ABSTRACT
Bacterial angular leaf spot (ALS) caused by Xanthomonas fragariae has become increasingly problematic to strawberry producers and nurseries. The use of genetic resistance is the most effective way to manage ALS. In our previous study, the major locus, FaRXf1, was identified for resistance to ALS in the linkage group 6D (LG6D). In this study, we developed subgenome-specific markers tightly associated with FaRXf1 and tested for their accuracy for high-throughput marker-assisted selection (HTP-MAS). Using the recently published octoploid reference genome sequence, we identified the location of FaRXf1 on chromosome Fvb6–2. The sequences of IStraw90 Axiom® SNP markers for FaRXf1 were used to design subgenome-specific high-resolution melting (HRM) markers. Two newly developed subgenome-specific HRM markers (Xf1HRM-8263 and Xf1HRM-8073) were consistent in their ability to correctly determine resistance and susceptibility of a total of 56 tested accessions. To validate the effectiveness of markers in HTP-MAS, we tested both CTAB purified DNA and NaOH crude extracts. The newly developed subgenome-specific marker Xf1HRM-8073 can be effectively used for the breeding of ALS resistance in cultivated strawberry.
Introduction
Bacterial angular leaf spot (ALS) caused by Xanthomonas fragariae is the most economically important bacterial disease in cultivated strawberry (F. ×ananassa) (Kennedy and King, Citation1962a, Citation1962b; Kim et al., Citation2016; Maas, Citation1984). It has been reported that ALS could reduce strawberry production and marketable yields up to 10% (Epstein, Citation1966; Kennedy and King, Citation1962b; Roberts et al., Citation1997). There is no commercially available strawberry variety that is resistance to ALS. It has been reported that a major locus, FaRXf1, controls the complete resistance to ALS (Roach et al., Citation2016). This FaRXf1 region is located in linkage group 6D (34.5–36.2 Mbp of F. vesca Genome v4.0).
Roach et al. (Citation2016) developed two high-resolution melting (HRM) markers, HRM6D_33.083 (SNP array probe AX-89898194) and HRM6D_33.110, tightly associated with the FaRXf1-mediated resistance. These two markers were able to select strawberry accessions possessing FaRXf1; however, they were not subgenome-specific markers and suitable for the use of marker-assisted seedling selection (MASS), since HRM curve patterns were not much different between resistance and susceptibility groups. There is great need to develop subgenome-specific markers for FaRXf1 and utilize it to MASS. The advantage of HRM analysis over other genotyping methods is that it is simple, accurate, high throughput, reproducible, and low cost (Noh et al., Citation2018; Simko, Citation2016). However, the two markers for the ALS resistance locus were developed from the diploid genome (Fragaria vesca) and are not subgenome-specific, which limits to the application of marker-assisted selection (MAS) in octoploid strawberry breeding. Non-subgenome-specific markers can target multiple positions and are not often able to distinguish alleles located in homeologous subgenomes (Mammadov et al., Citation2012). More importantly, non-subgenome-specific markers are not suitable for high throughput marker-assisted selection (HTP-MAS) combined with NaOH crude extracts. It is highly required to use subgenome-specific markers for an effective MAS in polyploid plants (Nekrutenko and Baker, Citation2003).
The cultivated strawberry (F. ×ananassa) is one of the most genetically complex allo-octoploid species (2n = 8x = 56), having originated from four different diploid ancestors (Edger et al., Citation2019; Shulaev et al., Citation2011). The first strawberry genome sequence was completed with diploid species F. vesca (2n = 2x = 14) (Shulaev et al., Citation2011). Although the information has been greatly useful for strawberry research, it still has limited use in octoploid strawberry. In 2019, the draft genome sequence of octoploid strawberry (F. ×ananassa, cv. ‘Camarosa’) was published (Edger et al., Citation2019). It was described that the four subgenomes of octoploid strawberry are derived from four wild diploid strawberries, F. iinumae, F. nipponica, F. viridis, and F. vesca, respectively. This study also reported that subegenomes originated from F. vesca are dominant in cultivated strawberry (F. ×ananassa) (Edger et al., Citation2019).
