 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The search for novel non-thermal preservation technologies is continuously growing, as traditional thermal processing may have undesirable effects on sensory and nutritional properties of fruit juices. Therefore, the aims of this study were to find the ultrasound, pomegranate extract and geraniol levels that simultaneously optimize different quality parameters of strawberry juice after 14 days of refrigerated storage (5°C) and evaluate the performance of the optimal combination of these treatmentsto control a contamination with Escherichia coli O157:H7. Response surface methodology was applied for optimization study where geraniol was applied at 0, 0.15 and 0.30 μL/mL; pomegranate extract, at 0, 180 and 360 μg/mL; and ultrasound treatments were performed at 40 kHz during 0, 15, and 30 min. The optimum combination resulted in 0.15 μL/mL of geraniol, 360 μg/mL of pomegranate extract and 30 min of ultrasound. This treatment was able to significantly reduce microbial counts, with a low impact on sensory attributes, while improving the antioxidant capacity of the product compared tountreated juice samples. Also, it allows reducing the contamination with E. coli to undetectable values. Thus, the optimized treatment resulted adequate for extending the shelf-life as well as increasing the safety of strawberry juice.
Introduction
The market of fresh, nutritious and ready-to-eat foods, such as unpasteurized fruit juices, is in continuous development in response to increasing consumer demands for healthier foods with fresh-like characteristics and free of synthetic additives (Jackson-Davis et al., Citation2018; Mosqueda-Melgar et al., Citation2012). In particular, strawberry juices have become more popular due to their noticeable organoleptic attributes and also for their outstanding nutritional properties, since this fruit is a good source of vitamins, minerals, fiber, and phytochemical compounds with beneficial effects in disease prevention and, thus, in human health (Correia et al., Citation2011; Giampieri et al., Citation2012; Oszmiański and Wojdyło, Citation2009).
Strawberry juice is highly susceptible to traditional thermal treatment, which seriously affects the quality of the product resulting in the loss of nutritional components and the detriment of organoleptic properties. For example, strawberry purees subjected to thermal processing showed a significant decrease in total phenols, anthocyanin and ascorbic acid content (Patras et al., Citation2009). Thus, and in line with consumer demands for fresh-like products, unpasteurized strawberry juices are gaining popularity. However, these products, which are usually consumed without further processing, have a short shelf-life associated with both microbial and enzymatic spoilage. Moreover, they can contain pathogenic microorganisms becoming a health risk (Jackson-Davis et al., Citation2018). Therefore, the development of non-thermal decontamination methods that are effective for the preservation of non-pasteurized strawberry juices, guaranteeing the safety and maintaining the freshness characteristics, is required (Santhirasegaram et al., Citation2016).
Amongst non-thermal technologies, ultrasound (US) is a preservation technique that subjects foods to frequencies between 20 and 500 kHz, inducing chemical and physical changes in the food product by cavitation phenomena (Awad et al., Citation2012). US has been studied in several fruit and vegetable juices as a suitable method for controlling microbial growth and improving quality parameters (Aadil et al., Citation2015; Anaya-Esparza et al., Citation2017; Ertugay and Başlar, Citation2014; Rawson et al., Citation2011; Saeeduddin et al., Citation2015). Nonetheless, ultrasound appears to be more effective when combined with other methods, such as natural antimicrobials (Abid et al., Citation2013).
The growth in consumer concerns about food safety issues encompasses not only aspects of microbiological safety but also the potential risks associated with synthetic preservatives. Therefore, natural compounds such as phytochemicals, essential oils, and fruit and vegetable extracts, are continuously being studied for their potential use as preservatives in the food industry. Pomegranate fruit (Punica granatum) extract and geraniol are good alternatives to synthetic preservatives as they are both considered GRAS (Generally Recognized as Safe) and have recognized antimicrobial activity (Chen and Viljoen, Citation2010; Ismail et al., Citation2012; Karimi et al., Citation2017; Molva and Baysal, Citation2015; Tomadoni et al., Citation2016b). Pomegranate extract is mainly obtained from wastes of the juice industry including peels, leaves, barks, pulp and seeds of pomegranate fruit (Ismail et al., Citation2012); and geraniol, a monoterpenoid, is a bioactive compound that is a common constituent of many aromatic herbs and essential oils and occurs naturally in Monarda fistulosa, ninde oil, rose oil, palmarosa oil and citronella oil, among others (Chen and Viljoen, Citation2010).
The use of several preservation techniques in combination (also known as hurdle technology) allows reducing the intensity of each hurdle avoiding the nutritional and sensory value losses while maintaining product safety. In a previous work, a combination of US and natural antimicrobials (vanillin and pomegranate extract) were studied and optimized (Tomadoni et al., Citation2016a). However, there is no previous reference to the combination of US with geraniol and pomegranate extract as hurdles for the treatment of unpasteurized strawberry juice. Furthermore, it is well known that when exploring new hurdles for a food product it is necessary to study the new system as different interactions and responses could be found. Thus, in this work, pomegranate extract, geraniol, and ultrasound treatments were employed as hurdles to develop unpasteurized strawberry juice.
The main objectives of the present investigation were: (a) to study the effects of pomegranate extract, geraniol, and ultrasound on different quality parameters of unpasteurized strawberry juice; (b) to find the optimum combination of these three hurdles using response surface methodology and the desirability function to simultaneously enhance microbiological, sensory and nutritional quality of strawberry juice; and (c) to determine the effectiveness of optimum treatment to control a contamination with Escherichia coli O157:H7.
