ABSTRACT
Pomegranate (Punica granatum. L) has gained much attention as a functional food and nutraceutical source. Pomegranate fruit quality depends on climate and growing conditions. The aim of this research was to evaluate the physical properties, phenols, flavonoids, and anthocyanins contents, mineral composition as well as antioxidant activity of ‘Gabsi’ pomegranates grown in different regions (northern, southern, and center of Tunisia). A significant variation was recorded for most quality attributes. However, weight, height, diameter, chroma (C), DPPH antioxidant activity and potassium content did not significantly differ among the three studied regions. The northern Tunisia gave colorful fruits with high zinc and copper contents, high anthocyanins, phenols contents and exhibited high antioxidant activity. Temperature has affected negatively total phenolic content and antioxidant activity, while a positive relationship was observed between them, rainfall, and relative humidity. Zerkine, a warmer and drier region, produced fruits with an intense red color, heavier and thicker skin and juice rich in flavonoids, sodium, and magnesium levels. However, pomegranates grown in center of Tunisia presented high aril yield, juice yield and high magnesium, sodium, and manganese contents. Also, a correlation analysis reported a significant relationship between sodium, magnesium contents, temperature, and relative humidity. This study provides a clear understanding on the impact of environment on bioactive compounds, antioxidant activity and allows guiding for a better selection of the growing location to improve pomegranate fruit quality.
Introduction
Interest in the nutritional quality of fruits is steadily increasing. People are becoming more and more aware of the need to consume healthy foods for a healthy life (Gündüz and Özbay, Citation2018). Pomegranate (Punica granatum L.), belongs to the Lythraceae family, has gained widespread popularity as a functional food and nutraceutical source. Pomegranates are well known source of nutritional and bioactive compounds such as dietary fibers, organic acids, minerals, and vitamins (El Falleh et al., Citation2011; Legua et al., Citation2016; Mena et al., Citation2012). A high antioxidative capacity due to high content of phenolic compounds such as anthocyanins, hydrolyzable tannins (punicalagins, punicalins), condensed tannins (proanthocyanidins), catechins, and phenolic acids (gallic, ellagic, and chlorogenic) characterize pomegranate juices (Nuncio-Jáuregui et al., Citation2015). Tunisia is the main Mediterranean producer, wich ‘Gabsi’ is the most known and commercial variety. Pomegranate fruit acceptability by consumers and processors depends on a combination of several attributes, including physical appearance (color and size), flavor (sugar content, acidity, and aroma) and nutritional compounds (phenols, minerals, vitamins). Several factors affect the external and internal quality of pomegranate fruit, including genotype, growing region, climate, and cultural practices (Mirdehgham and Rahemi, Citation2007; Schwartz et al., Citation2009). Several studies have focused on the effects of geographic origin and environmental conditions on the nutrient and phytochemical contents of pomegranate fruit (Mditshwa et al., Citation2013; Schwartz et al., Citation2009). It is suggested that climatic conditions include intense sunlight, mild winters, and hot dry summers without precipitation during the last stages of the fruit’s development are optimal for pomegranate fruit growth (Teixeira da Silva et al., Citation2013). Attanayake et al. (Citation2018) indicated that the accumulation of bioactive compounds and expression of few considered genes were relatively higher in drier and warmer climate than wetter and cooler conditions. However, Schwartz et al. (Citation2009) showed that pomegranate fruits grown in Mediterranean conditions are of higher quality than those grown in desert climate conditions. Yet limited information is available on the effects of environmental factors on quality of Tunisian pomegranate fruit. The objective of this study was to investigate physical and nutritional properties, as well as antioxidant activity of pomegranate fruit (Punica granatum L var. Gabsi) grown in three regions in Tunisia. In the present research, pomegranate fruits were sampled from northern, center, and southern Tunisia which differing in altitude and climatic conditions. Thus, we will look for a link between pomegranate fruit quality and measured environmental parameters and determine specific characteristics of pomegranates in each region in relation with climate.
Materials and Methods
Plant Material
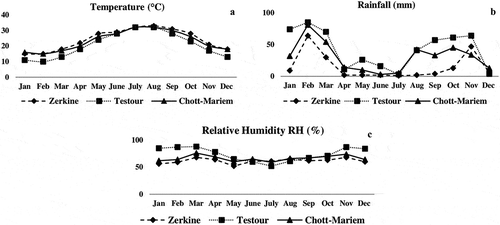
Pomegranate fruits were harvested from ‘Gabsi’ trees grown in commercial regular orchards located in Testour (Northern Tunisia, latitude 36° 33ʹ N, longitude 9° 26ʹ E), Chott-Mariem in center of Tunisia (latitude 35° 56ʹ N, longitude 10° 32ʹ E) and Zerkine in southeast Tunisia (latitude 33° 43ʹ N, longitude 10° 15ʹ E). Average monthly air temperature, rainfall, and relative humidity values in the three growing locations for the season 2015 are presented in . The three regions showed almost the same temperatures in June, July, and August and tend to be colder in Testour in September and October. The mean monthly rainfall is higher in Testour than in other regions. Also, Chott-Mariem and Testour are characterized by high relative humidity especially in winter.
