 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study was designed to compare biochemical characteristics in fruit, leaf and shoot bark among some Iranian pomegranate genotypes to determine for any probable correlation between different parts. Twenty genotypes were used in this experiment and biochemical characteristics such as vitamin C, anthocyanin, total phenolic content (TPC), titratable acidity (TA) and total soluble carbohydrate (TSC) were evaluated in leaf, arils, fruit peel, and shoot bark separately. Results of correlation analysis showed that there was significant correlation among anthocyanin, vitamin C, and TPC of arils with anthocyanin (r = 0.93), vitamin C (r = 0.90), and TPC (r = 0.95) of the leaf, respectively. Also, a positive correlation was observed between TA, vitamin C, anthocyanin, and TPC of arils with TSC of the shoot bark. Based on obtained results from this study, biochemical traits of the leaf such as anthocyanin, vitamin C and TPC can be used as a biochemical marker to determine superior genotypes in juvenile phase of plants.
Introduction
Pomegranate (Punica granatum L.) is an ancient fruit. This species has been traditionary included in the Punicaceae family which molecular phylogenetic analyzes supported the inclusion of Punica within the Lythraceae family (Currò et al., Citation2010). It is cultivated widely in Iran, Afghanistan, India and Mediterranean countries (Gözlekçi et al., Citation2011; Karimi and Mirdehghan, Citation2015). Due to high nutritional and medicinal value, recently the pomegranate fruits are attracted by many countries in the world (Holland et al., Citation2009; Karimi and Nowrozy, Citation2017). Organic acids, minerals (such as potassium), vitamins (C, A, and K), and phenolic compounds (which widely exist in the peel, pulp and seed) are the most important compounds of pomegranate fruits (Akhtar et al., Citation2015). The phenolic compounds found in fruit include anthocyanins, catechins, phenolic acids (gallic, ellagic and chlorogenic), hydrolyzable tannins (punicalagins and punicalins), and condensed tannins (proanthocyanidins) (Qu et al., Citation2012). The presence of phenolic compounds in different parts of the pomegranate fruit and its strong antioxidant activity, protect normal cells from various stimuli-induced oxidative stress and cell death. The peel has a higher content of phenolic than the arils (Shaban et al., Citation2013). Therefore, there is a wide variety of phenolic compounds differed of fruits among different genotypes (Gözlekçi et al., Citation2011).
Iran is the center of diversity of pomegranate and its variability among the genotypes can be attributed to the difference in leaf morphological characteristics, flower shape, fruit shape, fruit size and fruit flavor (sweet, sour, and mild) (Karimi, Citation2011; Karimi and Farahmand, Citation2011; Karimi and Mirdehghan, Citation2013). Biochemical compounds are considered as biochemical marker and were used as a reliable indicator in determining genetic diversity. There is a fundamental difference between genotypes regarding chemical composition (Ismail et al., Citation2014). Gözlekçi et al. (Citation2011) found that the phenolic compounds of juice, peel and the seed among four Turkish pomegranates were significantly different. There are several reports about biochemical diversity of pomegranate fruits in Iran (Khadivi-Khub et al., Citation2015), Turkey (Caliskan and Bayazit, Citation2013), and Egypt (Ismail et al., Citation2014). They concluded that biochemical traits could considered as a suitable marker for determining and distinguishing pomegranate genotypes. The amount of biochemical compounds especially phenolics varies in different organs and tissues. This variation also depends on species and genotypes and could be related to the difference in the enzyme activity responsible in these compounds biosynthesis (Kováčik and Klejdus, Citation2014). It has also been reported that there is a close relationship between the amount of phenolic compounds in different parts of the fruit (peel, aril and seed). Cultivars with a dark-red color peel and aril displayed a higher phenolic contents (Gözlekçi et al., Citation2011).
In fruit breeding programs, the long duration juvenile phase of plants increases the time and cost of final results (Karimi and Mirdehghan, Citation2013). Estimate of correlation coefficients allows comparison of indirect with the direct selection, computation of correlated response in a second trait if selection pressure is applied to the first, and establishment of selection strategy (Karimi and Mirdehghan, Citation2013). Correlations between vegetative and reproductive traits improved strategies and periods of breeding programs through superior seedlings in the first growth stages (Karimi and Mirdehghan, Citation2013). Correlation coefficients have been estimated in several fruit including pistachio (Karimi et al., Citation2009) and pomegranate (Karimi and Mirdehghan, Citation2013). Karimi and Mirdehghan (Citation2013) studied correlations between morphological characteristics of the leaf with titratable acidity and vitamin C of arils and reported that titratable acidity and vitamin C of fruit positively correlated with chlorophyll index and leaf weight. In another experiment, Sarkhosh et al. (Citation2009) studied correlations between fruit quantitative and qualitative traits of some pomegranate genotypes and reported that the anthocyanin contents of arils negatively correlated with fruit size. However, most correlations reported for pomegranate refer to fruit characteristics and there is not any report about the relationship between different part of fruits and others parts of trees. Therefore, this study aimed to study the genetic diversity of some Iranian native pomegranate genotypes based on biochemical markers, and to investigate and distribute the relationship of biochemical compounds in fruit, leaf, and shoot bark.