Materials and Methods
Plant Material and Juice Extraction
Strawberries (Fragaria x ananassa) used in this study were harvested from Sierra de los Padres (Mar del Plata, Argentina). Fruits with defects were discarded while those with adequate visual quality were washed with tap water and the calyx was removed by hand. Strawberry juice was obtained by squeezing fruits in a commercial juice extractor (XXL-SMITH SILVER, Moulinex, Argentina). The juice was homogenized and bottled into polyethylene terephthalate units (350 mL) and kept in refrigerated conditions until treatments were applied.
Treatment Optimization
In a first stage, treatment was optimized and validated with the objective to enhance the quality parameters of strawberry juice. As juice treatment, the application of combined treatments with ultrasound (US), pomegranate extract (PE) and geraniol (G) was proposed as non-thermal technologies.
US treatments were performed at 40 kHz frequency and 180 W, using an ultrasonic cleaning bath (TestLab, Argentina) with a rectangular container (290 x 150 × 150 mm) and a tank capacity of 6.5 L. The temperature in the ultrasonic bath was monitored at 20 ± 1°C using a thermometer. The juice level in the flasks was 2 cm below the water surface in the ultrasonic bath. Pomegranate extract was purchased from PureBulk (USA), while Geraniol (> 97%) was purchased from Sigma Aldrich (St. Louis, MO, USA). PE was previously characterized by HPLC analysis (Tomadoni et al., Citation2017b): 35% ellagic acid, 19% gallic acid, 10% punicalagin A, 5% punicalagin B, 2% caffeic acid. These natural antimicrobials were applied directly into the flasks. US time and concentration of PE and G were determined by the experimental design.
Experimental Design
Response Surface Methodology (RSM) was used to study the effects of ultrasonication time (x1, min), pomegranate extract concentration (x2, μg/mL) and geraniol concentration (x3, μL/mL) on the selected response variables. For this purpose, a Box-Behnken design () with fifteen experimental runs was applied to evaluate each independent variable at 3 different levels. US processing times were: 0, 15 and 30 min (selected according to (Tomadoni et al., Citation2017a). PE was applied at 0, 180 and 360 µg/mL of strawberry juice, while G was applied at 0, 0.15 and 0.3 µL/mL of juice. These concentrations were selected according to previous experiments (Alvarez et al., Citation2012; Tomadoni et al., Citation2015, Citation2016b). After treatment, samples were stored at 5°C for 14 days. On day 14, responses were measured for each trial and a second-order polynomial model was fitted to each response variable using the least-squares regression method (Tomadoni et al., Citation2016a).
Table 1. Box-Behnken experimental design matrix and mean values of all responses of untreated samples and samples treated under different experimental conditions after 14 d of storage at 5°C
Response Variables
Microbiological, nutritional and sensory quality indices were assessed at day 0 and after 14 days of refrigerated storage.
Microbiological quality
Yeast and molds (YM), and psychrophilic bacteria (PSY) were selected as indicators of microbiological quality as they are the main native microflora populations associated to refrigerated strawberry juice (Tomadoni et al., Citation2016b). The enumeration of these microbial populations was performed according to Ponce et al. (Citation2008). Yeast-Glucose-Chloramphenicol (Britania, Argentina) incubated at 25°C for 5 d and Plate Count Agar (Britania, Argentina) incubated at 7 °C for 7 d were used for YM and PSY enumeration, respectively. Microbial counts were performed by duplicate and expressed as log CFU/mL.
Nutritional quality
Antioxidant and phenolic compounds were extracted by homogenizing 2 mL of the sample with 10 mL of ethanol solution (80% v/v). The homogenate was then centrifuged (8000 rpm, 15 min, 4°C) and the supernatant was filtered using Whatman filter paper #1. This extract was maintained at −20°C until determinations.
The antioxidant activity was determined using the DPPH radical scavenging methodology proposed by Viacava et al. (Citation2015). Briefly, 0.1 mL from each extract was added to 3.9 mL of 100 μM ethanolic DPPH solution. The mixture was shaken and allowed to stand at room temperature in the dark. The decrease in absorbance at 517 nm was measured after 60 min using a UV-Vis spectrophotometer (1601 PC UV-visible, Shimadzu Corporation, Kyoto, Japan). The radical scavenging capacity was measured by duplicate and was expressed as the percentage of the DPPH radical inhibition (%RSC).
TPC was determined spectrophotometrically using the Folin–Ciocalteu reagent according to the methodology proposed by Viacava et al. (Citation2015) with slight modifications. Extract samples properly diluted were added to 1000 μL of FCR (diluted 1:10). After 3 min, 800 μL of 7.5% Na2CO3 solution was added and the reaction mixture was incubated for 2 h at room temperature. The absorbance was measured at 765 nm using a UV-Vis spectrophotometer (1601 PC UV-visible, Shimadzu Corporation, Kyoto, Japan) and TPC was calculated using gallic acid as standard. TPC was measured by duplicate and results were expressed as mg of gallic acid equivalents (GAE)/100 mL.
Sensory quality
Ten trained panelists evaluated the sensory quality of samples following the methodology described in Tomadoni et al. (Citation2016b). Briefly, samples labeled with 3-digit code numbers were randomly provided to panelists. The evaluated attributes were off-odor (fermentation and other atypical odors) and citric odor (lemon and citric odors), using a 5-point hedonic scale.
Simultaneous Optimization and Validation
For simultaneous optimization, the Desirability function (D) was used according to Tomadoni et al. (Citation2016a). Briefly, predicted values obtained from each model () were transformed into a dimensionless desirability scale (
) that ranges from 0 to 1, being d = 0 an unacceptable response value, and d = 1 a completely desirable one. The individual desirability functions are then combined to obtain the overall desirability D, defined as the geometric average of the individual desirability. An algorithm is then applied to this function in order to determine the set of values that maximize it (Bezerra et al., Citation2008).