The whole genome SNP genotyping platform IStraw90 Axiom® array and 35K Axiom® IStraw35 384HT array were developed using the information of F. vesca reference genome (Bassil et al., Citation2015; Verma et al., Citation2017). With the both SNP array platforms, major QTLs have been identified for resistance to angular leaf spot (FaRXf1: Roach et al., Citation2016), Colletotrichum crown rot (FaRCg1: Anciro et al., Citation2018), Phytophthora crown rot (FaRPc2: Mangandi et al., Citation2017), Fusarium wilt (Fw1: Pincot et al., Citation2018), and anthracnose fruit rot (FaRCa1: Salinas et al., Citation2018). So far, HRM markers for FaRPc2 have been developed for HTP-MAS and proven for the effectiveness of marker-assisted seedling selections to improve disease resistance (Lee et al., Citation2016a, Citation2016b; Noh et al., Citation2017, Citation2018; Whitaker et al., Citation2017). To facilitate strawberry breeding for fruit quality and disease resistance via MAS, it is important to develop subgenome-specific DNA markers for other known QTLs of fruit flavor, flowering, and disease resistance (Anciro et al., Citation2018; Chambers et al., Citation2014; Noh et al., Citation2017; Perrotte et al., Citation2016; Roach et al., Citation2016; Sánchez-Sevilla et al., Citation2014).
Here our study demonstrates for the first time how to develop subgenome-specific markers in octoploid strawberry. We physically located the SNP markers closely associated with FaRXf1 in the octoploid reference genome and developed subgenome-specific DNA markers. The newly developed markers were validated for marker efficacy and accuracy with elite breeding accessions. Finally, we successfully applied the marker for ALS resistance breeding via HTP-MAS in cultivated strawberry.
Materials and Methods
Plant Materials
A total of 14 accessions were used for the primer design test: nine resistant (K08-17, K12-10, 13.77–5, 13.78–57, 14.100–58, 14.100–59, 14.101–154, 14.101–200, and 14.101–225) and five susceptible accessions (‘Florida Brilliance’, ‘Florida Radiance’ ‘Strawberry Festival’, Sweet Sensation® ‘Florida 127ʹ, and ‘Treasure’). K08-17 and K12-10 resistant accessions are donors of the ALS resistance breeding populations and originate from the Agriculture and Agri-Food Canada, Kentville, NS, breeding program (Jamieson et al., Citation2013, Citation2014). Other resistant accessions were obtained from crossing families #13 (Family 13.77: ‘Strawberry Festival’ × K12-10; Family 13.78: ‘Strawberry Festival’ × K08-17) and #14 (Family 14.100: FL_11.28–24 × 13.78.57; Family 14.101: Sweet Sensation® ‘Florida 127ʹ × 13.77–5) described in our previous study (Roach et al., Citation2016).
After primer tests, a total of 56 accessions were used for DNA marker development and validation.
