ABSTRACT
Post-harvest loss negatively impacts food security, nutrition and economic stability of farmers, exporters, traders and consumers. Experiments were conducted to assess the effects of post-harvest techniques on the shelf life of Apple and Palmer mango cultivars under different storage conditions. Post-harvest losses of these fruit along the supply chain were also evaluated. A two-factors factorial experiment with six replications was used for each cultivar. Post-harvest techniques included dipping of fruit in hexanal solution (0.02% v/v), calcium chloride solution (2% w/v), smoke treatments and untreated fruit. The fruit were then stored at two different storage conditions namely: ambient temperature (28 ± 2°C) and cold storage (18 ± 2°C). Shelf life data was analyzed by using R-software. Mean separation was done by using Tukey Honestly Significant Difference at (p ≤ 0.05). Results showed that the major sites of post-harvest losses were at harvest, transport, wholesale and retail stages of supply chain. Furthermore, post-harvest treatments of fruit with hexanal and calcium chloride significantly increased shelf life and reduced disease incidences compared to untreated control and smoke-treated fruit. Cold storage significantly increased shelf life of mango fruit compared to ambient storage. Therefore, hexanal, calcium chloride and cold storage are recommended to extend fruit shelf life, maintain fruit firmness and to reduce disease incidences in mango fruit.
Introduction
Mango (Mangifera indica L.) is among the major fruit produced and consumed in Tanzania. The export potential and international trade of mango is limited by several factors such as its perishable nature, diseases (Singh et al., Citation2013) and insects infestations. Post-harvest loss refers to measurable quantitative and qualitative food loss in the post-harvest system (Kiaya, Citation2014). Post-harvest losses in developing countries are very high. Msogoya and Kimaro (Citation2011) recorded losses of 48–60% in fresh mango in Tanzania. Reduction of post-harvest losses and quality deterioration are essential in order to increase food availability from existing production (Kasso and Bekele, Citation2018).
Tanzanian traders normally buy fruit at farm gate for sell to local and international markets. However there are no specialized facilities for storage, treatment or transport. Losses are incurred at harvest, packing, transport, and while at wholesale and retail markets. Additional losses are suffered due to unforeseen delays at customs, offloading points or due to trucks breakdown. These necessitate the need to extend the shelf life of fruit to prevent further losses.
Smoke is commonly used by smallholder Tanzanian farmers to hasten fruit ripening (Baltazari et al., Citation2018). However, there is insufficient information on the effects of smoke on internal and external quality parameters and shelf life of mango fruit. Post-harvest fruit treatment using hexanal enhance freshness and shelf life of fruit such as apple, sweet cherry, peaches, mango, guava, banana and strawberry (Paliyath et al., Citation2008). Hexanal treatment significantly delay ripening and senescence of fruit and extended fruit shelf life (Paliyath and Droillard, Citation1992). Hexanal application has been reported to improve post-harvest fruit quality of guava, plum, grapes, sweet cherries, papaya, apples, pears, peaches, mango and strawberries (Paliyath, Citation2011; Paliyath et al., Citation2008) in India and Canada. However, post-harvest hexanal application has been tested in limited geographies, and in fruit cultivars from India and Canada (Paliyath et al., Citation2008). There is therefore no information on effects of hexanal on shelf life of fruit species and cultivars grown in the African tropics and specifically in Tanzania. Moreover, post-harvest techniques used by Tanzanian farmers are not well evaluated and documented. Mango is a climacteric fruit with export potential, its response to hexanal treatment for delayed ripening will be of interest to growers and exporters (Jincy et al., Citation2017).
Calcium chloride is another compound used for post-harvest treatment of fruit. Mishra (Citation2002) reported that calcium chloride delay ripening, reduce post-harvest decay and improve the nutritional quality of fruit and vegetables. Lara et al. (Citation2004) reported delayed fruit ripening, improved resistance to fungal attack and maintained structural integrity of strawberry cell walls after being dipped in 1% solution of calcium chloride.
Fruit production faces numerous post-harvest problems, that lead to both high quantitative and qualitative losses (Malik et al., Citation2004; Musasa et al., Citation2013). Post-harvest losses in the horticultural production chain are a challenge in both developing and developed countries (Musasa et al., Citation2013). Fruit are liable to damage and deterioration during harvesting, transportation, marketing, storage and consumption, if not properly handled (Ilyas et al., Citation2007; Murthy et al., Citation2009). The aim of this study was to evaluate the extent of post-harvest losses of mango fruit along the supply chain and to evaluate the effects of hexanal, calcium chloride and smoke treatment on quality of these fruit.