The validation of adjusted models was carried out through a new set of experiments using optimal operating conditions obtained with the Desirability function. The experimental and predicted values of the response variables were compared in order to determine the validity of the model.
Effectiveness of Optimal Treatment to Control a Contamination with Escherichia Coli O157:H7: Cell Inactivation and Evaluation of Survival during Storage
In a second stage, once the treatment was optimized, a new batch of strawberry juice was prepared as previously mentioned(see Plant Material and Juice Extraction section), and inoculated with E. coli O157:H7, simulating a contamination with this pathogen. After that, the juice was submitted to the optimal treatment in order to evaluate the performance of the treatment to control the contamination.
Culture Preparation
Non-toxigenic E. coli O157:H7 (FP 605/03, Malbran Institute, Buenos Aires, Argentina) was used. This strain was cultured in Brain Heart Infusion (BHI) broth (Britania, Argentina) for 24 h at 37°C. Then, 0.1 mL aliquot of the culture was transferred to 9.9 mL of BHI broth at two consecutive 24-h intervals followed by incubation at 37°C before each experiment to obtain cells in the stationary growth phase. A bacterial suspension (approximately 108 CFU/mL) was prepared by adding 10 mL of the E. coli culture to 90 mL of sterile peptonated water (0.1% w/v) (Britania, Argentina).
Samples Inoculation and Storage
Inoculation was carried out by adding the bacterial suspension to fresh strawberry juice to obtain the final desired concentration of cells (104–105 CFU/mL approximately). To do this, 100 mL of the bacterial suspension previously prepared were added to 10 mL of fresh strawberry juice. A batch of samples without inoculation was followed and evaluated to verify the absence of endogenous pathogens in the juice. Samples were submitted to the optimal treatment as was previously described. A control sample was inoculated with the microorganism but not submitted to the preservation treatment. After inoculation and treatment, samples were stored at 5°C for 14 days.
Sampling Procedure
Counts of inoculated E. coli were evaluated during storage using Eosin Methylene Blue (EMB) agar (Britania, Argentina) and the colonies were counted after incubation at 37°C for 24–48 h. E. coli colonies that were dark-centered, flat with a metallic sheen were taken into account.
Statistical Analysis
Results are reported as mean values accompanied by their standard errors. Data were analyzed using STATISTICA 7.1 (Statsoft, Citation2004). For optimization studies, the statistical analysis was performed using the analysis of variance (ANOVA) including the F-ratio and the determination coefficient R2. The Lack of Fit test was performed for each model with a 95% confidence level. The significance of fitted coefficients for each model were established according to the Student t-test with a 95% confidence level (Kuehl, Citation2001). For challenge test, data were subjected to ANOVA using the following sources of variation: TIME (storage time), TREATMENT (control or optimal treatment) and interaction TIME-TREATMENT. Differences were determined using the Tukey test. In all cases, a 95% confidence level was used to determine significant differences (Kuehl, Citation2001).
Results and Discussion
shows the results of the initial quality of strawberry juice (untreated) and values obtained after 14 days of refrigerated storage for untreated and treated samples under different experimental conditions.
Experimental data obtained for treated samples were used to fit a second order polynomial equation for each response variable. presents estimated coefficients of these fitted models with statistical parameters associated such as standard errors, t-value, and p-value, as well as the adjusted correlation coefficient (adjR2). Statistical analysis indicated that the obtained models resulted significant (p < .05) and with a good fit.
Table 2. Statistical parameters of RSM analysis of response variables
Influence of Hurdles on Microbial Quality
Geraniol concentration was the most important hurdle affecting the microbial quality of strawberry juices as linear and quadratic terms associated were significant for both yeast and molds (YM) and psychrotrophic bacteria (PSY) counts (). This effect is presented in which show the response surfaces obtained for YM and PSY counts, as a function of geraniol and ultrasound respectively. The negative coefficient for geraniol linear term in both models () indicates the antimicrobial effect of this compound on the native microflora studied: the higher the geraniol concentration, the higher the reduction of microbial counts. However, the significance of the quadratic term indicates a non-linear relationship between geraniol concentration and microbial counts, with an antimicrobial activity less effective as geraniol concentration increases.
Figure 1. Response surface for microbiological quality of strawberry juice. Variation of yeast and molds (a) and psychrophilic bacteria (b) with ultrasound time and geraniol concentration at 180 μg/mL of pomegranate extract
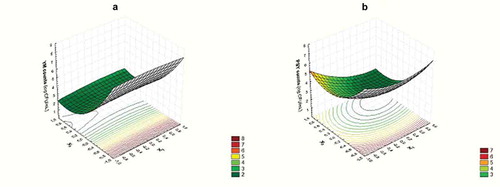
The efficiency of geraniol as a natural antimicrobial has been studied before, both in vitro and in vivo (Tomadoni et al., Citation2015, Citation2016b). Geraniol has the ability to alter bilayer properties of microbial cell membranes, making it more fluid, thus increasing its general permeability (Bard et al., Citation1988), leading to potassium loss from within the microbial cells. Hence, geraniol antimicrobial activity could be explained by the bilayer disorder and ion leakage that makes membrane-associated proteins inefficient, leading to at least inhibition of cell growth or even cell death (Dalleau et al., Citation2008).