Material and Methods
This study was performed during 2014 and 2015 on 26-years-old pomegranate trees grown at an experimental orchard located in the east of Nayriz, Fars province, Iran. These genotypes were: ‘PoustSabz-e-Pishva’, ‘Mahan’, ‘SiyahDaneh-e-Pishva’, ‘Gabri Daneh-e-Siyah’, ‘Tab o Larz’, ‘Atabaki’, ‘Chatrood’, ‘Malas-e-Aghda’, ‘Alak-e-Saveh’, ‘Zagh-e-Aghda’, ‘Malas-e-Mohamadzadeh’, ‘Torsh-e-Riz-e-Janati’, ‘Zoodras-e-Mohamadzadeh’, ‘Rabab-e-Kalh’, ‘Rabab- e-Khatdar’, ‘Rabab-e-Tahdar’, ‘Neyriz 68ʹ, ‘Neyriz 86ʹ, ‘Shyrin-e- Bi Hasthe’ and ‘Shirin-e-Hastedar’.Trees have been planted at 5 × 3 m distance and irrigated every 12 days. The experiment was conducted in Randomized Block Design (RBD) with three replications. Each tree was one replication. The samples of leaf, peel and arils were collected at the fruit ripening stage and immediately transported to the laboratory. Ten fruits were removed from each tree to evaluate the characteristics of the fruit.
Soluble Solid Content, Titratable Acidity and Vitamin C
The soluble solid content of juice was determined using a digital portable refractometer and expressed as %. Titratable acidity was measured by titration of 5 ml of fruit juice with 0.1 N of NaOH and expressed as a percentage of citric acid. Vitamin C in leaf, peel and bark shoot were determined according to de de Pinto et al. (Citation1999) method. Five gram of fresh leaf and fruit peel were ground with 10 mL meta-Phosphoric acid 5% and centrifuged at 10000 rpm for 15 min. Three hundred microliter of supernatant was mixed with 750 μl of potassium phosphate (1 M) and distilled water, respectively, and incubated in room temperature for 10 min. Finally, 600 μl tricarboxylic acid 10%, 600 μlrthophosphoric acid 44%, 600 Alpha, alpha’-dipyridyl 4% and 10 μl FeCl2 95% were added and vortexed. The samples were placed at 40°C for 20 min and the absorbance was read at 525 nm by a spectrophotometer (Hitachi, Instrument, Tokyo, Hapan, U-2000). Ascorbic acid was used as standard to produce the calibration curve, and results were expressed as mg.100 g−1fw.
Carbohydrate of Leaf and Peel
To determine the carbohydrate concentofleaf and peel, 0.5 g of youngest fully expanded leaf and fresh peel samples were homogenized with 5 ml of 95% ethanol. The insoluble parts of the extract were washed with 5 ml of 70% ethanol. Extracts were centrifuged at 3500 rpm for 10 min and the supernatant was separated and stored at 4ºC for carbohydrate determination. Ethanol extract (0.1 ml) mixed with 3 ml of fresh antron (200 mg antron plus 100 ml 72% sulfuric acid) in 10 ml falcons and was incubated in boiling water bath (90ºC) for 10 min. Falcons were cooled and light absorbance was measured at 625 nm by the spectrophotometer. Standard curves were prepared using pure glucose with different concentrations including; 0, 250, 500, 750, 1000, 1250, 1500, 1750, 2000, 2250 and 2500 mgl−1. The concentration of carbohydrate was expressed as mg g−1fw (Arrigoni et al., Citation1992).
Total Phenolic Contents of Aril, Peel, Leave and Bark Shoot
To determine TPC in arils, peel and bark shoot, 5 g of fresh tissue were homogenized with 10 ml phosphate buffer (pH 7.8) and centrifuged at 4800 rpm for 20 min. Then 100 μl of supernatant was mixed with 400 μl phosphate buffer and 2.5 ml Folin-Ciocalteu 50% and 2 ml sodium carbonate 7.5%. The samples were left in a water bath at 50°C for 5 min. The absorbance was measured at 760 nm using a spectrophotometer (Hitachi Instrument, Tokyo, Japan, U-2000) (Ayala-Zavala et al., Citation2004). For leaves, the extraction was carried out according to Isfendiyaroglu and Zeker (2002). Fresh plant material grounded with ethanol 95% (5 ml) and kept in dark condition for 48 h. One ml of ethanol 95% was added to 1 ml of supernatant, and total volume was adjusted to 5 ml by distilled waters, then 0.5 mL of Folin–Ciocalteu (50%) and 1 ml of calcium carbonate (5%) was added to samples. The samples were placed in a dark place for 1 h and TPC measured at 725 nm by a spectrophotometer (Hitachi Instrument, Tokyo, Japan, U-2000). TPC was calculated based on the standard curve and expressed as mg g−1 fw.
