature and humidity results in degenerative metabolic disorders brought about by cell membrane degradation (Basavarajappa et al., Citation1991). It has been reported that the capacity of a seed lot to survive artificial aging is strongly correlated to the physiological quality and storage potential of seeds in the lot (Bewley et al., Citation2013).
The results of the AA test are expressed as the percentage of normal seedlings (ISTA, Citation1995), and allow classifying artificially aged seed lots into the following categories: high vigor (germination higher than 80%), medium vigor (60–80%), and low vigor (germination lower than 60%) (Ayala-Garay et al., Citation2018). Patterns to distinguish normal from abnormal seedlings have been described for different species, among them J. nigra. These patterns are a highly valuable tool for AA vigor testing, among other tests (Flores et al., Citation2018).
The objective of this work was to develop a methodology to standardize the Accelerated Aging test for Juglans nigra seeds.
Materials and Methods
Fruits of J. nigra were obtained from 50-year-old trees at the J.F. Villarino Experimental Field at the Facultad de Ciencias Agrarias, Universidad Nacional de Rosario (College of Agricultural Sciences, Rosario National University), located in Zavalla (33°01´S, 60°53´W), Argentina. Fruits were harvested after natural fall at the end of March and mixed to form a single sample (FAO, Citation1991).
Fruits were macerated in water and washed thoroughly to remove their skin and pulp (Flores et al., Citation2013). After removal of both mesocarp and exocarp, nuts were disinfected for 2 min in a sodium hypochlorite solution (2% v/v) and rinsed with distilled water (Flores et al., Citation2011).
To overcome dormancy, seeds were subsequently stratified in cold-moist conditions by wrapping them in water-soaked paper and placing them in black polyethylene bags to avoid water loss. Bags were kept in a chamber at 5°C for 4 months (Flores et al., Citation2017).
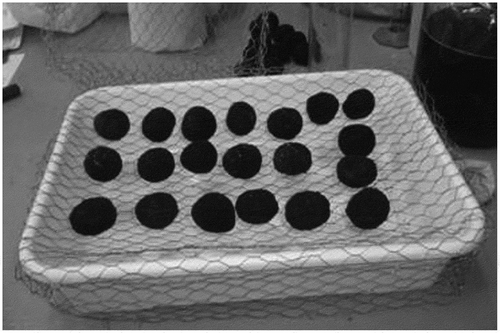
After the stratification period, the Accelerated Aging Test was performed using seeds with 12–14% internal moisture content (IMC). Plastic trays (30.0 x 21.0 × 8.0 cm) with 2000 cm3 of deionized water were used for the AA test. To avoid direct contact of seeds with water, a piece of wire mesh was fit on the bottom of each tray (Baskin, Citation1977). Seeds were spread uniformly on the mesh (ISTA (International Seed Testing Association), Citation1995; Marcos Filho et al., Citation2001) to ensure homogeneous water uptake ().
Each tray was sealed in a transparent bag and taken to the aging chamber at 44ºC ± 1ºC and 100% relative humidity.
The experiment was performed following a randomized complete block design with a factorial arrangement and five 40-seed replications for each treatment. The two factors evaluated were seed weight (with two levels: range 1: 12–16 g, and range 2: 21–26 g), and accelerated aging periods with four levels: zero days (control treatment), 3, 7, and 15 days.
At the end of each aging period, germination was tested following the International Seed Testing Association specifications (ISTA, Citation2003). Fruits were placed in trays on top of a 40 mm sand layer and covered with 20 mm of uncompressed wet sand. The trays were placed in a germination chamber (Forma Scientific, Model 3744) under 24 h of light and an alternating temperature regime of 20/30°C (16h/8h) (Brinkman et al., Citation1974; Ellis et al., Citation1985). Germination was evaluated weekly for 28 d and the results expressed as the percentage of germination of normal seedlings (AOSA Citation2002).
Germination velocity was evaluated using the germination velocity index (GVI) and mean time of maximum germination (MTMG) (Alzugaray et al., Citation2005). Germination (protrusion of the radicle through the seed coat) was recorded daily for 28 d, and GVI was calculated according to Kotowiski (Citation1926) as:
Where:
Ci: number of germinated seeds per day;
Ti: number of days since the beginning of the test;
N: total number of seeds in the sample.
