?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The effect of fermentation on the physicochemical (pH, titratable acidity, total soluble solids, color parameters, ethanol concentration), antioxidant (antioxidant activity (AA), total phenolic compounds (TFCs), total flavonoids (TFs), total monomeric anthocyanins (TMAs)), and sensory characteristics was evaluated in black cherry (Prunus serotina) juice and fermented beverages. The Fourier-Transform Infrared Spectroscopy (FTIR) spectra of black cherry juice and fermented beverages were also obtained. Three fermentation conditions were tested based on the total soluble solids (°Bx) content in fresh black cherry juice (FRJ): 10.9 °Bx in fresh juice (FEB1) and fresh juice adjusted to 17.5 (FEB2) and 25 (FEB3) °Bx. At the end of fermentation, the physicochemical and antioxidant characteristics of all beverages showed significant statistical differences (p ≤ 0.05). In the FTIR spectra, important changes were observed within juice and fermented beverages. A maximum concentration of 13.66% ethanol (v/v) was synthetized in FEB3. The AA, TPCs, TFs, and TMAs in FRJ and FEB3 beverage were 196.95 and 150.41 mg Trolox, 198.40 and 102.76 mg Gallic acid, 171.09 and 59.72 mg catechin and 1.64 and 0.72 mg cyanidin-3-O-glucoside/100 mL of beverage, respectively. Values for FEB1 and FEB2 are within values for FJR and FEB3. The averages of the sensory attributes were statistically different (p ≤ 0.05) for FRJ and FEBs. According to a 9-poins hedonic scale, fermented beverages “liked moderately” (7 in the scale). All beverages maintained their antioxidant characteristics in the range 34.90 ~ 76.40%.
Introduction
Black cherry (Prunus serotina subsp capuli) (capulín) is a fruit belonging to the Rosacea family. Black cherry is originally at America and it grows from Canada to Guatemala (Luna-Vázquez et al., Citation2013). Black cherry can be found with different names according to the region it grows; in the United States is known as black cherry, wild black cherry, rum cherry or mountain black cherry. In Mexico is known as “capulín” (Mc Vaugh, Citation1951). This fruit is a globose reddish-black pome of 12 to 20 mm in diameter; it has a sweet taste and a light astringent sensation. It has only one spherical stone (woody bone) surrounded by the endocarp (Ordaz-Galindo et al., Citation1999).
Black cherry, as most of the fruits, is rich in nutrients and antioxidants. It possesses a great variety of total phenolic compounds, such as flavonoids and tannins. Flavonoids (Amarowicz and Pegg, Citation2019; Dasgupta and Klein, Citation2014) in the fruit belongs to the anthocyanins group, mainly cyanidin-3-O-glucoside and cyanidin-3-O-rutinocide (Jiménez et al., Citation2011; Ordaz-Galindo et al., Citation1999). These compounds also provide a great antioxidant activity to the fruit (440 mg Trolox/100 g), even greater antioxidant activity than fruits such as blackberries, guavas, and grapes: 177.71, 205.99 and 230.51 mg Trolox/100 g, respectively (Hurtado and Pérez, Citation2014; Kuskoski et al., Citation2005).
Black cherry has been used since ancient times with medicinal purposes. Prunus serotina has been extensively used in the Mexican traditional medicine to treat various ailments such as diarrhea, respiratory discomforts (INI, Citation1994), among other sicknesses. It is prepared as an infusion for treating sore throat, stomachache, and inflammations (Hurtado and Pérez, Citation2014). Palomares-Alonso et al. (Citation2017) have reported that naringenin from P. serotine bark has cysticidal properties. Luna-Vázquez et al. (Citation2013) studied the antihypertensive properties of black cherry. Black cherry, being a seasonal fruit, is usually consumed fresh or for preparing products such as jams, jellies, preserves, fermented beverages, liqueurs, sweets, and food supplements due to its antioxidant properties (Villa, Citation2008). López et al. (Citation2017) discuss the use of a number of berries (raspberries, blackberries, blackcurrant, among other berries) for making spirits and liqueurs. After removing the flesh, the seeds of black cherry are dried and toasted to be eaten as a snack. However, huge amounts of fresh black cherry are wasted (SIAP (Servicio de Información Agroalimentaria y Pesquera), Citation2017) in the fields. Therefore, in order to minimize these losses, innovative products and methods for the processing of black cherry should be studied. Due to its nutritional, physicochemical and antioxidant properties, the black cherry fruit, as well as fruits other than grapes, could be used for obtaining fermented products, adding value to the product, improving their shelf life, and keeping antioxidant properties for benefiting the human health. It is known that during fermentation, many physical and chemical changes may occur throughout and until the end of the process (Gumienna et al., Citation2016) for delivering functional compounds (Mantzourani et al., Citation2018a, Citation2018b, Citation2020; Rios-Corripio et al., Citation2020). The differences in bioactive compounds before and after fermentation are very probably caused by polymerization, condensation, oxidation, hydrolysis and enzymatic activity reactions, among others (Gumienna et al., Citation2016).
The fermentation process of fruit juices is similar to that used for the production of wine. Depending on the fruit, some characteristics are very important to maintain the proper acceptance of the final product: color, aroma, flavor, some physicochemical characteristics, ethanol content and antioxidant properties (Berenguer et al., Citation2016; Mantzourani et al., Citation2018a). Ethanol is the main compound synthetized during fermentation; it is about 10-13% v/v and very important for the stability, aging and sensory properties of wines and similar beverages (Bozoglu et al., Citation2015).
The aim of this study was to evaluate the physicochemical, antioxidant and sensory characteristics of black cherry juice and alcoholic beverages obtained by fermentation of three initial total soluble solids content (10.9, 17.5 and 25°Bx) in the juice.