Anthocyanin Content of Aril, Peel and Leaf
The anthocyanin content of arils, peel and leaf were calculated according to Victor and Wrolstad (Citation1990) method. 0.25 g of fresh leaf, peel and arils were grounded with 10 ml acid-methanol (pure methanol and pure chloride acid in a volume ratio of 99:1) and kept in dark condition at 25°C for 24 h. Then samples were centrifuged at 4000 rpm for 10 min and the absorbance was measured at 550 nm with spectrophotometer (Hitachi Instrument, Tokyo, Japan, U-2000).
Chlorophyll of Leaf and Color Indices of Aril
In order to determine of chlorophyll of leaf, in last of July, 1 g of fresh leaf was removed from plants and was ground with 20 ml of 80% acetone and was centrifuged at 5000 rpm for 5 min, and the supernatant was transferred to a 100-ml volumetric flask. After extraction, the supernatant was diluted to the final volume of 100 ml by 80% acetone. The absorbance was measured at 663, 645 and 652 nm, using a spectronic spectrophotometer (Hasanpour et al., Citation2014). The chlorophyll a, chlorophyll b and total chlorophyll were calculated on an exponential basis, using the following equations:
In the above-mentioned equations, V and W represent the volumes of the acetone which is used and the leaf sample weight, respectively, and D represents the output of the spectrophotometer. Chroma a and L indices were recorded using colorimeter as color indices of aril. These parameters were used only in factor analysis.
Statistical Analysis
All data analysis was carried out using SAS software (ver.9.1; SAS Institute, Cary, NC, USA). One way analysis of variance (ANOVA) and multiple-range tests were used to evaluate the significance of differences among pomegranate sample composition. When analysis of variance (ANOVA) showed significant effects, Duncan test was applied to compare means at P < .05. Simple correlations, factor, and cluster analysis were carried out using SPSS (ver. 16) and Minitab (ver.17) software to reveal the relationships among the genotypes.
Results
Leaf Biochemical Traits
According to ANOVA results, vitamin C, anthocyanin, TSC and TPC of leaf significantly were affected by genotypes. The highest vitamin C (1.03 mg.100 g−1fw), anthocyanin (1.20 mg.100 g −1fw) and TPC (0.90 mg GA.g−1fw) were recorded in ‘Zoodras-e-Mohamadzadeh’, ‘Rabab-e-Kalh’ and ‘Chatrood’, respectively while as the lowest those were observed in ‘Shyrin Bi Hasth’, ‘Mahan’ and‘Torsh-e-Rizganati’, respectively ().
Table 1. Total phenolic content (TPC), anthocyanin and vitamin C in leaf of some Iranian pomegranate genotypes.
Biochemical Traits of Shoot Bark
Biochemical traits like vitamin C, TPC, TSC and anthocyanin were significantly affected by genotypes. The highest value of TSC (1.94 mg.g−1fw), vitamin C (1.57 mg.100 g−1fw), anthocyanin (0.40 mg.100 g−1fw) and TPC (6.44 mg GA.g−1fw) were observed in ‘Torsh-e-Riz-e-Janati’, ‘Zoodras-e-Mohamadzadeh’, ‘Alak-e-Saveh’ and ‘Poust Sabz-e-Pishva’, respectively. The minimum TSC and vitamin C were recorded in ‘Malas-e-Mohamadzadeh’. The genotypes of ‘Siyah Daneh-e-Pishva’ and ‘Chatrood’ had the lowest amount of TPC ().
Table 2. Total phenolic content (TPC), anthocyanin and vitamin C t in shoot bark of some Iranian pomegranate genotypes.