Materials and Methods
Hypothesis
Post-harvest losses of mango fruits are high and they vary with the stage of the supply chain
Post-harvest techniques such as hexanal, calcium chloride and smoke treatments improve the quality of treated mango fruit.
Description of Study Area
Mango fruit were harvested when mature but not ripe based on the fullness of the cheeks, skin glossiness, flesh color, development of the shoulders and number of days from bloom to harvest (Kapilan and Anpalagan, Citation2015; Mohammed et al., Citation2018). Fruit were harvested from Lunyala village in Mwalusembe ward (7.1435°S, 39.0542°E and 58.30 meters above sea level) in Mkuranga district, Coast region in eastern Tanzania during the December 2016 and December 2017 mango seasons. Apple and Palmer, the commonly grown cultivars in the study area were used.
After harvest, the marketable and unmarketable fruit were separated. The marketable quality of the fruit was subjectively assessed according to method by Mohammed et al. (Citation1999) with some modifications. On each sampling time, marketability of the fruit was assessed using a 1– rating scale, with 1 = unusable, 3 = unsalable (poor), 5 = fair, 7 = good, 9 = excellent; to evaluate the fruit quality. The mechanical damage, disease and insect freedom, softness, surface defects, and shrinkage were used as visual parameters for the rating. The ratings were averaged and the fruit that were rated of five and above were considered marketable, while those rated less than five were considered unmarketable.
Evaluation of Post-harvest Losses
Marketable fruit were loaded in the open truck cushioned by dry grass and transported from the Coast region to Sokoine University of Agriculture, Morogoro (234 km away). Upon arrival, the unmarketable fruit were sorted, counted and causes of damage were established. Marketable fruit were packed in bamboo crates cushioned with dry grass and placed under the shade for three days to simulate the wholesale market conditions. Fruit were then examined for damage and unmarketable fruit were counted and discarded. Remaining marketable fruit were once again placed under the shade for the six more days (making a total of nine days) to simulate the retail market conditions. On the 9th day after harvest, the fruit were evaluated and the number of unmarketable fruit with their respective cause of damage was recorded.
Post-harvest losses were evaluated according to the method by Msogoya and Kimaro (Citation2011) with some modifications. The post-harvest loss at each stage was calculated as the percentage of fruit loss specific to a defined point along the supply chain. A total of 5 000 mango fruit of each cultivar were harvested, sorted and graded to remove all the unmarketable fruit with their respective cause of damage being recorded. The causes of fruit damage were identified as either due to physical/mechanical damage, microbial decay, softening or insect damage and their percent losses were calculated. The experiments were repeated for 2 consecutive seasons of 2016 and 2017 mango seasons. The harvested fruit were sorted and graded to remove all the unmarketable fruit and their respective cause of damage was recorded. Unmarketable fruit were characterized by defects caused by insect infestations, microbial decay, broken and bruised skin, soft and shriveled fruit. The causes of fruit damage were identified and described as either due to mechanical damage, microbial decay, softening or due to insect infestations. The mechanical injury was scored as the number of fruits with cuts, scrapes, compressions and bruises (Brecht et al., Citation2010). The incidence of microbial decay was scored as the number of fruits with decay symptoms, and the causal agents of decay were identified according to Snowden (Citation2008). The insect infestations were scored upon the presence of larvae of fruit flies in the fruit followed method by Mwatawala et al., Citation2009).
Post-harvest Techniques and Design of Experiment
Thirty fruit of each cultivar were exposed to each post-harvest treatments and for shelf life, loss of firmness and disease incidence. Fruit were dipped in calcium chloride solution at (2% w/v) and hexanal solution at (0.02% v/v) for 5 min. Smoke was applied following a method by Baltazari et al. (Citation2018). The fruit were exposed to smoke obtained from burning 1.0 kg of dried banana leaves, which was done three times at intervals of 12 h in the chamber with a volume of 12 m3. The chambers were ventilated for 30 min by opening between smoke treatments. The chambers were located six meters from the source of smoke to reduce heat transfer to the fruit. The effectiveness of different concentrations of calcium chloride, hexanal and smoke in extending the shelf life, maintain fruit firmness and management of post-harvest diseases of mango cultivars were compared with the control under ambient (28 ± 2°C) and cold or reduced (18 ± 2°C) temperatures storage conditions. The experiments were replicated six times.
Shelf Life Assessment
The shelf life is the length of time for which an item remains usable, edible or salable. Shelf life was determined as the period (in days) through which the fruit remained marketable until 50% of the fruit was considered unmarketable. The fruit were assessed daily and when the half of the fruit were unmarketable, they were discarded and the time (number of days) recorded.