MTMG was calculated according to Edmond and Drapala (Citation1958) as:
Data were analyzed statistically with InfoStat (Di Rienzo et al., Citation2008), and analysis of variance and comparisons between treatment means were performed with Tukey’s test at 0.05 significance level. Normality was tested by hypothesis testing and graphical methods.
Results
In the AA test, no germination occurred at the 7- and 15-days accelerated aging periods in either seed weight range. For this reason, results were statistically analyzed using a two-factor factorial design, the two factors being seed weight (with both ranges, 12–16 and 21–26 g), and aging period with two levels (zero and three days).
Both variables had significant effects (F = 236.08, p < .0001 for the aging period; and F = 144.73, p < .0001 for seed weight), but no interaction between variables was observed. In the control treatment (0 days), the percentage of normal seedlings was 83.4% for bigger seeds, and 59% for smaller ones (30% lower than that of bigger seeds, ). In the 3-day accelerated aging treatment, only 54% of the bigger seeds and 30% of smaller ones germinated, which represents a germination decrease of 35% and 50%, respectively ().
Table 1. Percentage of normal seedlings, germination velocity index (GVI), and mean time of maximum germination (MTMG) after three days of accelerated aging (3d-aging) at 44°C and 100% relative humidity, for different weight ranges of J. nigra seeds.
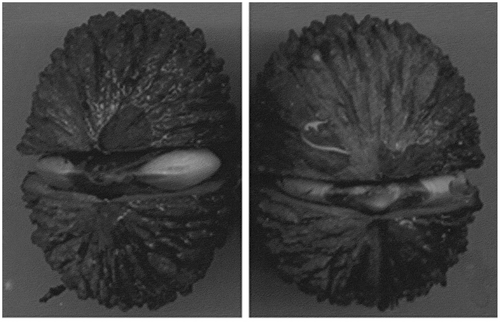
Seeds which failed to germinate in the 3-day period presented dark spots around the embryo ().
Figure 2. Dark stains around the embryo of non-germinated seeds from the 3-day accelerated aging treatment (44ºC ± 1ºC; 100% relative humidity).
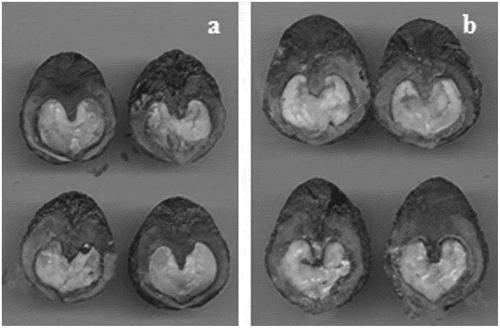
No germination occurred in the 7- and 15-days treatments, and non-turgid and decayed tissues were observed in these seeds ().
Figure 3. Non-turgid and decayed tissues in non-germinated seeds of weight range 1 (a) and 2 (b) after 7 and 15 days of accelerated aging (44ºC ± 1ºC; 100% relative humidity).
The GVI showed a significant effect of seed weight and AA treatments, with significant interactions between both variables. In the control treatment, bigger seeds had an average GVI of 15.8%, 58% higher than that of lighter seeds. In the 3-day AA treatment, the GVI of bigger seeds decreased from 18.5% to 11.4% (−38%) (F = 70,75; p < 0,0001), while no GVI change occurred in smaller seeds. This differing response accounts for the highly significant interaction (F = 17.47; p < .0001) between both variables ().
Results of the MTMG test also showed significant effects of AA treatments and seed size, with significant interactions between both variables (F = 4.48; p < .05). After the 3-day accelerated aging period, the MTMG of smaller seeds did not differ from that of Control seeds, reaching similar values at 13–14 days. In contrast, the MTMG of heavier seeds was almost doubled (9 days) after the 3-day aging period, compared to Control seeds (5 days) (F = 35,64; p < 0,0001), which explains the significant interaction between seed weight and aging period ().