DNA Extraction
Total genomic DNA was extracted using the simplified cetyltrimethylammonium bromide (CTAB) method described by Noh et al. (Citation2018) with minor modifications. Tissues from young green leaves were collected and ground to fine powders with liquid nitrogen using a Fisher Scientific™ PowerGen™ High-Throughput Homogenizer (Thermo Fisher Scientific Inc., DE, USA). 150 μl of extraction buffer A (EBA) [2% CTAB, 100 mM Tris (pH 8.0), 20 mM EDTA, 1.4 M NaCl, 4% polyvinylpyrrolidone (PVP), 0.1% ascorbic acid and 10 mM β-mercaptoethanol], 450 μl of extraction buffer B (EBB) [100 mM Tris-HCl (pH 8.0), 50 mM EDTA, 100 mM NaCl and 10 mM β-mercaptoethanol] and 50 μl of 20% sodium dodecyl sulfate (SDS, 4°C) were added and incubated at 65°C for 10 minutes. After the incubation, 205 μl of cold 5 M potassium acetate was added to the mixture and stored at −20°C for 20 minutes. The samples were centrifuged at 4,000 rpm for 20 minutes and 500 μl of the supernatant was transferred to a new tube. After adding 270 μl of cold absolute isopropanol, the samples were incubated at −20°C for 20 minutes, then centrifuged at 4,000 rpm for 20 minutes. The pellet was washed with 300 μl of 70% ethanol twice and was dissolved in 50 μl of 1× TE buffer pH 7.5 (IDTE, Coralville, IA). The DNA concentration was measured by Nanodrop 8000 UV-Vis Spectrophotometer (Thermo Fisher Scientific Inc., DE, USA) and the DNA were diluted to 10 ng/μl using 1× TE buffer pH 7.5 (IDTE, Coralville, IA) for further use. For crude DNA extraction, the rapid DNA extraction method was used as previously described (Noh et al., Citation2017). A single leaf disc (3 mm in diameter) per seedling was collected using a handheld hole punch (Harris Uni-Core, Sigma-Aldrich) and transferred into a 96-well PCR plate. 50 μl of Buffer A (100 mM NaOH and 2% Tween 20) was added to each well and incubated at 95°C for 10 minutes. Subsequently, 50 μl of Buffer B [100mM Tris-HCl (pH 8.0) and 2 mM EDTA] was added to the plate and stored at 4°C overnight. The crude DNA extracts were diluted 2-fold with sterile water and used for the HRM assay.
Development of Subgenome-Specific Marker
To develop subgenome-specific markers, we first selected nine IStraw90 Axiom® array SNP markers (AX-89898263, AX-89798107, AX-89840981, AX-89798089, AX-89810614, AX-89798073, AX-89898194, AX-89898137, AX-89840851) that are closely associated with FaRXf1 (Roach, Citation2015). Each SNP marker was localized in the octoploid reference genome (cv. ‘Camarosa’) using CLC Genomics Workbench 11.0 (https://www.qiagenbioinformatics.com/). Sequences were obtained to identify candidate polymorphisms for FaRXf1. These polymorphisms were used to design subgenome-specific HRM primers specifically located in the chromosome 6–2.
HRM Assay and Marker Data Analysis
PCR amplification was performed in a 5 µl reaction containing 0.5 µM of each primer set, 2× AccuStartTM II PCR ToughMix® (Quantabio, MA, USA), 1× LC Green® Plus HRM dye (BioFire, UT, USA), and 0.5 µl of DNA. PCR conditions were as follows: an initial denaturation at 95°C for 5 minutes; 45 cycles of denaturation at 95°C for 10 seconds, annealing at 62°C for 10 seconds, and extension at 72°C for 20 seconds. After PCR amplification, the samples were heated to 95°C for 1 minute and cooled to 40°C for 1 minute. Melting curves were obtained by melting over the desired range (60-95°C) at a rate of 50 acquisitions per 1°C. The HRM assay was performed with LightCycler® 480 System II (Roche Life Science, Germany). The HRM data was analyzed using the Melt Curve Genotyping and Gene Scanning Software (Roche Life Science, Germany). Analysis of HRM variants was based on differences in the shape of the melting curves and in Tm values. The HRM assay for each marker was repeated at least four times for a sample.