Peel Fruit Biochemical Traits
According to ANOVA results, TSC, vitamin C, anthocyanin and TPC of peel fruit significantly affected by genotypes.‘Torsh-e-Rizganati’, ‘Zoodras-e-Mohamadzadeh’, ‘Alak-e-Saveh’ and ‘Rabab-e-Kalh’ showed the highest amount of TSC (2.20 mg.g−1fw), vitamin C (3.13 mg.100 g−1fw), anthocyanin (9.0 mg.100 g−1fw) and TPC (10.02 mg GA.g−1fw), respectively. The lowest amount of TSC, vitamin C, anthocyanin and TPC were in ‘Malas-e- Mohamadzadeh’, ‘Alak-e-saveh’, ‘Poust Sabz-e-Pishva’ and ‘Neyriz 68ʹ genotypes, respectively. The highest coefficient of variation between leaf, bark and peel biochemical traits was observed about the anthocyanin, which indicates the role of this trait in the biochemical traits studied in evaluating genotypes and breeding actions ().
Table 3. Total phenolic content (TPC), TSC, anthocyanin and vitamin C in peel fruit of some Iranian pomegranate genotypes.
Aril Biochemical Traits
According to ANOVA results (), TSS of the arils was affected by the interaction of genotype and year whereas TA, TSS/TA, vitamin C, anthocyanin and TPC only were significantly affected by year and genotype (P < .05). The results also showed that the maximum TSS of aril was observed in ‘Zoodras-e-Mohamadzadeh’ (20.36) and ‘Shyrin-e-Bi Hasthe’ (20.20) genotypes in the second year and the minimum it was observed in ‘Malas-e-Aghda’ (11.13) genotype ( and ). The results also showed that the highest TA (1.79%), TSS/TA (39.39), vitamin C (40.72 mg.100 g−1fw), anthocyanin (6.49 mg.100 g−1fw) and phenol (7.17 mg GA.g−1fw) were recorded in ‘Zoodras-e-Mohamadzadeh’, ‘Shyrin-e-Bi Hasthe’, ‘Neyriz 86ʹ, ‘Chatrood’ and ‘Zoodras- e-Mohamadzadeh’, respectively. The lowest TA, TSS/TA, vitamin C, anthocyanin and TPC were observed in ‘Shyrin-e-Bi Hasthe’, ‘Chatrood’, ‘Atabaki’, ‘Alake-e-Saveh’, ‘Zagh-e-Aghda’ and ‘Tab o Larz’, respectively. Based on the coefficient of variation, vitamin C and TSS/TA showed the highest value but TA and anthocyanin traits showed the minimum. Therefore, vitamin C and TSS/TA were important biochemical factors of arils in determining genetic variation ().
Table 4. Total phenolic content (TPC), anthocyanin, vitamin C, TSS and TA ratio in arils of some Iranian pomegranate genotypes.
Table 5. Total soluble solid and TSS in leaf and aril of some Iranian pomegranate genotypes.
Factor Analysis
Factor analysis was used to determine the number of main factors to reduce the number of effective characteristics to discriminate between genotypes (). Based on factor analysis, the characteristics of fruit peel and arils accounted for 70.77% of the variance as the first main factor. For each factor, a factor loading of more than 0.59 was considered as being significant. For the first factor, characteristics including TSS, vitamin C, TPC and anthocyanin of arils and had a loading of more than 0.59 and defined 70.77% of the overall variance. The chlorophyll a, b and total were significant for the second factors with 10.58% of the overall variance. The third factor with 5.46% of the overall variance contributed to color aril (a, L and coroma indices).
Table 6. Cumulative variance and eigenvalue for three major factors obtained from factor analysis for studied pomegranate genotypes.
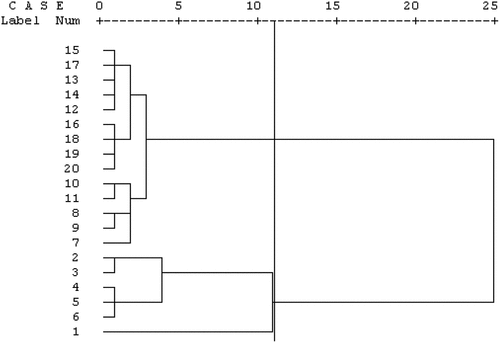
Cluster Analysis
The pomegranate genotypes were grouped according to three factors. Cluster analysis divided the genotypes into three groups. ‘PoustSabz-e-Pishva’ genotype was separated from other genotypes in first group. ‘Mahan’, ‘SiyahDaneh-e-Pishva’, ‘GabriDaneh-e-Siyah’, ‘Tab o Larz’ and ‘Atabaki’ were located in a similar group (second group). Other genotypes clustered in 30 groups ().