Figure 1. Climatic data of the three studied regions: (a) Temperature, (b) Rainfall, (c) Relative humidity (RH)
The pomegranate trees were 15 years old and an organic fertilization (manure application), drip irrigation were applied in all studied orchards.
Fruit Processing
From each orchard, 20 fruits (n = 4 fruits per tree) were harvested from all sides of each canopy including the top and inside the canopy. All samples were picked at mid October at early morning and were immediately transferred to the laboratory. The fruits were kept at 5°C until used for experiments. For each sample, fruits were washed and manually peeled and the juice was extracted from fleshy arils using a domestic blender (Moulinex LM 240, France). The juice was then filtered through muslin cloth. Juice was stored at −20°C for further analysis.
Physical Attributes
Individual fruit weight and skin weight (g) were measured using an electronic balance. Fruit height (mm) and fruit diameter (mm) were determined using a digital caliper with 0.001 mm accuracy. Fruit firmness (kg · cm−2) was measured at the equatorial region on two opposite sides of each pomegranate fruit using a digital penetrometer (FTA, model GS‐14, Guss Manufacturing Ltd., Strand, South Africa). Skin color was assessed according to grading scale (from 2: green to 18: dark red purple). Skin color was also measured in CIE L*a*b* coordinates (L*, a*, b*) on two opposite spots along the equatorial axis of each fruit using a calibrated Minolta Chroma Meter CR-400 (Minolta Corp, Osaka, Japan). The juice yield (%) was obtained by pressing 100 g of fleshy arils. All analyses were made for all individual fruits (n = 20) and results were expressed as means ± SE (standard error).
Total Phenolic Content
The total phenolic content (TPC) was determined in triplicate using the method described by Singleton et al. (Citation1999), with some modifications: 100 µL of the hydrophilic extract were mixed with 2.5 mL of Folin-Ciocalteu reagent and 400 µL phosphate buffer (50 mM, pH 7.8). The mixture was thoroughly vortexed and left for 2 min. Then, 2 mL of Na2CO3 (75 g L−1) was added to the mixture and vortexed again. The samples were thereafter left in a water bath at 50°C for 5 min. The absorbance was measured at 760 nm using a spectrophotometry (ThermoSpectronic Heλios γ, Cambridge, England) and the results (mean ± standard error) were expressed as mg of gallic acid equivalents per 100 mL.
Total Flavonoid Content
The total flavonoid content (TFC) in pomegranate juice was determined as described by Yang et al. (Citation2009). Briefly, 250 μL of pomegranate juice was mixed with distilled water (1.25 mL) and 5% sodium nitrite solution (75 μL). The mixture was thoroughly vortexed and left for 5 min. Subsequently, aluminum chloride (10%, 150 μL) was added to the mixture and allowed for further reactions for 6 min before adding sodium hydroxide (1 M, 500 μL) and distilled water (775 μL). The absorbance was measured at 510 nm by spectrophotometry (ThermoSpectronic Heλios γ, Cambridge, England). The measurements were conducted in triplicate and the total flavonoid content was expressed as mg of catechin equivalents per 100 mL.
Total Anthocyanin Content
Total anthocyanin content (TAC) was determined by the pH differential method (Cheng and Breen, Citation1991). Anthocyanins were analyzed in triplicate: 0.4 mL of pomegranate juice was diluted in 3.6 mL of two different buffers: potassium chloride (0.025 M, pH 1.0) and sodium acetate (0.4 M, pH 4.5). The absorbance (A) of two dilutions was determined at 510 and 700 nm using a spectrophotometry (ThermoSpectronic Heλios γ, Cambridge, England), where A = (A510 nm – A700 nm)pH 1.0 – (A510 nm – A700 nm)pH 4.5. The content of total anthocyanins (mg cyanidin-3-glucoside/L) was calculated as TAC = (A × MW ×DF × 1000)/(Ɛ × l), with a dilution factor (DF) of 10, an extinction coefficient (Ɛ) of 26,900 L mol−1 cm−1 and molecular weight (MW) of 449.2 g mol−1.