Results
Defining the Physical Location of FaRXf1 in the Octoploid Reference Genome
The physical locations of all nine IStraw90 Axiom® array SNP markers tightly associated with FaRXf1 were identified in the octoploid reference genome (Fragaria ×ananassa Camarosa Genome v1.0; https://www.ncbi.nlm.nih.gov/bioproject/515394) ( and ). The genomic region of FaRXf1 is between 30.77 Mb and 31.36 Mb, approximately 598 kb (). There are approximately 92 predicted genes present in the FaRXf1 genomic region (Table S1). Several genes for plant defense response such as kinase and NBS-LRR resistance (R) gene are located in the region. In the comparison of SNP probe sequences and octoploid reference genome (cv. ‘Camarosa’), four of nine IStraw90 Axiom® array SNP markers (AX-89898263, AX-89798107, AX-89798073, and AX-89898137) contained additional polymorphisms in the flanking sequence of the target SNP. Only the additional SNPs in AX-89898263, AX-89798073 and AX-89898137, are subgenome-specific for chromosome Fvb6–2. The other five IStraw90 Axiom® array SNP markers did not have any sequence polymorphisms around the target SNP (). The physical locations of HRM markers and primer sequences were determined in the reference genome (; Table S2). Finally, the subgenome-specific HRM markers were developed from AX-89898263, AX-89798073, and AX-89898137 for FaRXf1 (; ).
Figure 1. Physical locations of nine IStraw90 Axiom® array SNP markers in octoploid reference genome sequence (cv. Camarosa). The FaRXf1 genomic region (598 kb) is marked in the chromosome Fvb6–2, and subgenome-specific SNPs are highlighted in gray
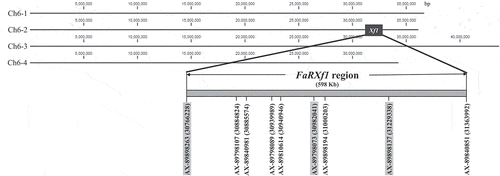
Table 1. List of the IStraw90 Axiom® array probes and sequences for FaRXf1 mapped to the reference genome ‘Camarosa’. The forward and reverse primer for each SNP probe are underlined. ‘Camarosa’ Chromosome 6–2 subgenome-specific SNPs are highlighted in gray
Development of Subgenome-Specific Markers for FaRXf1
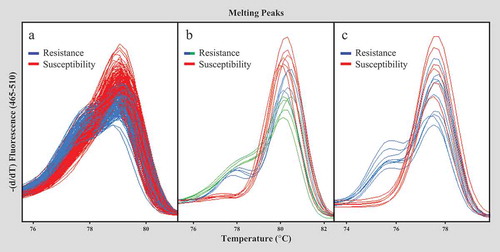
The HRM marker HRM6D_33.083 reported in our previous study (Roach et al., Citation2016) was tested for the crossing population of 14.100–59 (resistant) × Sweet Sensation® ‘Florida127ʹ (susceptible). The marker differentiates HRM genotype groups of resistance and susceptibility, but HRM melting pattern is not much different between two genotype groups and difficult to distinguish each genotype accurately (). As shown in , both subgenome-specific markers newly developed from this study, Xf1HRM-8263 and Xf1HRM-8073, were able to distinguish accessions among nine ALS resistant accessions (K08-17, K12-10, 13.77–5, 13.78–57, 14.100–58, 14.100–59, 14.101–154, 14.101–200, and 14.101–225) and five susceptible accessions (Sweet Sensation® ‘Florida127ʹ, ‘Florida Brilliance’, ‘Strawberry Festival’, ‘Treasure’, and ‘Florida Radiance’) (, ). For the Xf1HRM-8073 marker, there were two different patterns of HRM melting curve among 14 resistant accessions (). This is because of the presence of unknown mutation in the PCR amplified fragment. This finding also agrees with the previous study that the marker HRM6D_33.083 (AX-89898194) shows the different HRM curve pattern between K08-17 and K12-10 (Roach et al., Citation2016).