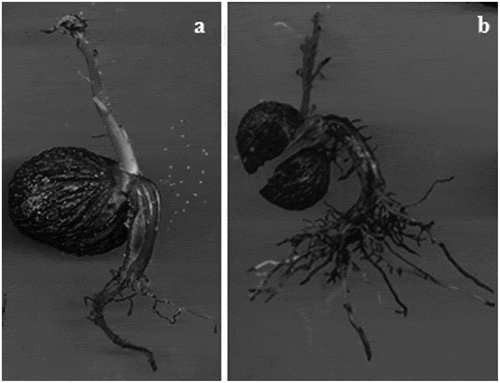
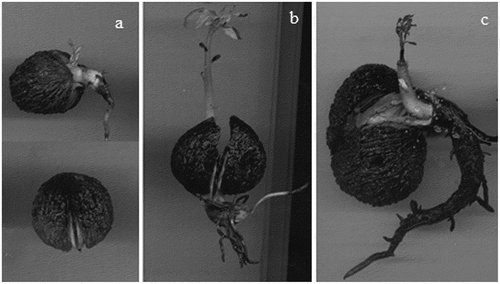
After three days of accelerated aging, weak normal seedlings were observed with a vigorous primary root, weak secondary roots, damaged terminal bud, and necrosis in some axillary buds (); there were also weak normal seedlings with vigorous primary and secondary roots, healthy terminal bud and necrosis in axillary buds (). Abnormal seedlings observed after this aging period presented some of the following characteristics: stubby primary root with no secondary roots (); absent or damaged primary root with necrosis in secondary roots and in primary leaves (); vigorous primary root, weak secondary roots and damaged terminal bud ().
Discussion
Our results have shown that black walnut seeds stored in high temperature and high relative humidity conditions undergo a deterioration process which deprives them of their germination capacity in just seven days. This trend has also been reported for lettuce seeds (Contreras and Barros, Citation2005).
The loss of germination capacity is the last stage of the natural seed aging process. Before reaching this stage, seeds undergo damages in the membrane system and in their breathing capacity, which in turn leads to lower energy output and a decrease in enzymatic activity. All these changes result in lower germination velocity, growth, and development of seedlings, decreased tolerance to adverse environmental conditions, and higher proportion of abnormal seedlings (Delouche, Citation2002).
The most frequent abnormality in the AA test was stubby seedlings with stubby primary root and no secondary roots. These results agree with previous studies and descriptions of Juglans nigra seedlings obtained from seeds stored in normal conditions (Flores et al., Citation2018). A remarkable finding was that the accelerated aging test increased the proportion of abnormalities in the aboveground part of seedlings (70%) when the usual response is a predominance of abnormalities at the root level (Flores et al., Citation2018). This shows a higher sensitivity of the aerial part of J. nigra seedlings to high-temperature stress.
Although the results of the AA test are customarily expressed as a percentage of germination normal seedling, it would be advisable to use other parameters, such as germination velocity and growth of seedlings (Carneiro and Guedes, Citation2002), which also occur before seed death (Bewley and Black, Citation1994; Delouche, Citation2002). For this reason, it has been suggested that other indices should be used to express the results of the AA test (Marcos Filho et al., Citation2009). In line with this suggestion, in the present work, the GVI and MTMG indices were used to quantify seed deterioration induced by AA treatments. These parameters showed high sensitivity of J. nigra seeds to accelerated aging procedures, although responses differed with seed size. Percentage of normal seedlings, GVI, and MTMG were most affected in heavier seeds, even though the values of each parameter were agronomically better in comparison with smaller seeds in all treatments. In many species, seed size is an indicator of physiological quality, with larger seeds normally presenting higher germination and vigor (Popinigis, Citation1985). Also, heavier seeds have a higher germination percentage and more vigorous seedlings, as has been reported for rice (Herrera, Citation1987), Impatiens wallerana (China sultani) (Li et al., Citation1996), and even earlier germination as in S. beneckei (cactus) (Ayala-Cordero et al., Citation2004). Probably the higher metabolic reserves of bigger seedlings confer a competitive advantage over later-emerging seedlings from lighter seeds, as has also been observed in Abutilon theophrasti (abutilon) (Baloch et al., Citation2001).
In the present work, heavier J. nigra seeds resulted in higher germination percentage and vigor, in both favorable and stressful storage conditions. Lighter seeds have limited reserves which are rapidly depleted shortly after germination begins (Bewley and Black, Citation1994), whereas heavier seeds, being more tolerant to adverse conditions, will have greater chances of survival and field establishment under suboptimal conditions (TeKrony et al., Citation1989).