Figure 1. Cluster analysis of studied pomegranate genotypes based on biochemical traits. 1: ‘Poust Sabz Pishva’, 2: ‘Mahan’, 3: ‘Siyah Daneh Pishva’, 4: ‘Gabri daneh Siyah’, 5: ‘Tab o Larz’, 6: ‘Atabaki’, 7: ‘Chatrood’, 8: ‘Malas e Aghda’, 9: ‘Alak e Saveh’, 10: ‘Zagh e Aghda’, 11: ‘Malas e Mohamadzadeh’, 12: ‘Torsh e Rizganati’, 13: ‘Zoodras e Mohamadzadeh’, 14: ‘Rabab e Kalh’, 15: ‘Rabab e Khatdar’, 16: ‘Rabab e Tahdar’, 17: ‘Neyriz 68ʹ, 18: ‘Neyriz 86ʹ, 19: ‘Shyrin Bi Hasthe’ and 20 ‘Shirin Hastedar’.
Correlation between Biochemical Traits in Arils and Leaf
Correlation between each pair of leaf and arils traits was calculated. It was found that anthocyanin, vitamin C and TPC of arils were significant correlated with anthocyanin (r = 0.93), vitamin C (r = 0.9) and TPC (r = 0.95) of leaf. As a conclusion, the genotypes had more leaf anthocyanin, vitamin C, and TPC also had more arils anthocyanin, vitamin C and TPC which could be used to select superior genotypes in the juvenile phase. The results also indicated that there was a negative correlation between chlorophyll a (r = −0.39) and b (r = −0.38) of the leaf with anthocyanin of arils. In addition, total chlorophyll of leaf was in negative correlation with TA/TSS (r = −0.41) and TPC (r = −0.38) of arils ().
Table 7. Bivalent correlations among some biochemical traits of leaf and aril in pomegranate genotypes.
Correlation between Biochemical Traits in Fruit Peel and Leaf
According to the correlation results of biochemical traits peel and leaf, there was a positive correlation between vitamin C of leaf and peel (r = 0.93). Also TPC (r = 0.88) and anthocyanin (r = 0.96) of peel were in positive correlation with TPC (r = 0.88) and anthocyanin of leaf, respectively (data not shown).
Correlation between Biochemical Traits in Fruit Peel and Arils
Correlation between biochemical traits of peel and arils showed that there was a positive correlation between vitamin C of arils and peel (r = 0.94), TPC of arils and peel (r = 0.44), anthocyanin of arils and peel and also TSC of peel and TSS of arils (r = 0.51) (data not shown).
Correlation between Biochemical Traits in Shoot Bark and Arils
Results of correlation between biochemical traits of shoot bark and arils showed that some biochemical traits of arils positively correlated with biochemical traits of shoot bark. Traits such as TA, vitamin C, anthocyanin and TPC of arils had a positive correlation with TSC of the shoot bark. Also, results showed that TPC, anthocyanin and vitamin C of fruit peel were in positive correlation with TPC, anthocyanin and vitamin C of shoot bark, respectively ().
Table 8. Bivalent correlation among some biochemical traits of shoot bark, aril and peel fruit in pomegranate genotypes.
Discussion
The results showed that leaf biochemical traits were significantly affected by genotypes. Similar results have been reported in litchi (Prasad et al., Citation2010) and pomegranate (Karimi and Mirdehghan, Citation2013). Change in the biochemical composition of the leaf has also been reported to be associated with different phenology of the plants. In addition, leaves are considered as a source of carbohydrate production site in plants due to the presence of pigments. Therefore, variation regarding leaf biochemical traits may be due to differences in the number of pigments and leaf photosynthesis activity (Prasad et al., Citation2010). Leaf total soluble carbohydrate and total phenolic compounds were used for classification of grape genotypes (Kato et al., Citation2012). Prasad et al. (Citation2010) classified litchi genotypes in terms of leaf total amino acid, TSC, and total soluble proteins. Evaluation of leaf biochemical traits is suitable not only for determining the resistance of genotypes to environmental conditions and variation but also can be used to studies the effects of medicinal and nutritional value on human (Picinelli et al., Citation1995). Several study focused on medicine and nutritional properties of olive (Pereira et al., Citation2006) and apple (Liaudanskas et al., Citation2014) leave composition. The antioxidant and anticancer properties of pomegranate leaves were reported (Nawwar et al., Citation1994; Zhang et al., Citation2010). According to the results of this study, it can be concluded that genotypes with highest phenolic compounds in leaves and fruits had more antioxidant properties. Hence, genotypes with higher anthocyanin leave have higher resistance to UV radiation, so it can be concluded that genotypes with higher levels of anthocyanin are recommended for higher altitude lands (Kato et al., Citation2012).
Our results also showed that peel biochemical traits were significantly affected by genotypes. These results were in agreement with the results of Mars and Marrakchi (Citation1999) and Khadivi-Khub et al. (Citation2015) in pomegranate genotypes. Peel color indices of pomegranate are one of the most traits that is affected by anthocyanin content in peel (Khadivi-Khub et al., Citation2015). Mars and Marrakchi (Citation1999) reported that peel color indices were significantly affected by pomegranate genotypes that were grown in Tunisia. Sarkhosh et al. (Citation2009) also suggested that peel color indices is one of the most important traits to determine variation in Iranian pomegranate genotypes.