Figure 2. HRM analysis of HRM6D_33.083 marker and two subgenome-specific markers, Xf1HRM-8073 and Xf1HRM-8263, associated with FaRXf1 using CTAB purified DNA. (A) HRM analysis result of HRM6D_33.083 marker with 384 breeding accessions in the 2017 HTP-MAS program; (B) and (C) HRM analysis results of Xf1HRM-8073 (B) and Xf1HRM-8263 (C) markers with 14 accessions (K08-17, K12-10, 13.77–5, 13.78–157, 14.100–58, 14.100–59, 14.101–154, 14.101–200, 14.101–225, ‘Florida Brilliance’, ‘Florida Radiance’, ‘Strawberry Festival’, Sweet Sensation® ‘Florida127ʹ, and ‘Treasure’). The blue and green curves indicate resistance and red curve indicates susceptibility
Validation of Subgenome-Specific Markers for FaRXf1
To validate the subgenome-specific markers, Xf1HRM-8263 and Xf1HRM-8073, we examined 56 breeding accessions used for FaRXf1 resistance breeding (). The resistance locus FaRXf1 originated from two resistant genotypes US4808 and US4809 has been substantially introgressed into the breeding germplasm throughout MASS. The three advanced selections, 17.68–110, 17.69–15, and 17.69–23 were originated from two crossing families #13 and #14, and share the same pedigree with other ALS resistant accessions (Figure S1). In the field inoculation test, these three accessions were highly resistant to ALS while other two susceptible varieties ‘Florida Festival’ and Sweet Sensation® ‘Florida 127ʹ were highly susceptible (Figure S1). For the HRM assay, all 56 accessions were tested with two markers, Xf1HRM-8263 and Xf1HRM-8073. Both markers were able to distinguish between resistant and susceptible accessions with CTAB purified DNA (Figure S2 and ). However, only Xf1HRM-8073 marker works well with NaOH extracted DNA (Figure S3 and ). Taken together, a subgenome-specific marker, Xf1HRM-8073 closely located to the FaRXf1-SNP marker AX-89898194 can be effectively used for the HTP-MAS.
Table 2. Subgenome specific-marker validation of Xf1HRM-8073 and Xf1HRM-8263 markers in five varieties and 51 UF advanced accessions using CTAB purified DNA and NaOH crude extracts
Discussion
For the last five years, DNA markers have been used to improve fruit quality and disease resistance in cultivated strawberry (Anciro et al., Citation2018; Chambers et al., Citation2014; Lee et al., Citation2016a, Citation2016b; Noh et al., Citation2017, Citation2018; Roach et al., Citation2016). In this study, we determined physical location of nine IStraw90 Axiom® array SNP markers associated with FaRXf1 in the octoploid reference genome. Our subgenome-specific DNA markers developed from this study are closely linked to the FaRXf1-mediated resistance and can be effectively used for ALS resistance breeding via MAS. The major locus conferring ALS resistance, FaRXf1, is located on chromosome 6–2 in octoploid reference genome (Edger et al., Citation2019; Roach et al., Citation2016). Two wild accessions, US4808 and US4809, are original donors of FaRXf1 in our ALS resistance breeding germplasm (Roach et al., Citation2016). We still do not understand if the resistance is controlled by one or few genes. The HRM marker HRM6D_33.083 (AX-89898194) was first developed for marker-assisted ALS resistance breeding and successfully applied for MAS. However, this marker is limited to use for MAS as it is not easy to interpret and less reproducible with large samples.
A high-throughput DNA test combined with rapid DNA extraction has been successfully applied for strawberry breeding to improve Phytophthora crown rot resistance and fruity aroma (gamma-decalactone) (Chambers et al., Citation2014; Noh et al., Citation2017, Citation2018). The use of crude DNA for MASS may reduce the quality of marker genotype result compared to using CTAB extracted DNA. However, when large scale of DNA test (>1,000 samples) is necessary, a high-throughput marker assay can greatly reduce time, labor and cost. Our current cost for in-house genotyping is about $0.20 per sample per datapoint. Overall, the marker accuracy is approximately 95% for routine marker-assisted seedling selection (>20,000 seedlings). More strawberry DNA tests should be available within a year. The strawberry DNA test handbook can be downloaded at Genome Database of Rosaceae (https://www.rosaceae.org/organism/Fragaria/x-ananassa). In addition, since the octoploid strawberry reference genome is now publicly available for research, it is now able to develop subgenome-specific markers for many known QTLs or characterized genes in cultivated strawberry. In addition, gene-specific markers can facilitate cloning and genetic engineering research such as CRISPR gene editing in strawberry.