Materials and Methods
Materials
Black cherry (Prunus serotina subsp. capuli) was obtained in June, at the beginning of the fruit season. Fruits were acquired from a local market in Cholula, Puebla, Mexico. Black cherries free from physical and microbiological injuries were chosen, washed with tap water and the remaining water removed with absorbent paper before obtaining the juice. Saccharomyces cerevisiae was obtained from the Microbiology Laboratory at Universidad de las Americas Puebla (UDLAP). To obtain the yeast´s biomass, S. cerevisiae was grown in maltose broth at 25°C, in a shaking bath model BT25 (Yamato Scientific®, Tokyo, Japan), until reaching the early stationary phase (Guerrero-Beltrán et al., Citation2009).
Methods
Juice
The black cherries juice was extracted using a laboratory Waring Commercial blender model 31BL40 (New Hartford, Connecticut, USA). Whole fruits were blended and then sieved throughout cheesecloth for eliminating all insoluble residues and pieces of seeds. The obtained turbid juice was centrifuged (HERMLE Z326K, Wehingen, Germany) at 6000 rpm for 30 min to eliminate tiny particles. The juice was analyzed in physicochemical, antioxidant and sensory characteristics. The yield of juice was calculated using EquationEq. (1)(1)
(1) :
Fermentation
Three fermentation conditions were tested based on the total soluble solids (TSS, °Bx) content (10.90 ± 0.01 °Bx) in fresh black cherry juice (FRJ): initial TSS in fresh juice (FEB1) and adjusted to 17.5 (FEB2) and 25 (FEB3) °Bx using standard sugar (Zafra S.A. de C.V., Mexico). The juice was filtered throughout cheesecloth and centrifuged (HERMLE Z326K, Wehingen, Germany) at 5500 rpm for 20 min. Then, five milliliters of inoculum [(2.60 ± 0.21) x 106 CFU/mL] were added to 1 L of FRJ or adjusted black cherry juice. The fermentation of juices was done at 25 ± 1°C in an OAKTON® incubator model 12501–10 (Vernon Hills, USA). The fermentation was performed until reaching a constant TSS (°Bx) content. Fermented beverages were centrifuged and analyzed in physicochemical, antioxidant and sensory characteristics. All experiments and analyzes were performed in triplicate.
Physicochemical Characteristics
Total soluble solids (TSS) content was measured with an Atago refractometer (Atago Co. LTD, Tokyo, Japan). pH was measured using a Conductronic pHmeter (Puebla, Mexico). Titratable acidity (TA) was measured by titration until reaching pH 8.1 using 0.1 N NaOH solution according to the 924.15 AOAC (Citation2000) method; acidity was calculated as citric acid (g/100 mL). Volatile (VA) and fixed (FA) acidities were measured according to the 11.047 and 981.12 AOAC (Citation2000) methods, respectively; FA was calculated as citric acid (g/100 mL) and VA as acetic acid (g/100 mL). The ethanol concentration was measured by specific gravity according to the 10.023 AOAC (Citation2000) method.
Color Parameters
A tristimulus Chroma Meter CR-400 colorimeter (Konica Minolta Sensing Inc., Osaka, Japan) was used to measure the L*, a*, and b* color parameters (CIELab* scale) in the transmittance mode. The total change in color (ΔE*) was calculated using EquationEq. (2)(2)
(2) :
where Lo*, ao*, and bo* are the color parameter values of fresh juice and L*, a*, and b* are values of the fermented beverages (FEBs). Hue (H°) and Chroma (C) of the black cherry beverages were also calculated.
Antioxidant Characteristics
Antioxidant activity (AA). It was analyzed according to the DPPH Brand-Williams et al. (Citation1995) method with some modifications. Briefly, an aliquot of 5 µL of sample was mixed with 1995 µL of ethanol (99.5%) and 2000 µL of DPPH (0.0039 g/100 mL of 99.5% ethanol) solution. The absorbance of the sample was measured at 517 nm using a JENWAY 6850 UV/Visible spectrophotometer (Stone, Staffordshire, UK). A standard curve was prepared at various concentrations of Trolox (6-hydroxy-2,5,7,8 tetramethylchrome-2 carboxylic acid 97%): 0–0.030 mg. The antioxidant activity was calculated with the percentage of inhibition using EquationEqs. (3)(3)
(3) and (Equation4
(4)
(4) ):
where I is inhibition (%), A is absorbance of the sample and Ac is absorbance of the control. Results were expressed as mg of Trolox equivalents (TE)/100 mL beverage:
where A is the absorbance of the sample, b is the intercept (1.486), m is the slope (3549.7 1/mg), and DF is the dilution factor of the sample.
Total phenolic compounds (TPCs). They were evaluated according to the Folin-Ciocalteu method described by Singleton et al. (Citation1999) with some modifications. An aliquot of 25 µL of sample (diluted 1:10 mL with distilled water) was mixed with 3975 µL of distilled water; then, 250 µL of Folin-Ciocalteu reagent and 750 µL of Na2CO3 (20%) added. This blend was perfectly mixed and left for 2 h in the dark. Absorbances were measured at 765 nm using a JENWAY 6850 UV/Visible spectrophotometer (Stone, Staffordshire, UK). A standard curve was prepared with different concentrations of Gallic acid (GA): 0–0.064 mg. Total phenolic compounds were calculated with EquationEq. (5)(5)
(5) and reported as mg GA/100 mL beverage:
where A is the absorbance of the sample, b is the intercept (0.0011), m is the slope (22.398 1/mg), and DF is the dilution factor of the sample.