Arils biochemical characteristic also affected significantly by different pomegranate genotypes. Variation of arils biochemical traits such as TA, TSS and anthocyanin content was reported in Turkish pomegranate genotypes (Caliskan and Bayazit, Citation2013). There are also many reports on fruits vitamin C content between genotypes and plant clones. It has been reported that genotypes with higher levels of TPC, vitamin C and TSC are more tolerant to biotic and abiotic stress conditions (Gill and Tuteja, Citation2010; Love et al., Citation2004; Wang and Gao, Citation2013). Also there are several reports in different amounts of pomegranate biochemistry such as acidity, fruit flavor, anthocyanin and fruit sugar which could be used as a marker for determining and distinguishing pomegranate genotypes from each other (Caliskan and Bayazit, Citation2013; Karimi and Mirdehghan, Citation2013; Khadivi-Khub et al., Citation2015; Ismail et al., Citation2014).
Fruit growth is affected by the relationship between carbohydrate sink and source. There is a close relationship between leaf as source and a fruit as a sink in which fruits growth and its quality is affected by the leaf (Smith and Stitt, Citation2007). A linear relationship between leaf carbohydrate and fruits carbohydrate had been reported in apple trees (Alan and Goffinet, Citation2013). In fact, the recognition of the relationship between leaf and fruit play an important role in determining the quality of the fruit. Therefore with using correlation coefficient there is a chance to find the compounds in fruits by evaluating the traits of the leaves (Nachtigall and Dechen, Citation2006).
Factor analysis shows that biochemical traits of the arils and peel provided the primary factor, confirming 70.77% of the total variance, which must be considered in distinguishing of pomegranate genotypes. In similar study, Karimi and Mirdehghan (Citation2013) reported that fruit traits were the most important factor in distinguishing of pomegranate genotypes.
There were positive and negative relationships between biochemical traits of leaf and arils. It means that the genotypes with high anthocyanin, vitamin C and TPC in leaf had more arils anthocyanin, vitamin C. Therefore TPC in arils could be used to select superior genotypes in the juvenile phase. Karimi and Mirdehghan (Citation2013) reported that titratable acidity (TA) and vitamin C in pomegranate leaves correlated with chlorophyll index and leaf weight.‘Shyrin-e-Bi Hasthe’ (seedless) genotypes had more phenolic compounds than ‘Shirin-e-Hastedar’ genotypes. High level of phenolic compounds in fruits caused stimulation of seedless fruits in tomato (Ingrosso et al., Citation2011) which are in agreements with our results. Also, Ryan et al. (Citation2002) showed that increasing of leaf phenolic compounds increased phenolic compounds in mesocarp of olive fruits. Peel color depends on the anthocyanin concentration. The higher anthocyanin content in fruit peel the higher color of fruit came to be. Similar results were reported by Sarkhosh et al. (Citation2009) on some Iranian pomegranate genotypes. The results of this study showed that arils biochemical traits correlated with peel biochemical traits so that the genotypes with more peel colorful display higher aril anthocyanin concentration. The positive correlations between peel and aril were reported in some Iranian (Khadivi-Khub et al., Citation2015) and Turkish (Gözlekçi et al., Citation2011) pomegranate genotypes which are in agreement with our results. The correlation between the peel and aril color is probably due to the activation of anthocyanin biosynthesis pathway genes that occur in both parts of the fruit.
Conclusion
In summary, our results showed that biochemical traits of aril, peel, leaves and shoot bark of pomegranate were significantly affected by genotypes, but these traits were not affected by years. It’ means that, biochemical traits such as vitamin C, TPC, TSS and TA in arils could be used as a suitable biochemical marker to determine variation between pomegranate genotypes. The results also indicated that there was apositive correlation between biochemical traits of shoot bark, leaves and peel with arils. Based on obtained results from this study, biochemical traits of the leaf such as anthocyanin, vitamin C and TPC can be used as a biochemical marker to determine superior genotypes in juvenile phase of plants. Therefore, it is possible to use these traits to select pomegranate seedlings in early stages of breeding.
Data archiving statement
The data is currently being submitted should still be included. The accession numbers are listed in .
Literature
- Akhtar, S., T. Ismail, D. Fraternale, and P. Sestili. 2015. Pomegranate peel and peel extracts: Chemistry and food features. Food. Chem. 174:417–425. doi: 10.1016/j.foodchem.2014.11.035.