Additionally, ultrasound time affected psychrotrophic bacteria counts as quadratic and interaction term with geraniol resulted significant in the model (). The inhibitory effect of US in the growth of the microorganisms could be attributed to the generation of H+ and OH− radicals during water sonolysis induced by cavitation phenomena (Mañas and Pagán, Citation2005), which produce oxidative damage leading to microbial inactivation. The interaction between ultrasound time and geraniol concentration indicates that the effect of geraniol is dependent on the US processing times: at longer ultrasound processing times the antimicrobial effect of geraniol on PSY counts is increased. This phenomenon is usually attributed to the intense pressure generated by US that favors the penetration of the chemical sanitizers through the cell membrane. In fact, the cavitation process facilitates the microorganisms disaggregation increasing the efficiency of the sanitization treatment (Gogate and Kabadi, Citation2009). Several studies have shown that ultrasound treatment also increased the efficiency of the sanitizing agents in reducing microbial populations on fruits and vegetables. It is interesting to note that YM was not affected by ultrasound. Cassani et al. (Citation2018) also observed this behavior when optimizing a preservation treatment for enriched strawberry juice with geraniol and ultrasound. Režek Jambrak et al. (Citation2018) and Ferrentino and Spilimbergo (Citation2015) stated that US manages to inactivate yeasts when it is applied in combination with other physical treatments such as low-grade temperature or some other non-thermal process. In our work, ultrasound did not add any improvement when combining with geraniol for YM reduction. Finally, pomegranate extract did not result significant for YM nor PSY. These results could be associated with the powerful antimicrobial effect of geraniol which makes negligible the influence of the other independent variables.
Influence of Treatments on Nutritional Quality
The three independent variables, i.e. ultrasound time, pomegranate concentration and geraniol concentration affect the nutritional quality of strawberry juices as linear, quadratic and interaction terms resulted significant in the models of antioxidant capacity (measured by DPPH scavenging activity) and total polyphenols content (TPC). For each response, two surface plots are presented to show the combined effects of ultrasound and the natural preservatives: for DPPH; and for TPC.
Figure 2. Response surface curves for nutritional quality of strawberry juice. (a): Variation of DPPH with ultrasound time and pomegranate extract concentration at 0.15 uL/mL of geraniol. (b): Variation of DPPH with ultrasound time and geraniol concentration at 180 ug/mL of pomegranate extract. (c): Variation of TPC with ultrasound time and pomegranate extract concentration at 0.15 uL/mL of geraniol. (d): Variation of TPC with ultrasound time and geraniol concentration at 180 ug/mL of pomegranate extract
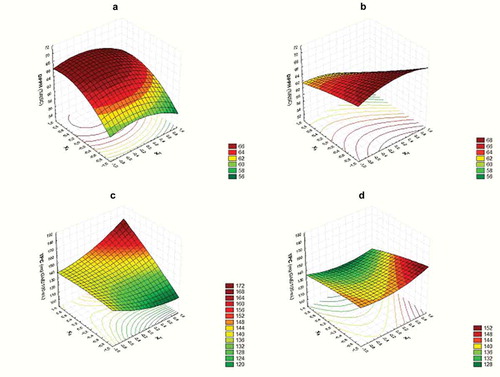
With regard to the DPPH model, linear and quadratic terms of ultrasound resulted significant (), indicating a non-linear relationship between ultrasound processing time and DPPH. The increase of antioxidant capacity on sonicated samples may be attributed to the release of antioxidant compounds from the cell wall, through the collapse via cavitation phenomenon (Cheng et al., Citation2007). However, the increase in ultrasound processing times beyond certain value became self-defeating as DPHH begin to decrease. This behavior, also reported by other authors (Wang et al., Citation2019), could be explained by the physical damage that long cavitation treatments could have on antioxidant constituents of fruit juices. DPPH radical scavenging activity was also affected by pomegranate extract concentration as linear and quadratic terms associated with this variable were significant in the model (). This non-linear relationship is clearly shown in where DPPH radical scavenging activity increases at lower rates when the concentration of the PE increases. The increment of DPPH with a higher concentration of pomegranate extract could be associated with the composition of the extract. Pomegranate extract used in this study is mainly composed by ellagic acid (35%), gallic acid (19%), punicalagin A (10%), punicalagin B (5%) and caffeic acid (2%). The highest antioxidant capacity of polyphenolic compounds toward DPPH free radical is shown on those phenols with a higher number of hydroxyl groups available ((Villaño et al., Citation2007), which explains the significant effect of pomegranate extract on the inhibition percentage of DPPH. Finally, it is interesting to note that geraniol has a negative effect on DPPH, even though this effect is not as important as pomegranate extract’s, as it can be seen when comparing . Furthermore, the interaction between geraniol and ultrasound time turned out to be significant (): at longer ultrasound processing times, the negative effect of geraniol in DPPH radical scavenging activity is more pronounced ().
As regards to TPC model, linear terms of pomegranate extract and geraniol were significant for this response. The effect of pomegranate was positive indicating that at higher concentrations of PE, TPC increases, as shown in . This result is associated with the composition of the extract being polyphenolic compounds the main components. Thus, increases in PE concentration result in higher TPC in the strawberry juice. On the other hand, geraniol exerted a negative effect on TPC that could be associated with some interaction between geraniol and polyphenols of strawberry juice. However, this effect is almost negligible in front of the positive effect of pomegranate extract, as can be seen in . Finally, the interaction term between ultrasound time and pomegranate extract concentration turned out to be significant as well. In this case, the interaction was expressed with TPC increases as increasing pomegranate extract concentration, and this behavior is more pronounced when longer ultrasound processing times are applied to strawberry juice (), demonstrating a synergic effect between these two hurdles. The increase of TPC on sonicated samples could be attributed to the release of these compounds from the cell wall, due to cavitation, as it was previously explained (Cheng et al., Citation2007). Also, Bhat et al. (Citation2011) stated that sonication produces the addition of hydroxyl radicals to the aromatic ring of phenolic compounds which could explain the increasing trend in phenolic content.