Total flavonoids (TFs). They were measured using the method reported by Dewanto et al. (Citation2002). An aliquot of 5 µL of sample was mixed with 3270 µL of distilled water and 75 µL of 5% NaNO2 solution. The blend was homogenized and stored in a dark environment. After 5 min, 150 µL of 10% AlCl3-6H20 were added and thoroughly mixed. After 6 min, 500 µL of 1 M NaOH was added and mixed. Immediately, the absorbance was measured at 510 nm in a JENWAY 6850 UV/Visible spectrophotometer (Stone, Staffordshire, UK). A standard curve was prepared with different concentrations of catechin (10 mg/100 mL of distilled water): 0–0.0180 mg). Total flavonoids were calculated with EquationEq. (6)(6)
(6) and reported as mg catechin (CAT)/100 mL beverage:
where A is the absorbance of the sample, b is the intercept (0.0002), m is the slope (2.9305 1/mg), and DF is the dilution factor.
Total monomeric anthocyanins (TMAs). They were analyzed by the pH differential method described by Giusti and Wrolstad (Citation2001). An aliquot of 1 mL of beverage was placed in an amber tube and mixed with 3 mL of pH 1.0 buffer (of potassium chloride) solution. In another amber tube, an aliquot of 1 mL of beverage was also placed and mixed with 3 mL of pH 4.5 buffer (of sodium acetate) solution. Both tubes were prepared in duplicate. The blends were mixed and allowed to stand at room temperature (25 ± 1°C) for 30 min. Absorbance of samples was measured at 520 and 700 nm using a JENWAY 6850 UV/Visible spectrophotometer (Stone, Staffordshire, UK). Total monomeric anthocyanins were calculated with EquationEqs. (7)(7)
(7) and (Equation8
(8)
(8) ) and results expressed as mg cyaniding-3-O-glucoside (C3OG)/100 mL beverage:
where MW is the molecular weight (449.2 g/mole) of cyanidin-3-O-glucoside, ɛ is the molar absorptivity coefficient (26,900 L/mole cm), F is the dilution factor, and 1 is the light pathway along the quartz cell (1 cm).
Infrared Spectroscopy (FTIR)
Mid-Infrared (MIR) spectra were obtained using a Bruker Vertex 70 FTIR spectrometer (Bruker, Vertex, Wisconsin, USA). Attenuated Total Reflectance (ATR) accessory and a Deuterated Triglycine Sulfate (DTGS) detector, which operates with a resolution of 4 cm−1, were used. A drop of sample was used for obtaining the spectra based on the selection of optimal signal to noise ratios. Each spectrum was collected and rotated against the background spectrum of the clean crystal surface to present the spectra in absorbance. Two spectra of each sample were obtained at room temperature. After each measurement, the crystal surface was cleaned with demineralized water and dried with a soft paper. Therefore, the next sample was analyzed.
Sensory Evaluation
A sensory evaluation was performed with a nine-point structured hedonic scale (Lim, Citation2011; Peryam and Pilgrim, Citation1957). The appearance, color, aroma, sweetness, flavor, and general acceptability were evaluated with 20 untrained consumers of wine (Larmond, Citation1982).
Statistical Analysis
Experimental data were analyzed using a Minitab v. 17 Statistical Software (Minitab Inc., State College, PA, USA). Differences within means of treatments were considered statistically significant for p ≤ 0.05 using ANOVA and Tukey tests. All measurements were completed in triplicate. The Pearson coefficients were calculated (Montgomery, Citation2017).
Results and Discussion
Yield
The yield of the juice obtained from whole black cherries was 31.66 mL/100 g. This percentage (v/w) was lower than that reported for other fruits since the fruit is small and has a large stone. The fruits are 2.2 cm in diameter approximately and the stone is of 1.1 cm approximately; therefore, this is way the yield was low compared to other fruits. For instance, for orange and pineapple juices, the yield has been 45.07 and 36.5 mL/100 g fruit, respectively (Jiménez et al., Citation2011). The percentage of yield is important in the food industry and can vary depending on the type of fruit, maturity, and other physicochemical characteristics.
Physicochemical Characteristics of Beverages
The physicochemical characteristics of FRJ and FEB black cherry beverages are shown in . The TSS, pH and total acidity values were characteristic of the FRJ fruit and similar to those reported by Jiménez et al. (Citation2011): 10.30 ± 0.09 °Bx, 3.9 ± 0.25 and 0.31 ± 0.02 g citric acid/100 g, respectively. These values are very important for performing the fermentation process; values in the averages 2.8–4.0 for pH and 0.25–0.40 g citric acid/100 g for acidity are recommended by the EC (Citation2009). The three initial musts with different total soluble solids content (10.9, 17.5 and 25 °Brix) had different fermentation times. Juice with the lower TSS content (FEB1) was the first in finishing the fermentation process: 7 days. FEB2 and FEB3 lasted 12 and 15 days for finishing the fermentation. Once fermented, the three beverages showed statistical differences (p ≤ 0.05) in their physicochemical characteristics (). The TSS content decreased; however, the beverage that finished with greater amount of TSS was the FEB3 beverage. All characteristics of FEBs were statistically different to those of fresh juice (p ≤ 0.05). pH decreased (3.70–3.66). TA increased at the end of the fermentation, this was expected since fermentation generate organic acids such as citric, malic, lactic, or acetic (Gumienna et al., Citation2016). The FA and VA were within the suggested ranges (0.45 ± 0.02 and 0.04 ± 0.00 g/100 mL) by the ICOP (International Code of Oenological Practices) (Citation2017) for fermented beverages. The maximum limit permitted for VA in wines is 0.12 g of acetic acid/L and the minimum limit permitted for FA is 0.4 g of tartaric acid/L. There was no statistical differences (p > .05) in FA within the fermented beverages. The VA in FEB3 was statistically different (p ≤ 0.05) to FEB1 and FEB2. FEB 3 was the beverage with the highest volatile acidity.