The complexity of the polyploid genome is the major bottleneck for DNA-informed breeding to improve strawberry fruit quality and disease resistance. The 90K Affymetrix® Axiom® array was the first whole genome SNP genotyping platform in strawberry (Bassil et al., Citation2015). This was developed from the sequences of diplioid (F. vesca) and octoploid cultivated strawberry (Bassil et al., Citation2015). Since the SNP markers were mapped to F. vesca genome, their locations were not precisely located in the specific subgenomes or chromosomes of octoploid strawberry. Therefore, Verma et al. (Citation2017) recently developed the 35K Axiom® IStraw35 384HT array using high-quality SNP markers that precisely located to the octoploid genetic linkage map. This new 35K SNP array has been successfully used for high-density scanning of large populations and has been used to identify many important QTLs for fruit quality and disease resistance (Anciro et al., Citation2018; Cockerton et al., Citation2018; Nellist et al., Citation2019; Noh et al., Citation2018; Pincot et al., Citation2018; Salinas et al., Citation2018). Because the octoploid reference genome is now available (Edger et al., Citation2019), it would be expected to have the SNP array based on F. ×ananassa reference genome, which can increase the subgenome specificity of SNP markers.
In conclusion, this is the first study to show the development of subgenome-specific markers using the newly available octoploid strawberry reference genome. The nine IStraw90 Axiom® array SNP markers associated with FaRXf1 were successfully located into the octoploid reference genome. Two subgenome-specific HRM markers, Xf1HRM-8263 and Xf1HRM-8073, linked to FaRXf1 accurately detect the resistant elite accessions in our breeding program. The marker Xf1HRM-8073 can be applied for HTP-MAS for ALS resistance breeding. Our findings here will be valuable for the development of new resistant varieties to ALS.
Supplemental Material
Download Zip (220.9 KB)Acknowledgments
The authors thank all members of the UF Strawberry Breeding Program, and Strawberry Molecular Genetics and Genomics Program for their technical assistance.
Supplementary Material
Supplemental data for this article can be accessed here.
Additional information
Funding
Literature cited
- Anciro, A., J. Mangandi, S. Verma, N. Peres, V.M. Whitaker, and S. Lee. 2018. FaRCg1: A quantitative trait locus conferring resistance to Colletotrichum crown rot caused by Colletotrichum gloeosporioides in octoploid strawberry. Theor. Appl. Genet. 131:2167–2177. doi: 10.1007/s00122-018-3145-z.
- Bassil, N.V., T.M. Davis, H. Zhang, S. Ficklin, M. Mittmann, T. Webster, L. Mahoney, D. Wood, E.S. Alperin, U.R. Rosyara, et al. 2015. Development and preliminary evaluation of a 90 K Axiom® SNP array for the allo-octoploid cultivated strawberry Fragaria ×ananassa. BMC Genom. 16:155. doi: 10.1186/s12864-015-1310-1.
- Chambers, A.H., J. Pillet, A. Plotto, J. Bai, V.M. Whitaker, and K.M. Folta. 2014. Identification of a strawberry flavor gene candidate using an integrated genetic-genomic-analytical chemistry approach. BMC Genom. 15:217. doi: 10.1186/1471-2164-15-217.
- Cockerton, H.M., R.J. Vickerstaff, A. Karlström, F. Wilson, M. Sobczyk, J.Q. He, D.J. Sargent, A.J. Passey, K.J. McLeary, K. Pakozdi, et al. 2018. Identification of powdery mildew resistance QTL in strawberry (Fragaria ×ananassa). Theor. Appl. Genet. 131:1995–2007. doi: 10.1007/s00122-018-3128-0.
- Edger, P.P., T.J. Poorten, R. VanBuren, M.A. Hardigan, M. Colle, M.R. McKain, R.D. Smith, S.J. Teresi, A.D.L. Nelson, C.M. Wai, et al. 2019. Origin and evolution of the octoploid strawberry genome. Nat. Genet. 51:541.