Differences found between DPPH and TPC behavior in response to hurdles under study could be associated to the fact that each phenolic compound presents different antioxidant properties, which depend mainly on its chemical structure and on the position of hydroxyl groups (Pokorný, Citation2003). Furthermore, other antioxidant compounds different from TPC that could be present in the extract could scavenge DPPH radical (Ju and Howard, Citation2003). Thus, these two indices do not always correlate.
Influence of Treatments on Sensory Quality
Both citric odor and off-odor responses were affected by geraniol as linear and quadratic terms resulted significant for these models. This effect is shown in which present the response surface for the combined effects of ultrasound time and geraniol concentration on citric odor and off-odor scores, respectively. Increases observed in citric odor as geraniol concentration increased were expected, as geraniol has an odor and tasty usually described as sweet floral rose, citrus with fruity and waxy nuances (Burdock, Citation2009). The non-linear relationship could be attributed to a saturation effect where further increases in geraniol concentration do not produce the same increase in the citric odor score. On the other hand, neither ultrasound time or pomegranate extract concentration produced any significant effect on this specific sensory attribute. At the concentrations used in this work, pomegranate extract was previously studied and showed no significant effect on odor characteristics of strawberry juice (Tomadoni et al., Citation2016b).
Figure 3. Response surface curves for sensory quality of strawberry juice. Variation of citric odor (a) and off-odor (b) with ultrasound time and geraniol concentration at 180 μg/mL of pomegranate extract
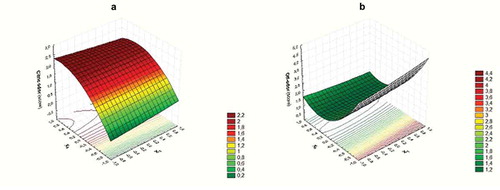
With regard to off-odor (), it decreases with increasing geraniol concentration. These results could be associated with the antimicrobial activity of geraniol, as microbial growth causes spoilage of the strawberry juice (the main cause of off-odors). In this case, the non-linear relationship is associated to the fact that increases in geraniol concentration beyond a certain value, is reflected in increases in its own citric odor (which is considered by panelists as a non-characteristic odor), leading to higher off-odor scores. The interaction term between ultrasound time and geraniol concentration also significantly affected the off-odor score, indicating that US improves the effect of geraniol avoiding the development of off-odors. This result could be related to psychrophilic bacteria growth, which was affected by ultrasound. Thus, lower PSY counts will lead to lower off-odor scores.
Simultaneous Optimization
Response variables under evaluation are differentially affected by changes in independent variables as was just analyzed. Thus, it is imperative to apply a simultaneous optimization criterion to obtain a compromise solution and to find the operating conditions that allow obtaining a safe strawberry juice with high nutritional and sensory quality during the entire shelf-life.
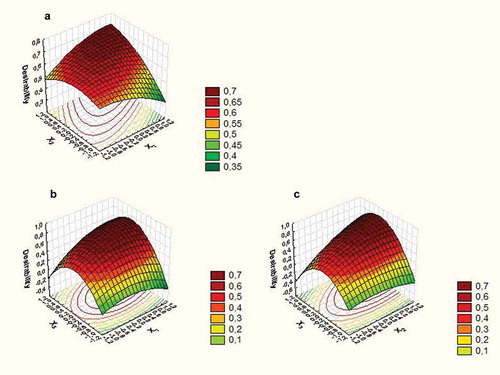
Simultaneous optimization () indicated that the optimal levels of the three preservation technologies studied resulted in X1 = 1 (30 min of ultrasound processing time), X2 = 1 (360 μg/mL of pomegranate extract), and X3 = 0 (0.15 μL/mL of geraniol). At this point, responses were predicted () as 2.97 and 2.83 log CFU/mL of YM and PSY counts, respectively; 63.77% inhibition of DPPH radical; 173.68 mg GAE/100mL of juice; and citric odor and off-odor scores of 1.82 and 1.82, respectively.
Figure 4. Response surface plots showing the combined effect of pomegranate extract concentration and ultrasound time (a), geraniol concentration and ultrasound time (b), and geraniol and pomegranate extract concentration (c) on the Desirability function with other variables constant at middle level
shows the results obtained for the response variables measured in the new set of strawberry juice treated under optimal levels obtained by simultaneous optimization (validation experiment). The responses for the untreated juice at day 0 (Fresh control) and at the end of refrigerated storage (day 14) are also shown in .
Table 3. Validation results
The scores found for citric-odor and off-odor in the validation experiments were in accordance with the values predicted by the model. However, predicted values for PSY, YM, DPPH and TPC were lower than those obtained in the validation experiment. Probably, strawberry fruits used for validation experiments presented higher initial microbial load and the growth during storage yielded higher values at day 14. As regards the antioxidant capacity and TPC, there is a notable variability in the concentration of polyphenol compounds present in strawberries, even in samples of the same variety and harvest date (Hossain et al., Citation2016), and it can be seen in that strawberries used for validation experiments had a higher concentration of antioxidant compounds, which resulted in higher DPPH and TPC values at day 14. An additional observation that is worth noting is related to the evolution of the TPC through storage time. In both and , it can be observed that TPC increases after 14 days of refrigerated storagein the untreated sample. This phenomenon has been previously observed by Puttongsiri and Haruenkit (Citation2010) when studying changes on TPC, antioxidant activity and ascorbic acid in tangerine juice through storage at different temperatures. They concluded that the increase in these compounds during storage is related to the liberation of free phenolic acids and free amino acids caused by the decomposition of the cell structure associated with the natural senescence.