Loss of Fruit Firmness
Fruit firmness was evaluated by using penetrometer (Wagner fruit test FT 20 Model, Wagner Instruments, Italy). Loss of firmness (softening) was established as change in firmness of fruit between harvest and after 12 days of storage.
Anthracnose Disease Incidence
The incidence of post-harvest diseases on fruit were determined according to method by (Dodd et al., Citation1991). The causal agents of decay/diseases were identified to species level with the aid of microscope based on symptoms, colony cultures, and their macro and micro-morphology, using a compendium of fruit diseases (Snowden, Citation2008).
Statistical Analyses
Two-way factorial ANOVA with six replications was used to analyze data. The factors were three post-harvest techniques (hexanal, calcium chloride and smoke treatment) and storage conditions (ambient (28 ± 2°C) and cold/reduced (18 ± 2°C) temperatures). The disease incidence data were arcsine percent transformed before analysis. Then, data for shelf life, change in firmness and disease incidences of each mango cultivars were separately analyzed by using R-software. Post hoc was performed using Tukey Honestly Significant Difference (HSD) at (p ≤ 0.05).
Results
Post-harvest Losses at Different Stages of Mango Supply Chain
We present results of two independent experiments for Apple and Palmer mango cultivars that were conducted for two consecutive seasons of 2016 and 2017. The post-harvest losses were evaluated from untreated fruit. The effects of post-harvest techniques namely hexanal, calcium chloride, smoke treatments and control on shelf life, loss of firmness and prevalence of post-harvest diseases were evaluated under ambient and cold/reduced temperature storage conditions.
Post-harvest losses (%) of Apple and Palmer mango fruit cultivars along the supply chain during 2016 and 2017 mango seasons are presented in . The Apple mango fruit had post-harvest losses of 49.02% and 44.11% during the 2016 and 2017 seasons, respectively, with an average loss of 46.57%. The Palmer mango cultivar suffered 43.16% post-harvest losses in 2016 and 41.55% in 2017 with an average loss of 42.36%. The apple mango cultivar suffered higher post-harvest losses than Palmer cultivar. Chi square (χ2) results showed that losses along the supply chain were significantly dependent on cultivar during 2016 (χ2 (1) = 5.095, p = .024) and 2017 season (χ2 (1) = 9.55, p = .002).
Table 1. Post-harvest losses of Apple and Palmer mango cultivars at different stages of supply chain during 2016 and 2017 mango seasons.
Cause of Losses at Different Stages of Mango Supply Chain
The main causes of post-harvest losses of Apple and Palmer mango cultivar fruit during 2016 and 2017 seasons are as shown on . The Apple cultivar fruit suffered highest post-harvest losses at transport stage while Palmer cultivar fruit suffered highest post-harvest losses at retail stage. The post-harvest losses were caused by crashing, bruising, infestation by insects such as fruit flies, over-ripening, post-harvest diseases and scratching by farm tools. Chi square test results showed that losses along the supply chain were significantly dependent on cultivar at transport (χ2 (1) = 6.038, p = .014) and wholesale stages (χ2 (1) = 4.96, p = .026) but not at harvest (χ2 (1) = 2.02, p = .155) and retail stages (χ2 (1) = 3.582, p = .058).
Table 2. Causes of post-harvest damage of Apple and Palmer mango cultivars at different stages of supply chain in 2016 and 2017 seasons.
Shelf Life of Mango Fruit
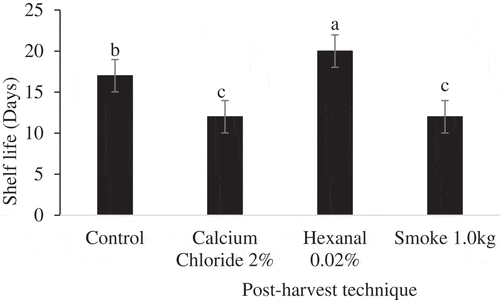
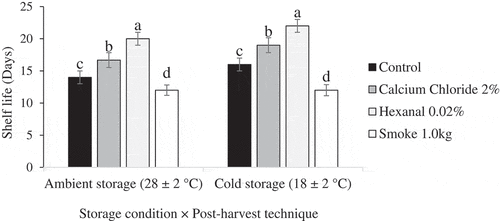
The two-way ANOVA results showed significant effects of post-harvest technique on shelf life of Apple and Palmer cultivars fruit. Post-harvest techniques × storage duration had significant effects on shelf life of fruit of Apple but not Palmer cultivar (). However, storage condition had non-significant effects on shelf life of fruit of both cultivars. Further analysis of simple means showed that post-harvest technique had significant effects on shelf life of Apple cultivar fruit under both ambient and cold/reduced temperatures storage conditions (). The hexanal-treated fruit had a shelf life of 20 and 22 days under ambient and cold/reduced temperature storage conditions, respectively. The smoke-treated fruit had the lowest shelf life under both storage conditions. analysis of variance for main means showed that post-harvest techniques had significant effects (p < .001) on shelf life of Palmer fruit () regardless of storage condition. Hexanal extended the shelf life Palmer cultivar fruit to 20 days while calcium chloride extended the shelf life to 17 days.