The AA treatments used in this work were too aggressive, as they resulted in tissue deterioration and seed death.
For a large number of species, some authors have suggested using a 41–43°C temperature range, and an aging period from 24 to 72 h (Hampton and Tekrony, Citation1995). The 72-h aging period recommended in the ISTA Vigor Manual ISTA (Citation1995) is not widely accepted by seed quality specialists from Brazil and Argentina on the grounds that it excessively lowers the germination capacity of seed lots. However, a 48-h stress period at 41°C is accepted as a suitable procedure for determining different vigor levels in seed lots sharing similar germination percentage (Marcos Filho et al., Citation2009; Salinas et al., Citation2001).
Accordingly, the AA test is to be adapted to each particular species so as to find the conditions that will allow vigor assessments without subjecting seeds to stress conditions more severe than those occurring in storage or in the field. Our results have clearly shown that exposure to stressful conditions for 72 h is excessive for J. nigra, in contrast to seeds of other species such as pumpkin and zucchini in which seed vigor potential can be determined at temperatures of 41°C for 96 h without compromising their germination capacity (Dutra and Vieira, Citation2006).
Vigor assessments of J. nigra seeds should not exceed 72 h of exposure to accelerated aging conditions. Further studies should be conducted to widen our knowledge of suitable assessment procedures by testing other time/temperature combinations.
Conclusions
Even though vigor of seeds subjected to the Accelerated Aging test is usually expressed in terms of percentage of germination of normal seedlings, it is advisable to perform germination speed tests as well, such as GVI and MTGM. As these tests are highly sensitive to the Accelerated Aging test, their results are representative of the physiological quality of seed lots. Heavier seeds had a higher percentage of vigor and normal seedlings. Our results are expected to have made a contribution towards the development of an improved procedure for testing seed vigor in this species.
Acknowledgments
The authors thank Mrs. Gabriela M. Venturi for her English assistance.
References
- Alizaga, R., D.C. Mello Vera, D.S.B. Dos Santos, and D. Irigon. 1994. Evaluación del vigor en semilla de Phaseolus vulgaris y su relación con la emergencia en el campo (Evaluation of vigor in Phaseolus vulgaris seeds and its relation with field emergence). Agron. Costarric 18(2):227–234.
- Alzugaray, C., N. Carnevale, A. Salinas, and R. Pioli. 2005. Observations on quality of Schinopsis balansae Engl. seeds. Seed Technol. 27:49–58.
- AOSA (Association of Official Seed Analysts). 2002. Seed vigor testing Handbook. AOSA. Contribution Nº 32. AOSA: Washington, DC, USA.
- Ayala-Cordero, G., T. Terraza, L. López-Mata, and C. Trejo. 2004. Variación en el tamaño y peso de la semilla y su relación con la germinación en una población de Stenocereus beneckei (Variation in seed size and weight in a Stenocereus beneckei population and its relationship with germination). Interciencia 29(12):692–697.
- Ayala-Garay, O.J., L.L. Pinzón-López, L. Latournerie-Moreno, A.V. Ayala-Garay, and S. Tovar-Carvajal. 2018. Adaptaciones metodológicas para evaluar la calidad fisiológica en semillas de chile habanero (Capsicum chinense Jacq.) (Methodological adaptations to evaluate physiological quality in seeds of habanero pepper). Agroproductividad 9(11):9–14.
- Baloch, H.A., A. Di Tommaso, and A.K. Watson. 2001. Intrapopulation variation in Abutilon theophrasti seed mass and its relationship to seed germinability. Seed Sci. Res. 11:333–345.
- Basavarajappa, B.S., H.S. Shetty, and H.S. Prakash. 1991. Membrane deterioration and other biochemical changes associated with accelerated ageing of maize seeds. Seed Sci. Technol 19(2):279–286.
- Baskin, C.C. 1977. Vigor test methods – Accelerated aging. Aosa 51:42–52.
- Bewley, J.D., and M. Black. 1994. Seeds: Physiology of development and germination. Plenum Press, New York. p. 445 p.
- Bewley, J.D., K.J. Bradford, W.H.M. Hilhorst, and H. Nonogaki. 2013. Seeds. Physiology of development, germination and dormancy. Third ed. Springer, New York. p. 392.