Table 1. Physicochemical characteristics of fresh and fermented black cherry beverages
At the end of fermentation, three different concentrations, statistically different (p ≤ 0.05), of ethanol were obtained; however, the one with the highest concentration of ethanol was FEB3; this was expected since it started with a higher content of TSS (25 °Bx). According to the Official Mexican Norm No. NOM-142-SSA1/SCFI-2014 (Secretaría, Citation2014), for alcoholic beverages in Mexico, this beverage would be classified as a medium alcoholic beverage. Ethanol in FEB 3 (13.66% v/v) was in the range similar to the amount in wine (10-13% v/v) (Bozoglu et al., Citation2015). FEB1 and FEB2 had 4.9 and 8.7% (v/v) of ethanol. This indicate that to reach an ethanol concentration similar to wine, the black cherry juice have to be adjusted in TSS to about 25 °Bx.
Color
The color parameters of FRJ and FEBs are shown in . The color parameters for FRJ were characteristic to the color of the black cherry (light brown color). Significant statistical differences (p ≤ 0.05) were found in all color parameters (L *, a *, b *, hue and Chroma) for FRJ and FEBs. FRJ had lower color parameters than those for FEBs; this indicates, according to the color space, a pale brown color. The luminosity increased at the end of the fermentation in the three FEBs, showing a lighter luminosity. The value of a* goes from green to red, as the lightness increases, generating less intense brown hues. The changes in hue could be due to the degradation of anthocyanins and flavonoids at the end of the fermentation process. The values obtained in this work were different to those reported by Jiménez et al. (Citation2011): L* = 14.60 ± 0.03, a* = 4.54 ± 0.06, b* = 13.83 ± 0.03, hue = 71.8° and Chroma = 14.56. According to the total change in color (ΔE), the condition with the greatest ΔE change was the FEB3 beverage (38.24); the one with the least ΔE change was the FEB1 (20.26). Some factors that influence the color change during the fermentation are anthocyanins, pigments found in black cherry. The conditions that may affect anthocyanins in the fermentation process are temperature, light, storage conditions, and oxygen, among others. Under the influence of these factors, the color change, in the range from orange to blue, could form yellow or colorless chalcones leading finally to brown polymers that may be an undesirable color from the point of view of the consumer (Gumienna et al., Citation2016).
Antioxidant Characteristics
The antioxidant characteristics of FRJ and FEB black cherry beverages are shown in . The antioxidant activity, total phenolic compounds, total flavonoids, and total monomeric anthocyanins in FRJ were 196.95 ± 0.18 mg Trolox/100 mL, 198.40 mg GA/100 mL, 171.09 ± 0.02 mg CAT/100 mL, and 1.64 ± 0.08 mg C3OG/100 mL, respectively. AA, TPCs, TF, and TMAs reduced in all FEBs as the initial TSS content increased in the juice. The remaining AA, TPCs, TF, and TMAs in FEB 3 was 76.36, 51.80, 34.91, and 43.90%, respectively. The antioxidant activity and total phenolic compounds in black cherry FRJ were lower than that reported by Luna-Vázquez et al. (Citation2013): 231.21 ± 0.15 mg Trolox, 362.2 ± 0.04 mg Gallic acid/100 mL, respectively. Rodríguez (Citation2011) reported 240.00 ± 0.02 mg Gallic acid/100 mL for phenolic compounds. The total phenolic compounds content found in this work was higher than that reported for grapes (160.9 ± 0.12 mg Gallic acid/100 mL). The content of total flavonoids in black cherry FRJ was lower than that reported by Luna-Vázquez et al. (Citation2013) (201.8 ± 5.2 mg catechin/100 mL) and higher than that reported by Rodríguez (Citation2011) (88.9 ± 0.10 mg catechin/100 mL). A higher content of total flavonoids was obtained in this work than that reported for grapes (121.2 ± 0.49 mg catechin/100 mL) (Rodríguez, Citation2011). The total monomeric anthocyanins content was lower in black cherry FRJ than that reported by Jiménez et al. (Citation2011) (3.08 ± 0.09 mg C3OG/100 mL) and higher than that reported by Cedillo-López et al. (Citation2006) (1.24 ± 0.03 mg C3OG/100 mL). On the other hand, in are shown the Pearson correlations within combinations of different antioxidant characteristics (antioxidant activity, total phenolic compounds, total flavonoids, and total monomeric anthocyanins). The reported Pearson correlations were calculated taking or not taking into account the black cherry juice antioxidant characteristics. It can be seen a correlation for antioxidant activity and the other antioxidant characteristics, as well as relationship combining the other antioxidant characteristics. However, it could not be a correlation between antioxidant activity and the other antioxidant characteristics. Not all phenolic compounds are antioxidants or are correlated with antioxidant activities (Conde-Hernández and Guerrero-Beltrán, Citation2014; Kiselova et al., Citation2006).
Table 2. Antioxidant characteristics of fresh and fermented black cherry beverages
Table 3. Pearson correlation coefficients for antioxidant activity (AA), phenolic compounds (TP), total flavonoids (TFs), and monomeric anthocyanins relationships of black cherry juice and alcoholic beverages
At the end of the fermentation process, there were significant statistical differences (p ≤ 0.05) in antioxidant activity, total phenolic compounds, total flavonoids and total monomeric anthocyanins. The four characteristics were reduced, mainly in the FEB3 beverage. This could be due to the longer processing time and/or the more drastic biochemical changes occurred in it. FEB3 had the lowest antioxidant activity: 150.4 ± 0.11 mg Trolox/100 mL (76.36 remaining) within all FEBs, including FRJ. At the end of fermentation, FEB3 had 102.76 ± 0.10 mg Gallic acid/100 mL, 59.72 ± 0.06 mg CAT/100 mL and 0.72 ± 0.17 mg C3OG/100 mL contents of TPCs, TFs, and TMAs, respectively. The reductions in the antioxidant characteristics at the end of fermentation were due to reactions of polymerization, condensation, oxidation, hydrolysis, enzymatic activity and by interactions of the yeasts with the bioactive compounds. Some researchers have pointed out that the content of polyphenols, including phenols, and anthocyanins at the end of the fermentation may decrease (Gumienna et al., Citation2016); the same was observed in this research. The remaining antioxidant characteristics were in the range 34.90–76.40% after fermentation. Therefore, all beverages still kept good amounts of their antioxidant properties.