Table 3. Effects of storage duration, storage conditions and post-harvest techniques on shelf life of Apple and Palmer mango cultivars.
Figure 1. Mean shelf life of Apple mango cultivar exposed to post-harvest techniques at ambient (F (3, 20) = 93.361, p < .001) and cold/reduced temperatures (F (3, 20) = 42.639, p < .001) storage condition.Post hoc test was done by Tukey HSD. Means with the same letters are not significantly different at p ≤ 0.05.
Loss of Fruit Firmness
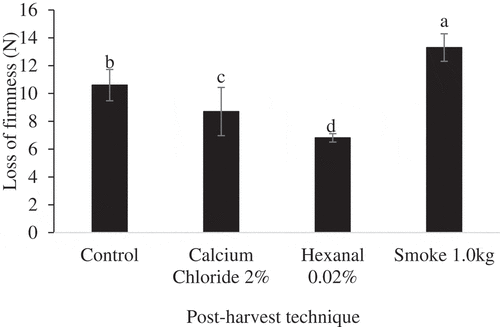
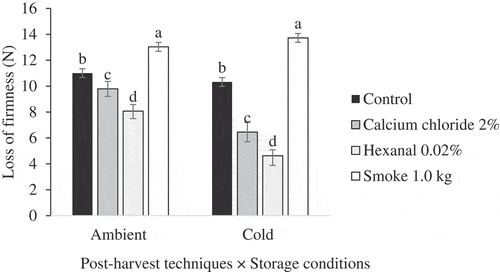
ANOVA results showed significant effects of post-harvest techniques on loss of firmness of Apple and Palmer mango cultivars fruits (). Furthermore, the effects of storage condition, and storage condition × post-harvest techniques were significant for Apple but insignificant for Palmer cultivar fruit. Analysis of simple means showed that post-harvest techniques had significant effects on loss of firmness of Apple cultivar fruit under both ambient and cold storage conditions (). Further analysis of main means showed that post-harvest techniques had significant effects on loss of firmness of Palmer mango fruit () regardless of storage condition. We recorded less changes in firmness in hexanal-treated Apple cultivar fruit stored under both ambient and cold conditions. We also recorded less loss of firmness of Palmer cultivar fruit treated with hexanal. Generally, loss of firmness was highest in smoke-treated fruit of both cultivars.
Table 4. Effects of storage duration, storage conditions and post-harvest techniques on loss of firmness of Apple and Palmer mango cultivars.
Figure 3. Mean Loss of firmness of Apple mango cultivar exposed to post-harvest techniques at ambient (F (3, 20) = 21.347, p = .001) and cold/reduced temperatures (F (3, 20) = 20.705, p = .017) storage condition.Post hoc test was done by Tukey HSD. Means with the same letters are not significantly different at p ≤ 0.05.
Anthracnose Disease Incidences
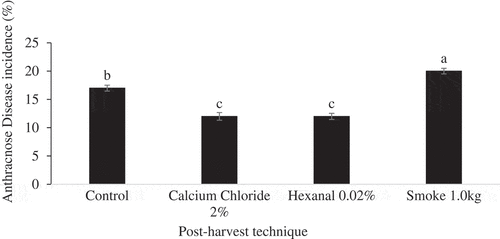
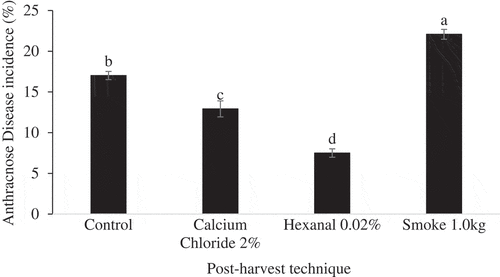
ANOVA results showed that storage condition and post-harvest technique had significant effects on the incidence of anthracnose disease on fruit of Apple and Palmer mango cultivars (). However, effects of their interactions were not significant (p > .05). Further analysis of main means showed that post-harvest techniques had significant (p ˂ 0.001) effects on incidence of anthracnose disease on Apple () and Palmer () mango cultivars regardless of storage condition. Hexanal-treated fruit had the lowest incidence of anthracnose disease, followed by calcium chloride while untreated fruit and smoke-treated fruit had the highest disease incidences.