- Bonner, F.T., J.A. Vozzo, W.W. Elam, and S.B. Land Jr. 1994. Tree seed technology training course. Instructor´s manual. USDA, Forest Service, New Orleans, Louisiana. p. 160 p.
- Brinkman, K.A., L. Juglans, and P. Walnut. 1974. Seeds of woody plants in the United States, p. 454–459. In: C.S. Schopmeyer (ed.). Agriculture Handbook. USDA, Washington, D.C.
- Carneiro, J.W.P., and T.A. Guedes. 2002. Dinâmica de ocorrências germinativas em amostras de sementes envelhecidas artificialmente (Dynamics of germination in artificially aged seed samples). Informativo ABRATES 12(1–3):44–51.
- Contreras, S., and M. Barros. 2005. Pruebas de vigor en semillas de lechuga y su correlación con emergencia (Vigor tests in lettuce seeds and their correlation with emergence). Rev. Cien. Inv. Agr 32(1):3–11. doi: 10.7764/rcia.v32i1.301.
- Delouche, J.C. 2002. Germinación, deterioro y vigor de semillas (Seed germination, deterioration and vigor). Seed News 6(6). 03 May 2013. http://www.seednews.inf.br/espanhol/seed66/artigocapa66a_esp.shtml.
- Delouche, J.C., and C.C. Baskin. 1973. Accelerated ageing techniques for predicting the relative storability of seed lots. Seed. Sci. Technol. 1:427–452.
- Delouche, J.C., and W.P. Caldwell. 1962. Seed vigor and vigor tests. Proceedings of the Association of Official Seed Analysts, 50:136-140.
- Di Rienzo, J.A., F. Casanoves, M.G. Balzarini, L. Gonzalez, M. Tablada, and C.W. Robledo. 2008. InfoStat, versión 2008, Grupo InfoStat, FCA. Universidad Nacional de Córdoba, Argentina.
- Dutra, A.S., and V.D. Vieira. 2006. Accelerated aging test to evaluate seed vigor in pumpkin and zucchini seeds. Seed Sci. Technol. 34:209–214. doi: 10.15258/sst.2006.34.1.24.
- Edmond, J.B., and W.J. Drapala. 1958. The effect of temperature, sand and soil, and acetone on germination of okra seed. Proc. Amer. Soc. Hort. Sci. 71:728–734.
- Ellis, R.H., T.D. Hong, and E.H. Roberts. 1985. Handbook of seed technology for genebanks. Vol. II. Juglandaceae. IBPGR, Rome, Italy.
- FAO. 1991. Planning seed collection, p. 33–63. In: R.L. Willan (ed.). A guide to forest seed handling. FAO For. Paper 20/2, Rome.
- Flores, P.C., D. Poggi, S.M. García, M. Catraro, and N.F. Gariglio. 2017. Ruptura de la dormición y exigencias de luz para la germinación de semillas de Juglans nigra (Dormancy breakup and light requirements of Juglans nigra seeds). FAVE Sección Ciencias Agrarias 16(2):33–46. ISSN: 2346-9129. doi: 10.14409/fa.v16i2.7018.
- Flores, P.C., D. Poggi, S.M. García, M. Catraro, and N.F. Gariglio. 2018. Descripción de patrones normales y anormales de plántulas de Juglans nigra (Description of normal and abnormal seedling patterns of Juglans nigra). FAVE Sección Ciencias Agrarias 17(2):23–37. ISSN: 2346-9129.
- Flores, P.C., D. Poggi, S.M. García, and N.F. Gariglio. 2011. Topographic tetrazolium testing of black walnut (Juglans nigra) seeds. Seed Sci. Technol. 39:230–235. doi: 10.15258/sst.2011.39.1.23.
- Flores, P.C., D. Poggi, S.M. García, and N.F. Gariglio. 2013. Fruit pulp of Juglans nigra affects seed germination and root growth. Seed Technol. 35(1):41–53. ISSN 1096-0724.
- Hammons, B.K. 1998. World Consumption and Production Trends. Published by International Nut Council. Hammons Products Company: Stockton, Missouri. p. 25–28.