FTIR Analysis
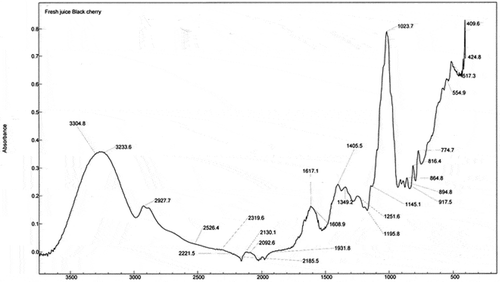
display the FTIR spectra of fresh and fermented black cherry beverages in the region 3500–500 cm−1. The FTIR technique provided important information about changes in fresh juice after fermentation. The spectrum of FRJ black cherry () shows the characteristic peaks that allow to distinguish the presence of glucose, fructose, and sucrose in the range of 1400 to 950 cm−1 (Kelly et al., Citation2004). The bands between 1153 and 900 cm−1 are typical of C-O and C-C bonds, while those between 1400–1199 cm−1 are of O-C-H, C-C-H and C-O-H bonds (Wiercigroch et al., Citation2017). The C-O-C stretching bond indicates the presence of glycosidic bonds. As for fructose, its bond is observed at 917 cm−1, corresponding to the C-H stretching bond (Tewari and Irudayaraj, Citation2004). Sucrose is also observed in the band at 1349 cm−1 (Rosas and Fernández, Citation2012). The region of 1650–1500 cm−1 corresponds to the phenolic compounds, where two extra bands were presented at 1617 and 1608 cm−1. Fresh juices are mostly composed of water, therefore the presence of OH groups in FRJ black berry corresponding to the band observed at 3233 cm−1 (Plyler, Citation1952). In the FRJ black berry spectrum, the 1617 cm−1 band corresponds to a C = C double bond of the aromatic ring in the anthocyanin structure (Ortega et al., Citation2007) which is reduced in the FEBs (–).
Figure 2. FTIR spectrum of fermented black cherry beverage (FRB1) from juice with 10.9% of total soluble solids
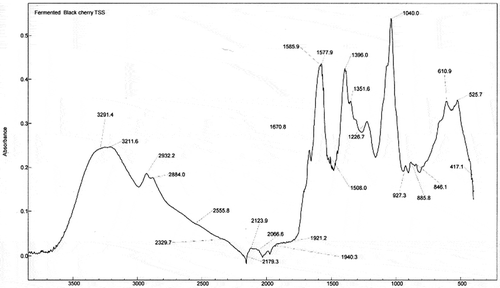
Figure 3. FTIR spectrum of fermented black cherry beverage (FRB2) from juice with 17.5% of total soluble solids
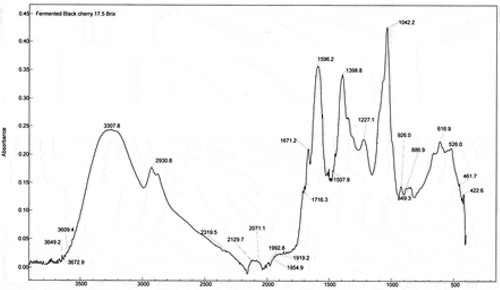
Figure 4. FTIR spectrum of fermented black cherry beverage (FRB3) from juice with 25% of total soluble solids
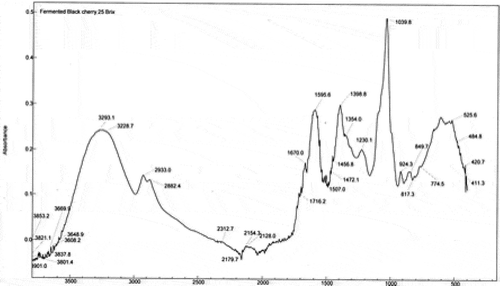
The main changes in the FTIR spectra of fermented beverages are observed in the region 1850–1150 cm−1. The FTIR spectrum of the FEB1 beverage () depicts differences in the baseline compared to the spectrum of FRJ black cherry (). These differences lie mainly in the region of 2000 to 700 cm−1 and are mostly due to the fermentation process and the formation of new chemicals such as alcohol, organic acids, phenols and glycerol. The bands observed in the spectrum of FEBs at 3307 cm−1 are due to the presence of the hydroxyl group that indicates the presence of water (Stuart, Citation2004); however, the band decreased due to the formation of ethanol in FEB beverages. As for FRJ, the presence of the bonds C = O, C-O, OH is observed (Ortega et al., Citation2007). Similarly, it is observed the presence of organic acids (C-OH and OH). The most relevant vibration band of organic acids corresponds to the C = O bond of the carboxylic acid at 1720 cm−1 for tartaric and malic acids. The OH bond of the carboxylic acid is at 1400 cm−1 (Bauer et al., Citation2008).
The spectra of the FEB2 and FEB3 beverages are very similar to that for FEB1, mainly due to the formation of new compounds during the fermentation process such as ethanol, phenolic compounds and organic acids. The main bands showing similarity were found at 1670, 1716, 1595, 1398 and 1042 cm−1. Both FEBs spectra are alike. This could indicate that the samples have a greater similarity of compounds formed during fermentation. Within these bands, the 1398 cm−1 band corresponds to the formation of citrate (Bauer et al., Citation2008). Regarding the spectrum of the sample FEB1, a new band is observed in the region 1716 cm−1, corresponding to the C = O bond of carboxylic acids. Sometimes, due to the complexity of the spectrum, it is not possible to determine the position of certain bands since they are overlapped with adjacent bands.