Table 5. Effects of storage duration, storage conditions and post-harvest techniques anthracnose disease incidences of Apple and Palmer mango cultivars.
Discussion
Post-harvest losses of mango cultivars recorded in our study were higher at retail, wholesale and transport stages than at harvest. The total post-harvest losses of mango reached 44.56%. Gebre-Mariam (Citation1999) reported lack of post-harvest and marketing infrastructure for packaging, cold storage, treatment and washing as the major causes of post-harvest loss of fruit. The post-harvest losses observed in this study harvest were mainly caused by mechanical damage such as bruises due to mishandling and insects infestations. Generally, post-harvest damages are influenced by fruit cultivar, storage temperature, water content, specific gravity and degree of maturity of the fruit (Blahovec, Citation2001; Sudheer and Indira, Citation2007). Post-harvest losses incurred by smallholder farmers are relatively high mostly due to inappropriate crop handling, processing and storage technologies (Rugumamu, Citation2009). We recorded high post-harvest losses at retail stage when fruit were soft and easily infected by diseases such as mango anthracnose caused by Colletotrichum gloeosporioides.
Total post-harvest losses encountered along the mango supply chain from harvest to retail were high in both Apple and Palmer cultivars. Post-harvest losses vary greatly among commodities, production areas and seasons (Kitinoja et al., Citation2011). We observed mechanical damages and bruises, fruit rot and insect infestations as major causes of loss at harvest stage. The fruit damages at wholesale market (three days after fruit harvesting) were mainly due to diseases and mechanical injuries. while losses at retail stage were due Post-harvest diseases and reduced firmness. The fruit loose water and become unmarketable as they shrivel and soften during storage (Kawada and Kitagawa, Citation1984).
The current study showed that hexanal extended shelf life of both mango cultivars relative to smoke and control. Hexanal enhances the shelf life of fruits by maintaining fruit firmness of treated fruits. Previous research showed that hexanal extended the shelf life of several fruit such as apple, banana, cherry, peach, strawberry, mango and vegetables such as broccoli and tomato (El Kayal et al., Citation2017; Paliyath and Subramanian, Citation2008; Preethi et al., Citation2018). The current study also showed that calcium chloride extended shelf life of the two mango cultivars compared to the control and smoke-treated fruit. Hexanal and calcium chloride extend shelf life of fruit through delayed senescence, ripening and reduction of post-harvest decay in fruits such as apple, strawberry, sweet cherry and mango (Anusuya et al., Citation2016; Mishra, Citation2002; Sharma et al., Citation2010).
Interestingly, smoke treated fruit in the current study had a shorter shelf life compared to the control. During ripening, the internal concentration of ethylene rises to saturation levels. As a consequence, additional application of exogenous ethylene has no further promotive effect on ripening of climacteric fruit such as mango (Porat et al., Citation1999; Saltveit, Citation1999). According to Saltveit (Citation1999) reduced shelf life of mango is caused by ethylene which enhances ripening and softening of climacteric fruit. Thakur et al. (Citation2017) pointed out that delayed softening extends shelf life of fresh produces. The internal factors like respiratory processes, ethylene evolution, enzymatic starch hydrolysis and other carbohydrate hydrolysis lead to cell wall softening and subsequent post-harvest deterioration in fruit (Preethi et al., 2018).
Apple cultivar fruit stored under cold condition maintained their firmness much longer compared than those stored under ambient condition. According to Thakur et al. (Citation2017), digestion of cell wall by enzymes is responsible for cell wall digestion, and this process increase with storage temperature. Similar results were reported by Katsiferis et al. (Citation2008) in orange, Tabatabaekoloor (Citation2014) in kiwifruit, and by Hertog et al. (Citation2004) in tomato. Generally, storage period, temperature, ethylene levels and maturity influence the ripening rate of fruit (Ritenour et al., Citation1999). Fruit firmness is one of the primary parameters used to evaluate the overall fruit quality (Fekete, Citation1994).
There were significant effects of post-harvest treatments on loss of firmness of both Apple and Palmer mango cultivars. Interestingly, smoke-treated fruit suffered higher loss of firmness compared with untreated fruit. We recorded lower loss of firmness in hexanal-treated fruit compared with calcium chloride, smoke-treated and the untreated fruit. Hexanal inhibits the activity of Phospholipase D which is responsible for initiation of catabolic events that leads to the eventual deterioration of the membrane and therefore fruit softening (El Kayal et al., Citation2017; Thakur et al., Citation2017). Our results further suggest that application of calcium chloride is an effective way to delay ripening and maintain firmness of fruit (see also El Kayal et al., Citation2017; Farahi and Goodarzi, Citation2008). It was generally noted that fruit stored under ambient conditions had higher loss of firmness than cold stored fruit. Hexanal-treated fruit had suffered less loss of firmness than the untreated fruit regardless of storage condition.