Even though the strawberries used in the validation experiment () had a different initial composition and microbial load than those in the RSM experiment (), the general conclusions did not change. The optimum treatment did reduce significantly both YM and PSY counts compared to the untreated sample at day 14 (3.64 and 3.70 log reductions, respectively). Furthermore, the optimum treatment achieved similar increments on antioxidant capacity and TPC compared to control at day 14 (16.25% and 13.28%, respectively) than those predicted with the simultaneous optimization (10.72 and 33.28%, respectively).
Effect of Optimal Treatment on Escherichia Coli O157: H7 Inoculated in Strawberry Juice
The typical acidity of strawberry juices is lethal to most bacterial species that can contaminate these products. However, several outbreaks associated with the consumption of unpasteurized fruit juices contaminated with Escherichia coli O157: H7 have been reported (Shahbaz et al., Citation2018; Vojdani et al., Citation2008). Thus, it is important to evaluate the performance of the optimal treatment to control an eventual contamination with this pathogen.
shows counts of E. coli O157:H7 in control (untreated) and treated (at optimal conditions) strawberry juice along with refrigerated storage. It is interesting to note that significant reductions were observed (p < .05) in the counts of the pathogen inoculated in the untreated samples, reducing their counts by 1.85 log during 14 days. These reductions in E. coli over time may be due to the sum of various effects such as the low temperature (5°C), the low pH and the competitive microflora present in strawberry juice. However, when the inoculated juice is submitted to the optimal treatment a slight initial reduction is observed in E. coli counts and thereafter these samples showed higher reductions of the pathogen counts through the storage time than that observed for the untreated juice, reaching values below the limit of detection (< 2.00 log) at 14 days. In the treated samples, in addition to the aforementioned factors (low pH, refrigeration temperature and competitive microflora), the greater decrease in E. coli is attributed to the own effect of the combined treatments of biopreservatives and ultrasound.
Table 4. Counts of inoculated Escherichia coli O157:H7 (log CFU/mL) in control and treated strawberry juice stored at 5°C
Conclusions
The combination of ultrasound with two different natural compounds (geraniol and pomegranate extract) was studied to enhance the quality of strawberry juice, avoiding traditional thermal treatments. Simultaneous optimization, through response surface methodology and desirability function, was successful in finding the optimal levels of each proposed hurdle technology to improve the strawberry juice quality: 30 min of ultrasound processing time + 360 μg/mL of pomegranate extract + 0.15 μL/mL of geraniol, being these conditions a compromise solution between the different responses studied, maximizing the antioxidant properties of the product, while simultaneously reducing the microbial load and minimizing the impact on sensory attributes. The optimum combination was able to significantly reduce both psychrophilic bacteria and yeast and molds counts, with a low impact on sensory attributes, while improving the antioxidant capacity of the product compared to control. Finally, this treatment was effective to control a contamination with Escherichia coli O157:H7, a pathogen that generates concern for the health of consumers of unpasteurized juices.
Therefore, hurdle technology, combining two natural preservatives, i.e. pomegranate extract and geraniol, with a non-thermal processing technology, i.e. ultrasound, optimized through statistical tools, such as RSM and desirability function, resulted adequate for extending the shelf-life as well as increasing the safety of strawberry juice.
Additional information
Funding
Literature cited
- Aadil, R.M., X.A. Zeng, Z.H. Zhang, M.S. Wang, Z. Han, H. Jing, and S. Jabbar. 2015. Thermosonication: A potential technique that influences the quality of grapefruit juice. Int. J. Food Sci. Techn 50(5):1275–1282. doi: 10.1111/ijfs.12766.
- Abid, M., S. Jabbar, T. Wu, M.M. Hashim, B. Hu, S. Lei, … X. Zeng. 2013. Effect of ultrasound on different quality parameters of apple juice. Ultrason Sonochem 20(5):1182–1187. doi: 10.1016/j.ultsonch.2013.02.010.
- Alvarez, M.V., M.R. Moreira, and A. Ponce. 2012. Antiquorum sensing and antimicrobial activity of natural agents with potential use in food. J. Food Saf. 32(3):379–387. doi: 10.1111/j.1745-4565.2012.00390.x.
- Anaya-Esparza, L.M., R.M. Velázquez-Estrada, A.X. Roig, H.S. García-Galindo, S.G. Sayago-Ayerdi, and E. Montalvo-González. 2017. Thermosonication: An alternative processing for fruit and vegetable juices. Trends in Food Sci. Tech. 61:26–37. doi: 10.1016/J.TIFS.2016.11.020.
- Awad, T.S.S., H.A.A. Moharram, O.E.E. Shaltout, D. Asker, and M.M.M. Youssef. 2012. Applications of ultrasound in analysis, processing and quality control of food: A review. Food Res. Int. 48(2):410–427. doi: 10.1016/j.foodres.2012.05.004.
- Bard, M., M.R. Albrecht, N. Gupta, C.J. Guynn, and W. Stillwell. 1988. Geraniol interferes with membrane functions in strains of Candida and Saccharomyces. Lipids 23(6):534–538. doi: 10.1007/BF02535593.
- Bezerra, M.A., R.E. Santelli, E.P. Oliveira, L.S. Villar, and L.A. Escaleira. 2008. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76(5):965–977. doi: 10.1016/j.talanta.2008.05.019.
- Bhat, R., N.S.B.C. Kamaruddin, L. Min-Tze, and A.A. Karim. 2011. Sonication improves kasturi lime (Citrus microcarpa) juice quality. Ultrason Sonochem 18(6):1295–1300. doi: 10.1016/j.ultsonch.2011.04.002.