Sensory Characteristics
shows the results of the sensory evaluation using a structured nine points hedonic scale. For all attributes, a minimum of 5 and a maximum of 8 were observed. A number 5 corresponds to “neither like nor dislike” and number 8 corresponds to “like very much”. There were not statistical differences (p > .05) in appearance for FRJ and FEBs beverages; however, differences (p ≤ 0.05) were observed in the other attributes. As usual, the fermentation process affected all these parameters. Within FEBs, there were no significant statistical differences (p > .05) in appearance, color and sweetness; but differences (p ≤ 0.05) were observed in aroma, flavor and general acceptability. In general, FRJ, FEB2 and FEB3 were very well accepted. It is important to say that the flavor of black cherry is very light, light sweet and have “grass” notes in flavor. In addition, few people use to “taste” it since it is not consumed regularly. However, FRJ black cherry was well accepted in sweetness, flavor and general acceptability. The higher the TSS content, the higher the preference of beverages by the judges. In the attribute of aroma, sample FEB2 and FEB3 were well accepted; this may indicate that the volatile compounds generated in the fermentation (due to the generation of alcohol, acidity, phenols, among others.) liked to consumers. In flavor, the best qualified sample was FRJ beverage; however, there were no statistical differences (p > .05) within FRJ and FEB2 and FEB3 beverages. The sample that did not like too much was FEB1 ().
Table 4. Sensory evaluation of fresh and fermented black cherry beverages
At this point, it should also be highlighted that ethanol concentration could provide sensory attributes to a product such as sweetness (Mattes and DiMeglio, Citation2001). In wine, in addition to sweetness, astringency, due to tannins, acidity is also important; however, high acidity may make the wine non-pleasurable (Baker and Ross, Citation2014). In addition to the concentration of ethanol, the phenolic compounds have their own sensory attributes. They can produce a sensation of astringency (Baker and Ross, Citation2014). If their concentration is high, the acceptance in taste of the beverage may decrease. If their concentration is balanced, it is better accepted. However, it is very well known that the perception of consumers, regarding alcoholic beverages, depends on many characteristics of the beverage. In red wines for instance, previous researching has shown that flavor compounds can interact with nonvolatile and volatile components in fermented beverages, including alcohol and phenolic compounds, affecting aromatic compounds in wine and, therefore, sensitivity (Baker and Ross, Citation2014; Villamor et al., Citation2013).
Conclusions
These physicochemical characteristics (TSS, pH, acidity) of black cherry were apt to carry out the fermentation process. In the three studied conditions (FEB1, FEB2 and FEB3), there were important changes at the end of fermentation. According to the results for each FEB condition, it is very important to consider the TSS adjustment to obtain a fermented black cherry beverage, similar to wine, in alcoholic content, or even to similar to a beer, also in alcoholic content. The FTIR analysis allowed to observe the changes occurred before and after fermentation. This indicates important changes in compounds (ethanol, organic acids and phenols, mainly). The condition with the greatest changes was the FEB3 beverage. All beverages maintained important antioxidant characteristics. Fermentation generated sensorial changes in the black cherry FRJ, being FEBs moderately accepted by evaluators.
Highlights
Black cherry is a potential source of anthocyanins and polyphenols (antioxidants).
Black cherry alcoholic beverages still have good antioxidant characteristics.
Black cherry beverages had 4.94, 8.66, and 13.66% ethanol, as beer or wine.
Black cherry with 13.66% ethanol is similar as wine.
All black cherry beverages were well accepted by consumers.
Acknowledgments
Gabriela Rios-Corripio thanks to Universidad de las Americas Puebla (UDLAP) for the scholarship granted to complete her doctoral studies. We want to thank to Dra. Rios-Corripio, M. A. for the advice in the analyses of FTIR and the Chemical-biological Sciences department of UDLAP for the support received to carry out the FTIR analysis.
References
- Amarowicz, R., and R.B. Pegg.2019. Natural antioxidants of plant origin. p. 1–81. In: eds. I.C.F.R. Ferreira and L. Barros. Advances in Food and Nutrition Research. Vol. 90, Academic Press, USA.
- AOAC. 2000. Official Methods of Analysis. Association of Analytical Chemist. Inc, Washington, D.C., USA.
- Baker, A.K., and C.F. Ross. 2014. Sensory evaluation of impact of wine matrix on red wine finish: A preliminary study. J. Sens. Stu. 29(2):139–148. doi: 10.1111/joss.12089.
- Bauer, R., H. Nieuwoudt, F.F. Bauer, J. Kossmann, K.R. Koch, and K.H. Esbensen. 2008. FTIR Spectroscopy for grape and wine analysis. Analyt. Chem. 80(5):1371–1379. doi: 10.1021/ac086051c.
- Berenguer, M., S. Vegara, E. Barrajón, D. Saura, M. Valero, and N. Martí. 2016. Physicochemical characterization of pomegranate wines fermented with three different Saccharomyces cerevisiae yeast strains. Food Chem. 190:848–855. doi: 10.1016/j.foodchem.2015.06.027.
- Bozoglu, M.D., S. Ertunc, B. Akay, N. Bursali, N. Vural, H. Hapoglu, and Y. Demirci. 2015. The effect of temperature, pH and SO2 on ethanol concentration and sugar consumption rate (SCR) in apple wine process. J. Chem. Soc. Pak. 37(3):431–439.
- Brand-Williams, W., M.E. Cuvelier, and C. Berset. 1995. Use of free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 28:25–30. doi: 10.1016/s0023-6438(95)80008-5.