The incidence of Anthracnose disease on mango fruit was evaluated throughout the supply chain. It was observed that the Anthracnose disease was dominant during storage. It was noted the cold storage of mango fruit significantly delayed and reduced the incidence anthracnose disease regardless of cultivar. The reduced incidence of anthracnose indicated that calcium chloride and hexanal have antifungal activity (Fallik et al., Citation1998; Hamilton-Kemp et al., Citation1992). Similar, previous studies showed that hexanal can control of fungal diseases such as green mold (Archbold et al., Citation1999) and inhibit the growth of hyphae of Penicillium expansum and Botrytis cinerea in vitro and on apple slices (Song et al., Citation1996). Mango fruit treated with smoke had higher incidences of anthracnose disease than hexanal, calcium chloride-treated and the untreated fruit. Smoke produces ethylene which hastens the ripening of climacteric fruit like mango and exposes them to diseases.
This study concludes that post-harvest losses of mango were occured at harvest, transport, whole sale and retail stage of supply chain. The causes of post-harvest losses at different stages include physical damage, insect infestations, over-ripening and post-harvest diseases such as anthracnose. Hexanal under both storage conditions. Hexanal- and calcium chloride-treated fruit maintained their firmness for a relatively longer time during storage compared to untreated and smoke-treated fruit. This study recommends application of hexanal and calcium chloride under cold storage in order to extend fruit shelf life, maintain fruit firmness and reduce disease incidences in mango fruit.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Additional information
Funding
Literature cited
- Anusuya, P., R. Nagaraj, G.J. Janavi, K.S. Subramanian, G. Paliyath, and J. Subramanian. 2016. Pre-harvest sprays of hexanal formulation for extending retention and shelf-life of mango (Mangifera indica L.) fruits. Sci. Hortic. 211:231–240. doi: 10.1016/j.scienta.2016.08.020.
- Archbold, D.D., T.R. Hamilton-Kemp, A.M. Clements, and R.W. Collins. 1999. Fumigating crimson seedless’ table grapes with (E)-2-hexenal reduces mold during long-term post-harvest storage. HortScience 34(4):705–707. doi: 10.21273/HORTSCI.34.4.705.
- Baltazari, A., H.D. Mtui, M.W. Mwatawala, L.M. Chove, A. Sullivan, G. Paliyath, and J. Subramanian. 2018. Effects of smoke, hexanal, and calcium chloride on post-harvest quality of oranges [Citrus x sinensis (L.) Osbeck] cvs Msasa and Jaffa under different storage durations and conditions in Tanzania. Trop. Agric. 95(1):71–81.
- Blahovec, J. 2001. Static mechanic and texture of fruit and vegetables. Res. Agr. Eng. 47(4):144–169.
- Brecht, J.K., S.A. Sargent, A.A. Kader, E.J. Mitcham, F. Maul, P.E. Brecht, and O. Menocal. 2010. Mango postharvest best management practices manual. Univ. of Fla. Horticultural Sciences Department, Gainesville. p. 78.
- Dodd, J. C., R. Bugante, I. Koomen, P. Jeffries, and M. J. Jeger. 1991. Pre - and post-harvest control of mango anthracnose in the philippines. Plant Path 40(4):576-583.
- El Kayal, W., I. El-Sharkawy, C. Dowling, G. Paliyath, J.A. Sullivan, and J. Subramanian. 2017. Effect of preharvest application of hexanal and growth regulators in enhancing shelf life and regulation of membrane-associated genes in strawberry. Can. J. Plant. Sci. 97(6):1109–1120.
- Fallik, E., D.D. Archbold, T.R. Hamilton-Kemp, A.M. Clements, R.W. Collins, and M.M. Barth. 1998. (E)-2-hexenal can stimulate Botrytis cinerea growth in vitro and on strawberries in vivo during storage. J. Am. Soc. Hortic. Sci. 123(5):875–881. doi: 10.21273/JASHS.123.5.875.
- Farahi, M.H., and K. Goodarzi. 2008. Effect of calcium chloride application on firmness and post-harvest retention of berry grape (Vitis vinifera, L.) cv. Askari. J. Sci. Technol. Agri. Nat. Res. 12(45):183–190.
- Fekete, A. 1994. Elasticity characteristics of fruit Int. Agrophys. 8:411–414.