- Hampton, J.G., B.J. Brunton, G.M. Pemberton, and J.S. Rowarth. 2004. Temperature and time variables for accelerated ageing vigour testing of pea (Pisum sativum L.) seed. Seed Sci. Technol 32(1):261–264. doi: 10.15258/sst.
- Herrera, J. 1987. Efecto de la gravedad específica de la semilla sobre el desarrollo y la producción de arroz cv. CR1113 (Effect of seed specific gravity on the development and production of rice cv. CR1113). Agron. Costarric 11(2):453–461.
- International Seed Testing Association, 1995. Handbook of vigour test methods. 3rd edition. Eds. Hampton J. G. and D.M. Tekrony Chairperson and Deputy Chairperson, Vigour Test Comitte. Zurich. p. 117.
- ISTA (International Seed Testing Association). 2003. International rules for seed testing. ISTA, Zurich. p. 333.
- Kotowiski, F. 1926. Temperature relations to germination of vegetable seed. Amer. Soc. Hort. Sci. Proc. 23:176–184.
- Li, A., J. Herrera, and R. Barboza. 1996. Efecto del envejecimiento acelerado sobre la germinación y el vigor de la semilla de China sultani (Impatiens wallerana) en almácigo (Effect of accelerated aging on germination and vigor of Impatiens wallerana seeds planted in a seedbed). Agron. Costarric 20(2):173–180.
- Marcos Filho, J., A.D. Novembre, and H.M. Chamma. 2001. Testes de envelhecimiento acelerado e de deterioraçâo controlada para avaliaçâo do vigor de sementes de soja (Accelerated aging and controlled deterioration tests to evaluate soybean seed vigor). Sci. Agric. 58:421–426. doi: 10.1590/S0103-90162001000200029.
- Marcos Filho, J., A.L. Pereira Kikuti, and L. Baptista de Lima. 2009. Métodos para avaliação do vigor de sementes de soja, incluindo a análise computadorizada de imagens (Methods for assessing soybean seed vigor, including computerized image analysis). Rev. Bras. Sementes 31(1):102–112. doi: 10.1590/S0101-31222009000100012.
- Popinigis, F. 1985. Fisiologia das sementes (Seed physiology). Ministério de Agricultura-AGIPLAN, Brasília. p. 289.
- Reid, W., M. Coggeshall, H. E. Garrett, J. Van Sambeek. 2009. Growing black Walnut for Nut production. Agroforestry in Action. Columbia, MO: University of Missouri Center for Agroforestry. p. 16.
- Rodo, A.B., and J. Marcos Filho. 2003. Accelerated aging and controlled deterioration for thedetermination of the physiological potential of onion seeds. Sci. Agric. 60(3):465–469. doi: 10.1590/S0103-90162003000300008.
- Romano, A., I. Teves, N. Torres, and L. Cazón. 2008. Variaciones en la calidad de semillas de poroto (Phaseolus vulgaris L.) por efectos del daño mecánico y su influencia en el vigor de las plántulas (Variations in quality of bean seeds (Phaseolus vulgaris L.) due to mechanical damage and their influence on seedling vigor). Idesia 26(2):83–87.
- Salinas, A.R., A.M. Yoldjian, M.L. Dietrich, R.M. Craviotto, and V. Bisaro. 2001. Pruebas de vigor y calidad fisiológica de semillas de soja (Vigor tests and physiological quality of soybean seeds). Pesqui. Agropecu. Bras 36(2):371–379. doi: 10.1590/S0100-204X2001000200022.
- Tekrony, D.M. 1995. Accelerated ageing, p. 816–822.In: Congress of the international seed testing association, Copenhagen. Seed vigour testing: Contributions to a seminar. International Seed Testing Association, Zurich.
- TeKrony, D.M. 2005. Accelerated aging test: Principles and procedures. Seed Technol. 27(1):135–146.
- TeKrony, D.M., D.B. Egli, and D.A. Wickham. 1989. Corn seed vigour effect on no tillage field performance. Crop Sci. 29:1523–1528. doi: 10.2135/cropsci1989.0011183X002900060042x.
- Woodworth, R.H. 1930. Meiosis of microsporogenesis in the Juglandaceae. Am. J. Bot. 17(9):863–869. doi: 10.1002/j.1537-2197.1930.tb04927.x.