- Burdock, G.A. 2009. Fenaroli’s Handbook of flavor ingredients. 6th ed. Boca Raton: CRC Press.
- Cassani, L., B. Tomadoni, M.R. Moreira, and M.V. Agüero. 2018. Improving quality parameters of functional strawberry juices: Optimization of prebiotic fiber enrichment and geraniol treatment. Food and Bioproducts Process. doi: 10.1007/s11947-018-2170-x.
- Chen, W., and A.M. Viljoen. 2010. Geraniol - A review of a commercially important fragrance material. South African Journal of Botany 76(4):643–651. doi: 10.1016/j.sajb.2010.05.008.
- Cheng, L.H., C.Y. Soh, S.C. Liew, and F.F. Teh. 2007. Effects of sonication and carbonation on guava juice quality. Food Chem 104(4):1396–1401. doi: 10.1016/j.foodchem.2007.02.001.
- Correia, P.J., M. Pestana, F. Martinez, E. Ribeiro, F. Gama, T. Saavedra, and P. Palencia. 2011. Relationships between strawberry fruit quality attributes and crop load. Sci. Hortic. 130(2):398–403. doi: 10.1016/J.SCIENTA.2011.06.039.
- Dalleau, S., E. Cateau, T. Berges, J.-M. Berjeaud, and C. Imbert. 2008. In vitro activity of terpenes against Candida biofilms. Int. J. Antimicrob. Agents 31(6):572–576. doi: 10.1016/j.ijantimicag.2008.01.028.
- Ertugay, M.F., and M. Başlar. 2014. The effect of ultrasonic treatments on cloudy quality-related quality parameters in apple juice. Innovat. Food Sci. Emerging Tech. 26:226–231. doi: 10.1016/j.ifset.2014.06.013.
- Ferrentino, G., and S. Spilimbergo. 2015. High pressure carbon dioxide combined with high power ultrasound pasteurization of fresh cut carrot. J Supercrit Fluids. 105:170–178. doi: 10.1016/J.SUPFLU.2014.12.014.
- Giampieri, F., S. Tulipani, J.M. Alvarez-Suarez, J.L. Quiles, B. Mezzetti, and M. Battino. 2012. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 28(1):9–19. doi: 10.1016/J.NUT.2011.08.009.
- Gogate, P.R., and A.M. Kabadi. 2009. A review of applications of cavitation in biochemical engineering/biotechnology. Biochem. Eng. J. 44(1):60–72. doi: 10.1016/j.bej.2008.10.006.
- Hossain, A., P. Begum, M. Salma Zannat, M. Hafizur Rahman, M. Ahsan, and S.N. Islam. 2016. Nutrient composition of strawberry genotypes cultivated in a horticulture farm. Food Chem. 199:648–652. doi: 10.1016/j.foodchem.2015.12.056.
- Ismail, T., P. Sestili, and S. Akhtar. 2012. Pomegranate peel and fruit extracts: A review of potential anti-inflammatory and anti-infective effects. J Ethnopharmacol 143(2):397–405. doi: 10.1016/J.JEP.2012.07.004.
- Jackson-Davis, A., A. Mendonca, S. Hale, J. Jackson, A. King, and J. Jackson. 2018. Microbiological Safety of Unpasteurized Fruit and Vegetable Juices Sold in Juice Bars and Small Retail Outlets. Food and Feed Saf. Sys. Anal. 213–225. doi: 10.1016/B978-0-12-811835-1.00012-9.
- Ju, Z.Y., and L.R. Howard. 2003. Effects of Solvent and Temperature on Pressurized Liquid Extraction of Anthocyanins and Total Phenolics from Dried Red Grape Skin. J. Agric. Food Chem. 51(18):5207–5213. doi: 10.1021/jf0302106.
- Karimi, A.R., M. Tarighatjoo, and G. Nikravesh. 2017. 1,3,5-Triazine-2,4,6-tribenzaldehyde derivative as a new crosslinking agent for synthesis of pH-thermo dual responsive chitosan hydrogels and their nanocomposites: Swelling properties and drug release behavior. Int. J. Biol. Macromol. 105:1088–1095. doi: 10.1016/j.ijbiomac.2017.07.128.
- Kuehl, R. 2001. Diseño de experimentos. 2nd ed. México D.F.: Thompson Learning Intl.
- Mañas, P., and R. Pagán. 2005. Microbial inactivation by new technologies of food preservation. J. Appl. Microbiol. 98(6):1387–1399. doi: 10.1111/j.1365-2672.2005.02561.x.
- Molva, C., and A.H. Baysal. 2015. Evaluation of bioactivity of pomegranate fruit extract against Alicyclobacillus acidoterrestris DSM 3922 vegetative cells and spores in apple juice. LWT - Food Sci Tech 62(2):989–995. doi: 10.1016/j.lwt.2015.02.021.
- Mosqueda-Melgar, J., R.M. Raybaudi-Massilia, and O. Martín-Belloso. 2012. Microbiological shelf life and sensory evaluation of fruit juices treated by high-intensity pulsed electric fields and antimicrobials. Food and Bioproducts Process. 90(2):205–214. doi: 10.1016/j.fbp.2011.03.004.
- Oszmiański, J., and A. Wojdyło. 2009. Comparative study of phenolic content and antioxidant activity of strawberry puree, clear, and cloudy juices. Euro. Food Res. Tech. 228(4):623–631. doi: 10.1007/s00217-008-0971-2.