- Alan, N.L., and M.C. Goffinet. 2013. Apple fruit growth. New York Fruit Q. 21:11–14.
- Arrigoni, O., L. De Gara, F. Tommasi, and R. Liso. 1992. Changes in the ascorbate system during seed development of Vicia faba L. Plant Physiol. 99:235–238. doi: 10.1104/pp.99.1.235.
- Ayala-Zavala, F., Y. Wang, C.Y. Wangm, and G. Gonzalez-Aguilar. 2004. Effect of storage temperatures on antioxidant capacity and aroma compounds in strawberry fruit. LWT- Food Sci. Technol. 37:687–695. doi: 10.1016/j.lwt.2004.03.002.
- Caliskan, O., and S. Bayazit. 2013. Morpho-pomological and chemical diversity of pomegranate accessions grown in Eastern Mediterranean region of Turkey. J. Agric. Sci. Technol. 15:1449–1460.
- Currò, S., M. Caruso, and G. Distefano. 2010. New microsatellite loci for pomegranate, Punicagranatum L. (Lythraceae). Am. J. Bot. 97:58–60. doi: 10.3732/ajb.1000143.
- de Pinto, M.C., D. Francis, and L. de Gara. 1999. The redox state of the ascorbate-dehydroascorbate pair as a specific sensor of cell division in tobacco BY-2 cells. Protoplasma 209:90–97. doi: 10.1007/BF01415704.
- Gill, S.S., and N. Tuteja. 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48:909–930. doi: 10.1016/j.plaphy.2010.08.016.
- Gözlekçi, Ş., O. Saraçoğlu, E. Onursal, and M. Özgen. 2011. Total phenolic distribution of juice, peel, and seed extracts of four pomegranate cultivars. Pharmacogn Mag. 7:161. doi: 10.4103/0973-1296.80681.
- Hasanpour, Z., H.R. Karimi, and S.H. Mirdehghan. 2014. Effects of salinity and water stress on echophysiological parameters and micronutrients concentration of pomegranate (Punuca granatum L.). J. Plant Nutr. 38:1–13.
- Holland, D., K. Hatib, and I. Bar‐Ya’akov. 2009. Pomegranate: Botany, horticulture, breeding. Hortic Rev. 35:127–191.
- Ingrosso, I., S. Bonsegna, S. De Domenico, B. Laddomada, F. Blando, A. Santino, and G. Giovinazzo. 2011. Over-expression of a grape stilbene synthase gene in tomato induces parthenocarpy and causes abnormal pollen development. Plant Physiol. Biochem. 49:1092–1099. doi: 10.1016/j.plaphy.2011.07.012.
- Ismail, O.M., R.A. Younis, and A.M. Ibrahim. 2014. Morphological and molecular evaluation of some Egyptian pomegranate cultivar. Afr. J. Biotechnol. 13:154–167.
- Karimi, H.R., A. Zamani, A. Ebadi, and M.R. Fatahi. 2009. Morphological diversity of Pistacia species in Iran. Genet. Resour. Crop Evol. 56:561–571. doi: 10.1007/s10722-008-9386-y.
- Karimi, H.R., and M. Nowrozy. 2017. Effects of rootstock and scion on graft success and vegetative parameters of pomegranate. Sci. Hortic. 214:280–287. doi: 10.1016/j.scienta.2016.11.047.
- Karimi, H.R., and S.H. Mirdehghan. 2013. Correlation between the morphological characters of pomegranate (Punica granatum L.) traits and their implications for breeding. Turk. J. Bot. 37:355–362.
- Karimi, H.R., and S.H. Mirdehghan. 2015. Effects of self, open and supplementary pollination on growth pattern and characteristics of pomegranate fruit. Int. J. Fruit Sci. 15:382–391. doi: 10.1080/15538362.2015.1009974.
- Karimi, R. 2011. Stenting (cutting and grafting): A new technique for propagation pomegranate (Punicagranatum L.). J. Fruit Ornamental Plant Res. 19:73–79.
- Karimi, R., and H. Farahmand. 2011. Study of pomegranate (Punica granatum L.) propagation using bench grafting. J. Fruit Ornamental Plant Res. 19:67–72.
- Kato, C.G., C.D. Tonhi, and E. Clemente. 2012. Antocianinas de uvas (Vitis vinifera L.) produzi da semsistema convencional. Revisa Brasileira De Techologia Agroindustrial 6:809–821.
- Khadivi-Khub, A., M. Kameli, N. Moshfeghi, and A. Ebrahimi. 2015. Phenotypic characterization and relatedness among some Iranian pomegranate (Punica granatum L.) accessions. Trees 29:893–901. doi: 10.1007/s00468-015-1172-9.