- Cedillo-López, D., M.C. Beltrán-Orozco, and M.P. Salgado-Cruz 2006. Cuantificación y estabilidad de los pigmentos presentes en 2 variedades del fruto del capulín (Prunus serotina Ehrenb. subs. capuli (Cav.) McVaught). Paper presented at IV Congreso Internacional de Ingeniería Bioquímica and XV Congreso Nacional de Ingeniería Bioquímica. Colegio Mexicano de Ingenieros Bioquímicos, A. C. Morelia, Michoacán, México. 4-7 April.
- Conde-Hernández, L.A., and J.A. Guerrero-Beltrán. 2014. Total phenolics and antioxidant activity of Piper auritum and Porophyllum ruderale. Food Chem. 142(1):455–460. doi: 10.1016/j.foodchem.2013.07.078.
- Dasgupta, A., and K. Klein. 2014. Fruits, vegetables, and nuts: Good sources of antioxidants, p. 209–235. In: A. Dasgupta and K. Klein (eds.). Antioxidants in food, vitamins and supplements. Elsevier, San Diego, CA, USA.
- Dewanto, V., X. Wu, K.K. Adom, and R.H. Liu. 2002. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 50:3010–3014. doi: 10.1021/jf0115589.
- EC. 2009. Commission Regulation No. 606/2009. Council Regulation (EC) No 479/2008 as regards the categories of grapevine products, oenological practices and the applicable restrictions. 12 March, 2016. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32009R0606
- Giusti, M.M., and R.E. Wrolstad. 2001. Anthocyanins: Characterization and measurement with UVvisible spectroscopy, F1.2, p. 1–13. In: R.E. Wrolstad (ed.). Current protocols in food analytical chemistry. John Wiley & Sons Inc, New York, USA.
- Guerrero-Beltrán, J.A., J.S. Welti-Chanes, and G.V. Barbosa-Cánovas. 2009. Ultraviolet-C light process of grape, cranberry, and grapefruit juices to inactivate Saccharomyces cerevisiae. J. Food Process. Eng. 32(6):916–932. doi: 10.1111/j.1745-4530.2008.00253.x.
- Gumienna, M., A. Szwengiel, and B. Górna. 2016. Bioactive components of pomegranate fruit and their transformation by fermentation processes. Eu. Food Res. Technol. 242(5):631–640. doi: 10.1007/s00217-015-2582-z.
- Hurtado, H.N., and N. Pérez. 2014. Identificación, estabilidad y actividad antioxidante de las antocianinas aisladas de la cáscara del fruto de capulí (Prunus serotina spp. capuli (Cav) Mc. Vaug Cav). Inf. Tecnol. 25(4):131–142. doi: 10.4067/s0718-07642014000400015.
- ICOP (International Code of Oenological Practices). 12, December 2017. Annex maximum acceptable limits. http://www.oiv.int/public/medias/3741/e-code-annex-maximum-acceptable-limits.pdf
- INI. 1994. Atlas de las Plantas de la Medicina Tradicional Mexicana. Vol. I. Instituto Nacional Indigenista, Mexico.
- Jiménez, M., I. Castillo, E. Azuara, and C.I. Beristain. 2011. Actividad antioxidante y antimicrobiana de extractos de capulín (Prunus serotina subsp Capulí). Rev. Mex. Ing. Quim. 10:29–37.
- Kelly, D.J., G. Downey, and V. Fouratier. 2004. Initial study of honey adulteration by sugar solutions using midinfrared (MIR) spectroscopy and chemometrics. J. Agric. Food Chem. 52:33–39. doi: 10.1021/jf034985q.
- Kiselova, Y., D. Ivanova, T. Chervenkov, D. Gerova, B. Galunska, and T. Yankova. 2006. Correlation between the in vitro antioxidant activity and polyphenol content of aqueous extracts from Bulgarian herbs. Phytother. Res. 20:961–965. doi: 10.1002/ptr.1985.
- Kuskoski, E.M., A.G. Asuero, A.M. Troncoso, J. Mancini-Filho, and R. Fett. 2005. Aplicación de diversos métodos químicos para determiner actividad antioxidante en pulpa de frutos. Ciênc. Tecnol. Aliment. Campinas 25(4):726–732.
- Larmond, E. 1982. Laboratory methods for sensory evaluation of food. Canada Department of Agriculture. Ottawa. Can. Publication 1637:23–24.
- Lim, J. 2011. Hedonic scaling: A review of methods and theory. Food Qual. Prefer. 22:733–747. doi: 10.1016/j.foodqual.2011.05.008.
- López, F., J.J. Rodríguez-Bencomo, I. Orriols, and J.R. Pérez-Correa. 2017. Fruit brandies, p. 531–556. In: M.R. Kosseva, V.K. Joshi, and P.S. Panesar (eds.). Science and technology of fruit wine production. Academic Press, London, UK.
- Luna-Vázquez, J.F., C. Ibarra-Alvarado, A. Rojas-Molina, J.I. Rojas-Molina, E. Yahia, M.D. Rivera-Pastrana, A. Rojas-Molina, and M.A. Zavala-Sánchez. 2013. Nutraceutical value of black cherry Prunus serotina Ehrh. Fruits: Antioxidant and antihypertensive properties. Molecules 18(11):14597–14612. doi: 10.3390/molecules181214597.
- Mantzourani, I., S. Kazakos, A. Terpou, A. Mallouchos, A. Kimbaris, A. Alexopoulos, E. Bezirtzoglou, and S. Plessas. 2018a. Assessment of volatile compounds evolution, antioxidant activity, and total phenolics content during cold storage of pomegranate beverage fermented by Lactobacillus paracasei K5. Fermentation 4(4):95. doi: 10.3390/fermentation4040095.