- Patras, A., N.P. Brunton, S. Da Pieve, and F. Butler. 2009. Impact of high pressure processing on total antioxidant activity, phenolic, ascorbic acid, anthocyanin content and colour of strawberry and blackberry purées. Innovat. Food Sci. Emerging Tech. 10(3):308–313. doi: 10.1016/j.ifset.2008.12.004.
- Pokorný, J. 2003. 3 – Natural antioxidants. In Peter Zeuthen and Leif Bogh-Sorenson (Eds.), Food Preservation Techniques (pp. 31–48). Cambridge: Woodhead Publishing. doi: 10.1533/9781855737143.1.31.
- Ponce, A.G., M.V. Aguero, S.I. Roura, C.E. Del Valle, and M.R. Moreira. 2008. Dynamics of indigenous microbial populations of butter head lettuce grown in mulch and on bare soil. J. Food Sci. 73(6):M257–63. doi: 10.1111/jfds.2008.73.issue-6.
- Puttongsiri, T., and R. Haruenkit. 2010. Changes in ascorbic acid, total polyphenol, phenolic acids and antioxidant activity in juice extracted from coated kiew wan tangerine during storage at 4, 12 and 20??c. Kasetsart J. Nat Sci 44(2):280–289.
- Rawson, A., B.K. Tiwari, A. Patras, N. Brunton, C. Brennan, P.J. Cullen, and C. O’Donnell. 2011. Effect of thermosonication on bioactive compounds in watermelon juice. Food Res. Int. 44(5):1168–1173. doi: 10.1016/j.foodres.2010.07.005.
- Režek Jambrak, A., M. Šimunek, S. Evačić, K. Markov, G. Smoljanić, and J. Frece. 2018. Influence of high power ultrasound on selected moulds, yeasts and Alicyclobacillus acidoterrestris in apple, cranberry and blueberry juice and nectar. Ultrasonics. 83:3–17. doi: 10.1016/J.ULTRAS.2017.02.011.
- Saeeduddin, M., M. Abid, S. Jabbar, T. Wu, M.M. Hashim, F.N. Awad, … X. Zeng. 2015. Quality assessment of pear juice under ultrasound and commercial pasteurization processing conditions. LWT - Food Sci Tech 64(1):452–458. doi: 10.1016/j.lwt.2015.05.005.
- Santhirasegaram, V., Z. Razali, and C. Somasundram. 2016. Safety improvement of fruit juices by novel thermal and nonthermal processing. Food Hygiene and Toxicology in Ready-to-Eat Foods 209–223. doi: 10.1016/B978-0-12-801916-0.00012-1.
- Shahbaz, H.M., J.U. Kim, S.-H. Kim, and J. Park. 2018. The Inactivation of Pathogens in Fruit Juice: Escherichia coli O157: H7,Salmonella Typhimurium, and Listeria monocytogenes. Fruit Juices 341–361. doi: 10.1016/B978-0-12-802230-6.00018-7.
- Statsoft, I. 2004. STATISTICA (data analysis software system). <www.statsoft.com>.
- Tomadoni, B., L. Cassani, M.R.R. Moreira, and A. Ponce. 2015. Efficacy of vanillin and geraniol in reducing Escherichia coli O157: H7on strawberry juice. LWT - Food Sci Tech 64(2):554–557. doi: 10.1016/j.lwt.2015.06.039.
- Tomadoni, B., L. Cassani, A. Ponce, M.R. Moreira, and M.V. Agüero. 2016a. Optimization of ultrasound, vanillin and pomegranate extract treatment for shelf-stable unpasteurized strawberry juice. LWT - Food Sci Tech. doi: 10.1016/j.lwt.2016.05.024.
- Tomadoni, B., L. Cassani, G. Viacava, M.D.R. Moreira, and A. Ponce. 2017a. Effect of ultrasound and storage time on quality attributes of strawberry juice. J. Food Process. Eng. 40:5. doi: 10.1111/jfpe.12533.
- Tomadoni, B., M.D.R. Moreira, J.P. Espinosa, and A. Ponce. 2017b. Individual and Combined Effects of Pomegranate Extract and Ultrasonic Treatments on Kiwifruit Juice Quality Parameters. J. Food Process. Eng. 40(1):e12339. doi: 10.1111/jfpe.12339.
- Tomadoni, B., G. Viacava, L. Cassani, M.R. Moreira, and A. Ponce. 2016b. Novel biopreservatives to enhance the safety and quality of strawberry juice. J Food Sci Technol 53(1):281–292. doi: 10.1007/s13197-015-2068-9.
- Viacava, G.E., S.I. Roura, and M.V. Agüero. 2015. Optimization of critical parameters during antioxidants extraction from butterhead lettuce to simultaneously enhance polyphenols and antioxidant activity. Chemometrics Intel. Lab.Sys. 146:47–54. doi: 10.1016/j.chemolab.2015.05.002.
- Villaño, D., M.S. Fernández-Pachón, M.L. Moyá, A.M. Troncoso, and M.C. García-Parrilla. 2007. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 71(1):230–235. doi: 10.1016/j.talanta.2006.03.050.
- Vojdani, J.D., L.R. Beuchat, and R.V. Tauxe. 2008. Juice-associated outbreaks of human illness in the United States, 1995 through 2005. J. Food Protec. 71:356–364. doi: 10.4315/0362-028X-71.2.356.
- Wang, J., J. Wang, J. Ye, S.K. Vanga, and V. Raghavan. 2019. Influence of high-intensity ultrasound on bioactive compounds of strawberry juice: Profiles of ascorbic acid, phenolics, antioxidant activity and microstructure. Food Control. 96:128–136. doi: 10.1016/J.FOODCONT.2018.09.007.