- Kováčik, J., and B. Klejdus. 2014. Induction of phenolic metabolites and physiological changes in chamomile plants in relation to nitrogen nutrition. Food. Chem. 142:334–341. doi: 10.1016/j.foodchem.2013.07.074.
- Liaudanskas, M., P. Viškelis, R. Raudonis, D. Kviklys, N. Uselis, and V. Janulis. 2014. Phenolic composition and antioxidant activity of Malus domestica leaves. Sci. World J. 2014:1–10. doi: 10.1155/2014/306217.
- Love, S.L., T. Salaiz, B. Shafii, W.J. Price, A.R. Mosley, and R.E. Thornton. 2004. Stability of expression and concentration of ascorbic acid in North American potato germplasm. Hortic. Sci. 39:156–160.
- Mars, M., and M. Marrakchi. 1999. Diversity of pomegranate (Punica granatum L.) germplasm in Tunisia. Genet. Resour. Crop Evol. 46:461–467. doi: 10.1023/A:1008774221687.
- Nachtigall, G.R., and A.R. Dechen. 2006. Seasonality of nutrients in leaves and fruits of apple trees. Scientia Agricola 63:493–501. doi: 10.1590/S0103-90162006000500012.
- Nawwar, M.A., S.A. Hussein, and I. Merfort. 1994. Leaf phenolics of Punica granatum L. Phytochemistry 37:1175–1177. doi: 10.1016/S0031-9422(00)89552-7.
- Ismail, O. M., A. A. Younis, and A. M. Ibrahim. 2014. Morphological and molecular evaluation of some egyptian pomegranate cultivars. African Journal of Biotechnology 13:226–237.
- Pereira, J.A., A.P. Pereira, I.C.F. Ferreira, P. Valentao, P.B. Andrade, R. Sebra, L. Estevinho, and A. Bento. 2006. Table olives from Portugal: Phenolic compounds, Antioxidant potential and antimicrobial activity. J. Agric. Food Chem. 54:8425–8431. doi: 10.1021/jf061769j.
- Picinelli, A., E. Dapena, and J.J. Mangas. 1995. Polyphenolic pattern in apple tree leaves in relation to scab resistance. A preliminary study. J. Agr. Food Chem. 43:2273–2278. doi: 10.1021/jf00056a057.
- Prasad, A., B. Das, and M. Gerad. 2010. Differentiation litchi genotypes based on leaf biochemical parameters: A feasibility study. Biospectra 5:105–110.
- Qu, W., A.P. Breksa, Z. Pan, and H. Ma. 2012. Quantitative determination of major polyphenol constituents in pomegranate products. Food. Chem. 132:1585–1591. doi: 10.1016/j.foodchem.2011.11.106.
- Ryan, D., M. Antolovich, P. Prenzler, K. Robards, and S. Lavee. 2002. Biotransformations of phenolic compounds in Olea europaea L. Sci. Hortic. 92:147–176. doi: 10.1016/S0304-4238(01)00287-4.
- Sarkhosh, A., Z. Zamani, R. Fatahi, and H. Ranjbar. 2009. Evaluation of genetic diversity among Iranian soft-seed pomegranate accessions by fruit characteristics and RAPD markers. Scientia Horticultureae 121:313–319. doi: 10.1016/j.scienta.2009.02.024.
- Shaban, N.Z., M.A. El-Kersh, F.H. El-Rashidy, and N.H. Habashy. 2013. Protective role of Punica granatum (Pomegranate) peel and seed oil extracts on di-ethylnitrosamine and phenobarbital-induced hepatic injury in male rats. Food. Chem. 141:1587–1596. doi: 10.1016/j.foodchem.2013.04.134.
- Smith, A.M., and M. Stitt. 2007. Coordination of carbon supply and plant growth. Plant Cell Environ. 30:1126–1149. doi: 10.1111/j.1365-3040.2007.01708.x.
- Victor, H., and R.E. Wrolstad. 1990. Characterization of anthocyanian-containing colours and fruit juice by HPLC/photodiode arrary detection. J. Agric. Food Chem. 38:698–708. doi: 10.1021/jf00093a025.
- Wang, S.Y., and H. Gao. 2013. Effect of chitosan-based edible coating on antioxidants, antioxidant enzyme system and postharvest fruit quality of strawberries (Fragaria × aranassa Duch.). LWT- Food Sci. Technol. 52:71–79. doi: 10.1016/j.lwt.2012.05.003.
- Zhang, L., Y. Gao, Y. Zhang, J. Liu, and J. Yu. 2010. Chang in bioactive compounds and antioxidant activities in pomegranate leaves. Sci. Hortic. 123:543–546. doi: 10.1016/j.scienta.2009.11.008.