- Gebre-Mariam, S. 1999. Banana production and utilization in Ethiopia. Ethiopian Agricultural Research Organization, Addis Ababa, Ethiopia. http://www.eiar.gov.et
- Hamilton-Kemp, T.R., C.T. McCracken, J.H. Loughrin, R.A. Andersen, and D.F. Hildebrand. 1992. Effects of some natural volatile compounds on the pathogenic fungi Alternaria alternata and Botrytis cinerea. J. Chem. Ecol. 18(7):1083–1091. doi: 10.1007/BF00980064.
- Hertog, M., R. Ben-Arie, E. Roth, and B.M. Nicolai. 2004. Humidity and temperature effects on invasive and non-invasive firmness measures. Postharvest Biol. Technol. 33:79–91. doi: 10.1016/j.postharvbio.2004.01.005.
- Ilyas, M.B., M.U. Ghazanfar, M.A. Khan, C.A. Khan, and M.A.R. Bhatti. 2007. Post-harvest losses in apple and banana during transport and storage. Pak. J. Agr. Sci. 44(3):534–539.
- Jincy, M., M. Djanaguiraman, P. Jeyakumar, K.S. Subramanian, S. Jayasankar, and G. Paliyath. 2017. Inhibition of phospholipase D enzyme activity through hexanal leads to delayed mango (Mangifera indica L.) fruit ripening through changes in oxidants and antioxidant enzymes activity. Sci. Hortic. 218:316–325. doi: 10.1016/j.scienta.2017.02.026.
- Kapilan, R., and V.C. Anpalagan. 2015. Determination of optimum maturity of north Sri Lankan Kilichondan Mango fruits (Mangifera indica L.) based on their biochemical properties. Adv. Appl. Sci. Res. 6(10):105–113.
- Kasso, M., and A. Bekele. 2018. Post-harvest loss and quality deterioration of horticultural crops in Dire Dawa Region, Ethiopia. J. Saudi Soc. Agric. Sci. 17(1):88–96.
- Katsiferis, T., N. Zogzas, and V.T. Karathanos. 2008. Mechanical properties and structure of unripe oranges during processing of spoon sweets. J. Food Eng. 89:149–155. doi: 10.1016/j.jfoodeng.2008.04.014.
- Kawada, K., and H. Kitagawa. 1984. Deformation of’marsh1 grapefruit as affected by fruit orientation at packing. Annu. Meet. Fla. State Hort. Soc. 97:138–140.
- Kiaya, V. 2014. Post-harvest losses and strategies to reduce them. Technical Paper on Post-harvest Losses, Action Contre la Faim (ACF). p. 25.
- Kitinoja, L., S. Saran, S.K. Roy, and A.A. Kader. 2011. Post-harvest technology for developing countries: Challenges and opportunities in research, outreach and advocacy. J. Sci. Food Agric. 91(4):597–603. doi: 10.1002/jsfa.4295.
- Lara, I., P. García, and M. Vendrell. 2004. Modifications in cell wall composition after cold storage of calcium-treated strawberry fruit. Postharvest Biol. Technol. 34:331–339. doi: 10.1016/j.postharvbio.2004.05.018.
- Malik, A.U., Z. Singh, B.A. Saleem, and M.N. Khan. 2004. Post-harvest handling of fresh citrus fruit: An overview. Proceedings of International Conference on Citriculture. Faisalabad, Pakistan, p. 223–230.
- Mishra, S. 2002. Calcium chloride treatment of fruit and vegetables. Tetra technologies Inc, The Woodlands, Texas, USA. p. 25.
- Mohammed, M., J. Mpagalile, V. Lopez, and K. Craig. 2018. Mango value chain in Trinidad and Tobago, Guyana and St. Lucia: Measuring and reducing postharvest losses. JPHT 6(2):1–13.
- Mohammed, M., L.A. Wilson, and P.I. Gomes. 1999. Postharvest sensory and physiochemical attributes of processing and non-processing tomato cultivars. J. Food Qual. 22(2):167–182. doi: 10.1111/jfq.1999.22.issue-2.
- Msogoya, T.J., and E.S. Kimaro. 2011. Assessment and management of post-harvest losses of fresh mango under small-scale business in Morogoro. Tanzania. J. Anim. Plant Sci. 11(1):1358–1363.
- Murthy, D.S., T.M. Gajanana, M. Sudha, and V. Dakshinamoorthy. 2009. Marketing and post-harvest losses in fruit: Its implications on availability and economy. Marketing 64(2):259–275.
- Musasa, S.T., B.M. Mvumi, F.A. Manditsera, J. Chinhanga, S. Musiyandaka, and C. Chigwedere. 2013. Post-harvest orange losses and small-scale farmers’ perceptions on the loss causes in the fruit value chain: A case study of Rusitu Valley, Zimbabwe. Food Sci. Qual. Manage. 18:1–8.