- Mantzourani, I., C. Nouska, A. Terpou, A. Alexopoulos, E. Bezirtzoglou, M.I. Panayiotidis, A. Galanis, and S. Plessas. 2018b. Production of a novel functional fruit beverage consisting of cornelian cherry juice and probiotic bacteria. Antioxidants 7(11):163. doi: 10.3390/antiox7110163.
- Mantzourani, I., A. Terpou, A. Bekatorou, A. Mallouchos, A. Alexopoulos, A. Kimbaris, E. Bezirtzoglou, A.A. Koutinas, and S. Plessas. 2020. Functional pomegranate beverage production by fermentation with a novel synbiotic L. paracasei biocatalyst. Food Chem. 308:125658. doi: 10.1016/j.foodchem.2019.125658.
- Mattes, R.D., and D. DiMeglio. 2001. Ethanol perception and ingestion. Physiol. Behav. 72(1–2):217–229. doi: 10.1016/s0031-9384(00)00397-8.
- Mc Vaugh, R. 1951. A revision of the North American black cherries (Prunus serotina Ehrh) and relatives. Brittonia. 7:279–315. doi:10.2307/280469.
- Montgomery, D.C. 2017. Design and analysis of experiments. 9th ed. John Wiley & Sons Inc., USA.
- Ordaz-Galindo, A., P. Wesche-Ebeling, R.E. Wrolstad, L. Rodriguez-Saona, and A. Argaiz-Jamet. 1999. Purification and identification of capulin (Prunus serotina Ehrh) anthocyanins. Food Chem. 65:201–206. doi: 10.1016/S0308-8146(98)00196-4.
- Ortega, G., A. Bermello, M. Guerra, G. Michelena, G. Castillo, S. Armenteros, G. Mieres, E. Carreras, D. Crespo, S. Matos, et al. 2007. Estudios de separación y caracterización de pigmento en caldos de fermentación de Botryodiplodia theobromae. ICIDCA. Sobre Los Derivados De La Caña De Azúcar. 41:27–34.
- Palomares-Alonso, F., I.S. Rojas-Tomé, G. Palencia Hernández, M.A. Jiménez-Arellanes, M.L. Macías-Rubalcava, A. González-Maciel, A. Ramos-Morales, R. Santiago-Reyes, N. Castro, I. González-Hernández, et al. 2017. In vitro and in vivo cysticidal activity of extracts and isolated flavanone from the bark of Prunus serotina: A bio-guided study. Acta Trop. 170:1–7. doi: 10.1016/j.actatropica.2017.02.023.
- Peryam, D.R., and F.J. Pilgrim. 1957. Hedonic scale method of measuring food preferences. Food Technol 11:9–14.
- Plyler, E. 1952. Infrared spectrum of methanol Ethanol, and N-propanol. J. Res. Natl. Bur. Stand. 48(4):281–286. doi: 10.6028/jres.048.036.
- Rios-Corripio, G., J. Welti-Chanes, V. Rodríguez-Martínez, and J.A. Guerrero-Beltrán. 2020. Influence of high hydrostatic pressure processing on physicochemical characteristics of a fermented pomegranate (Punica granatum L.) beverage. Innov. Food Sci. Emerg. Technol. 59:102249. doi: 10.1016/j.ifset.2019.102249.
- Rodríguez, D.L.E. 2011. Determinación de la actividad antioxidante del fruto sin semilla del capulín mexicano (Prunus serotina) e identificación de sus fenoles marcadores mediante CLAR-EM. Depto. Ingeniería en Alimentos, Universidad Autónoma de Querétaro, Querétaro, Mexico, M.Sc. Thesis.
- Rosas, M.E., and M.J. Fernández. 2012. FTIR aplicada durante la deshidratación osmótica de mango Ataulfo (Magnifera indica L.). Sociedad Mexicana De Ciencia Y Tecnología De Superficies Y Materiales 21:8–13.
- Secretaría, D.S. 2014. Norma Oficial Mexicana. NOM-142-SSA1/SCFI-2014. Bebidas alcohólicas. Especificaciones sanitarias, Mexico, 12 December. 2017. http://www.dof.gob.mx/nota_detalle.php?codigo=5386313&fecha=23/03/2015
- SIAP (Servicio de Información Agroalimentaria y Pesquera). 2017. Mexico, 01 December. 2017. http://www.siap.gob.mx/cierre-de-la-produccion-agricola-por-cultivo
- Singleton, V.L., R. Orthofer, and R.M. Lamuela-Raventos. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. 1999. Meth. Enzymol. 299:152–178. doi: 10.1016/s0076-6879(99)99017-1.
- Stuart, B.H. 2004. Infrared Spectroscopy: Fundamentals and applications. In: Analytical Techniques in the Sciences. John Wiley & Sons, Ltd, USA.
- Tewari, J., and J. Irudayaraj. 2004. Quantification of saccharides in multiple floral honeys using Fourier transform infrared microattenuated total reflectance spectroscopy. J. Agric. Food Chem. 52(11):3237–3243. doi: 10.1021/jf035176+.
- Villa, D.T.F. 2008. Frutos del capulín (Prunus serotina) como fuentes potenciales de compuestos bioactivos. Depto. Ingeniería en Alimentos, Universidad Autónoma de Querétaro, Querétaro, Mexico, M.Sc. Thesis.
- Villamor, R.R., M.A. Evans, and C.F. Ross. 2013. Effects of ethanol, tannin, and fructose concentrations on sensory properties of model red wines. Ame. J. Enology Vitic. 64(3):342–348. doi: 10.5344/ajev.2013.12118.
- Wiercigroch, E., E. Szafraniec, K. Czamara, M.Z. Pacia, K. Majzner, K. Kochan, and K. Malek. 2017. Raman and infrared spectroscopy of carbohydrates: A review. Spectrochim. Acta Part A. 185:317–335. doi: 10.1016/j.saa.2017.05.045.