- Epstein, A.H. 1966. Angular leaf spot of strawberry. Plant Dis. Report. 50:167.
- Jamieson, A.R., P.D. Hildebrand, and W.E. Renderos. 2013. Breeding strawberry plants resistant to angular leafspot disease. Int. J. Fruit Sci. 13:28–35. doi: 10.1080/15538362.2012.696959.
- Jamieson, A.R., P.D. Hildebrand, W.E. Renderos, and S.A.E. Fillmore. 2014. Resistance to angular leaf spot disease of strawberry: Influence of seedling age. Acta Hortic. 1049:187–192. doi: 10.17660/ActaHortic.2014.1049.18.
- Kennedy, B.W., and T.H. King. 1962a. Angular leaf spot of strawberry caused by Xanthomonas fragariae sp. nov. Phytopathology 52:873–875.
- Kennedy, B.W., and T.H. King. 1962b. Studies on epidemiology of bacterial angular leafspot on strawberry. Plant Dis. Report. 46:360–363.
- Kim, D.R., G.H. Gang, C.W. Jeon, N.J. Kang, S.W. Lee, and Y.S. Kwak. 2016. Epidemiology and control of strawberry bacterial angular leaf spot disease caused by Xanthomonas fragariae. Plant Pathol. J. 32:290. doi: 10.5423/PPJ.OA.01.2016.0007.
- Lee, S., Y.-H. Noh, J.A. Roach, J. Mangandi, S. Verma, V.M. Whitaker, and K.R. Cearley. 2016a. A high-throughput genotyping system combining rapid DNA extraction and high-resolution melting analysis in allo-octoploid strawberry, Acta Hortic. 1156:89–94.
- Lee, S., Y.-H. Noh, S. Verma, and V.M. Whitaker. 2016b. DNA, technology, and Florida strawberries. University of Florida IFAS Extension. Nov. 2016.<https://edis.ifas.ufl.edu/hs1287>.
- Maas, J.L. 1984. Compendium of strawberry diseases. Amer. Phytopathol. Soc., St. Paul, MN.
- Mammadov, J., R. Aggarwal, R. Buyyarapu, and S. Kumpatla. 2012. SNP markers and their impact on plant breeding. Int. J. Plant Genomics. 2012:1–11. doi: 10.1155/2012/728398.
- Mangandi, J., S. Verma, L. Osorio, N.A. Peres, E. van de Weg, and V.M. Whitaker. 2017. Pedigree-based analysis in a multiparental population of octoploid strawberry reveals QTL alleles conferring resistance to Phytophthora cactorum. G3: Genes Genom. Genet. 7:1707–1719. doi: 10.1534/g3.117.042119.
- Nekrutenko, A., and R.J. Baker. 2003. Subgenome-specific markers in allopolyploid cotton Gossypiumhirsutum: Implications for evolutionary analysis of polyploids. Gene. 306:99–103. doi: 10.1016/S0378-1119(03)00427-X.
- Nellist, C.F., R. Vickerstaff, M.K. Sobczyk, C. Marina-Montes, P. Brain, F.M. Wilson, D.W. Simpson, A.B. Whitehouse, and R. Harrison. 2019. Quantitative trait loci controlling Phytophthora cactorum resistance in the cultivated octoploid strawberry (Fragaria ×ananassa). Hort. Res. 6:60. doi: 10.1038/s41438-019-0136-4.
- Noh, Y.H., S. Lee, V.M. Whitaker, K.R. Cearley, and J.S. Cha. 2017. A high-throughput marker-assisted selection system combining rapid DNA extraction high-resolution melting and simple sequence repeat analysis: Strawberry as a model for fruit crops. J. Berry Res. 7:23–31. doi: 10.3233/JBR-160145.