- Mwatawala, M.W., M. De Meyer, R.H. Makundi, and A.P. Maerere. 2009. Host range and distribution of fruit-infesting pestiferous fruit flies (Diptera, Tephritidae) in selected areas of Central Tanzania. Bull. Entomol. Res 99(6):629–641. doi: 10.1017/S0007485309006695.
- Paliyath, G., and J. Subramanian. 2008. Phospholipase D inhibition technology for enhancing shelf life and quality, p. 240–245. In: G. Paliyath, D.P. Murr, A.K. Handa, and S. Lurie (eds.). Post-harvest biology and technology of fruit, vegetables, and flowers. 1st ed. Wiley-Blackwell, New Jersey.
- Paliyath, G. 2011. Phospholipase D inhibition technology for enhancing the shelf life and quality of fruit, vegetables and flowers. University of Guelph, Ontario, Canada. p. 79.
- Paliyath, G., K. Tiwari, H. Yuan, and B.D. Whitaker. 2008. Structural deterioration in produce-Phospholipase D, membrane and senescence, p. 195. In: G. Paliyath, D.P. Murr, A.K. Handa, and S. Lurie(eds.), Post-harvest biology and technology of fruit, vegetables and flowers. Wiley-Blackwell, New Jersey.
- Paliyath, G., and M.J. Droillard. 1992. The mechanisms of membrane deterioration and disassembly during senescence. Plant Physiol. Biochem. 30:89–812.
- Porat, R., B. Weiss, L. Cohen, A. Daus, R. Goren, and S. Droby. 1999. Effects of ethylene and 1-methylcyclopropene on the post-harvest qualities of ‘Shamouti’oranges. Postharvest Biol. Technol. 15(2):155–163. doi: 10.1016/S0925-5214(98)00079-9.
- Preethi, P., K. Soorianathasundaram, A. Sadasakthi, K.S. Subramanian, G. Paliyath, and J. Subramanian. 2018. Influence of Hexanal formulation on storage life and post-harvest quality of mango fruits. J. Environ. Biol. 39(6):1006–1014. doi: 10.22438/jeb/39/6/MRN-777.
- Ritenour, M.A., C.H. Crisosto, D.T. Garner, D.W. Cheng, and J.P. Zoffoli. 1999. Temperature, length of cold storage and maturity influence the ripening rate of ethylene preconditioned kiwifruit. Postharvest Biol. Technol. 15:107–115. doi: 10.1016/S0925-5214(98)00074-X.
- Rugumamu, C.P. 2009. Assessment of post-harvest technologies and gender relations in maize loss reduction in Pangawe village, Tanzania. Tanz. J. Sci. 35:67–76.
- Saltveit, M.E. 1999. Effect of ethylene on quality of fresh fruit and vegetables. Postharvest Biol. Technol. 15:279–292. doi: 10.1016/S0925-5214(98)00091-X.
- Sharma, M., J.K. Jacob, J. Subramanian, and G. Paliyath. 2010. Hexanal and 1-MCP treatments for enhancing the shelf life and quality of sweet cherry (Prunus avium L.). Sci. Hortic. 125(3):239–247. doi: 10.1016/j.scienta.2010.03.020.
- Singh, Z., R.K. Singh, V.A. Sane, and P. Nath. 2013. Mango-post-harvest biology and biotechnology. Crit. Rev. Plant Sci. 32(4):217–236. doi: 10.1080/07352689.2012.743399.
- Snowden, A.L. 2008. Post-harvest diseases and disorders of fruit and vegetables: Volume 1: General Introduction and fruit. Manson Publishing Ltd, London. p. 320.
- Song, J., R. Leepipattanawit, W. Deng, and R.M. Beaudry. 1996. Hexanal vapor is a natural, metabolizable fungicide: Inhibition of fungal activity and enhancement of aroma biosynthesis in apple slices. J. Am. Soc. Hort. Sci 121(5):937–942. doi: 10.21273/JASHS.121.5.937.
- Sudheer, K.P., and V. Indira. 2007. Post-harvest technology of horticultural crops. Vol. 7. New India Publishing, New Delhi, India. p. 290.
- Tabatabaekoloor, R. 2014. Bio-mechanical Behavior of kiwifruit as affected by fruit orientation and storage conditions. Proceedings International Conference of Agricultural Engineering, 6–10 July, Zurich, Switzerland. p. 1–8.
- Thakur, J.P., P.P. Gothwal, and I. Singh. 2017. Post-harvest treatments for extension of mango fruit var. Dashehari (Mangifera indica, L.). Int. J. Food Sci. Nutr. 2(4):156–162.