- Noh, Y.H., Y. Oh, J. Mangandi, S. Verma, J.D. Zurn, Y.T. Lu, Z. Fan, N. Bassil, N. Peres, G. Cole, et al. 2018. High-throughput marker assays for FaRPc2-mediated resistance to Phytophthora crown rot in octoploid strawberry. Mol. Breeding. 38:104. doi: 10.1007/s11032-018-0861-7.
- Perrotte, J., A. Gaston, A. Potier, A. Petit, C. Rothan, and B. Denoyes. 2016. Narrowing down the single homoeologous FaPFRU locus controlling flowering and fruit production in the cultivated octoploid strawberry using a selective mapping strategy. Plant Biotech. J. 14:2176–2189. doi: 10.1111/pbi.12574.
- Pincot, D.D., T.J. Poorten, M.A. Hardigan, J.M. Harshman, C.B. Acharya, G.S. Cole, T.R. Gordon, M. Stueven, P.P. Edger, and S.J. Knapp. 2018. Genome-wide association mapping uncovers Fw1, a dominant gene conferring resistance to Fusarium wilt in strawberry. G3: Genes Genom. Genet. 8:1817–1828. doi: 10.1534/g3.118.200129.
- Roach, J.A. 2015. Genetic mapping of a locus conferring resistance to Xanthomonas fragariae in ontoploid strawberry. Florida, University of Florida, PhD Thesis
- Roach, J.A., S. Verma, N.A. Peres, A.R. Jamieson, W.E. van de Weg, M.C. Bink, N.V. Bassil, S. Lee, and V.M. Whitaker. 2016. FaRXf1: A locus conferring resistance to angular leaf spot caused by Xanthomonas fragariae in octoploid strawberry. Theor. Appl. Genet. 129:1191–1201. doi: 10.1007/s00122-016-2695-1.
- Roberts, P.D., R.D. Berger, J.B. Jones, C.K. Chandler, and R.E. Stall. 1997. Disease progress, yield loss, and control of Xanthomonas fragariae on strawberry plants. Plant Dis. 81:917–921. doi: 10.1094/PDIS.1997.81.8.917.
- Salinas, N., S. Verma, N. Peres, and V.M. Whitaker. 2018. FaRCa1: A major subgenome-specific locus conferring resistance to Colletotrichum acutatum in strawberry. Theor. Appl. Genet. 132:1109–1120.
- Sánchez-Sevilla, J.F., E. Cruz-Rus, V. Valpuesta, M.A. Botella, and L. Amaya. 2014. Deciphering gamma-decalactone biosynthesis in strawberry fruit using a combination of genetic mapping, RNA-Seq and eQTL analyses. BMC Genomics. 15:218. doi: 10.1186/1471-2164-15-218.
- Shulaev, V., D.J. Sargent, R.N. Crowhurst, T.C. Mockler, O. Folkerts, A.L. Delcher, P. Jaiswal, K. Mockaitis, A. Liston, S.P. Mane, et al. 2011. The genome of woodland strawberry (Fragaria vesca). Nat. Genet. 43:109. doi: 10.1038/ng.740.
- Simko, I. 2016. High-resolution DNA melting analysis in plant research. Trends Plant Sci. 21:528–537. doi: 10.1016/j.tplants.2016.01.004.
- Verma, S., N.V. Bassil, E. van de Weg, R.J. Harrison, A. Monfort, J.M. Hidalgo, I. Amaya, B. Denoyes, L. Mahoney, T.M. Davis, et al. 2017. Development and evaluation of the Axiom® IStraw35 384HT array for the allo-octoploid cultivated strawberry Fragaria ×ananassa. Acta. Hortic. 1156:75–82. doi: 10.17660/ActaHortic.2017.1156.10.
- Whitaker, V.M., S. Lee, L.F. Osorio, S. Verma, J.A. Roach, J. Mangandi, Y.-H. Noh, S. Gezan, and N. Peres. 2017. Advances in strawberry breeding at the University of Florida, Acta Hortic. 1156:1–6.