Total Antioxidant Activity
Total antioxidant activity was determined using ABTS, FRAP, and DPPH methods according to Re et al. (Citation1999), Benzie and Strain (Citation1996) and Brand-Williams et al. (Citation1995), respectively. For DPPH assay, 10 µL of the supernatant were mixed with 40 µL of MeOH and added to 950 µL of DPPH solution. After 50 min of reaction, the absorbance was measured at 515 nm. Regarding FRAP assay, 990 µL of FRAP reagent were added to 10 µL of the supernatant. After 10 min of reaction, the absorbance was measured at 593 nm. The 2,2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) was also performed: 990 µL of ABTS reagent were added to 10 µL of the supernatant. After 6 min of reaction, the absorbance was measured at 734 nm using a spectrophotometry (ThermoSpectronic Heλios γ, Cambridge, England). All measurements were in triplicate and the results were expressed as trolox (mM) equivalent per 100 mL.
Mineral Content
The mineral content in pomegranate juice was determined as described by Simón-Grao et al. (Citation2014). The pomegranate juice was centrifuged at 9,500 rpm for 15 min and the supernatant was passed through a filter of 0.45 µm. Dilutions were prepared in triplicate using ultra high-purity deionized water and samples were stored at 4°C until analysis. Determination of K, Ca, Mg, Na, Cu, Zn, Mn, and Fe was carried out using a Solar 969 atomic absorption-emission spectrometer (Unicam Ltd, Cambridge, UK). K and Na were analyzed by atomic emission, while the other elements were analyzed by atomic absorption.
Statistical Analyses
Statistical analyses were performed using one-way analysis of variance ANOVA, and the significant differences between means were determined by Duncan’s multiple range test using SPSS 20 software. Significance was defined at P < .05. Pearson’s correlation test was used to determine relationships between the climatic parameters and quality attributes. Statistical significance was given at P < .01. Principal component analysis (PCA) was also carried out using XLSTAT version 2018.1 in order to discriminate between the localities on the basis of pomegranate fruit physical and nutritional properties.
Results and Discussion
Physical Properties
Fruit size is a varietal characteristic that may fluctuate depending on climatic and agricultural conditions (Zaouay et al., Citation2012). Fruit weight of pomegranates from different regions ranged between 428.13 and 479.69 g (). These values were significantly higher than those (309.7–330.8 g) recorded in other Tunisian (Zaouay et al., Citation2012), Iranian (Tehranifar et al., Citation2010) and South African cultivars (Mditshwa et al., Citation2013). According to International Standards for Fruit and Vegetables: Pomegranate (OECD, Citation2014), the fruit size determined by the equatorial diameter showed that fruits from different regions belonging to class A (diameter greater than 81 mm). However, no significant differences were observed for fruit weight, fruit height, and fruit diameter between studied regions. In other studies, climate was found significantly affected the pomegranate fruit size (Mditshwa et al., Citation2013; Schwartz et al., Citation2009).
Table 1. Physical properties of pomegranate fruits var “Gabsi” from different regions
The skin weight varied significantly according to geographical origin. Pomegranates from Testour and Zerkine showed the highest skin weight (220.5 g and 230.1 g, respectively). However, the lowest value (175.4 g) was recorded in pomegranates from center of Tunisia (Chott-Mariem). A significant difference in skin thickness was also observed among growing locations, which fruits from Southern Tunisia having the thickest skin (4.7 mm) and those from center presented the thinnest one (3.05 mm). The ‘Gabsi’ pomegranates showed thinner skin than other Tunisian cultivars (Zaouay et al., Citation2012). These values were also lower than those recorded in other studies (Martinez et al., Citation2012; Viyar et al., Citation2017). Generally, consumers prefer pomegranate fruit with thinner skin which are being easily peeled and having an edible part more important.
Firmness, which is an important fruit textural property, has a direct impact on the fruit’s consumer acceptance (Khodabakhshian et al., Citation2015). The region showed a significant impact on fruit firmness (). The fruits grown in Northern and Southern Tunisia were firmer (4.29 kg · cm−2 and 4.45 kg · cm−2, respectively) than those from center of Tunisia (3.50 kg · cm−2). Increase in firmness has been suggested to be the result of tissue lignification (Dangcham et al., Citation2008). The content of lignin in plant tissues was significantly increased in the process of cold acclimation, following an increase of the C3 H gene expression that may affect the cell wall rigidity and water permeability (Liu et al., Citation2018; Wei et al., Citation2006). In our study, it appears that climate had no clear influence on fruit firmness since the pomegranates originating from two regions with different climates showed statistically similar firmness values. The high firmness values of pomegranates produced in Northern and Southern Tunisia could be due to higher skin thickness of these fruits.
The aril yield is an important industrial property. It revealed a significant variation from region to another (). The fruits from center of Tunisia (Chott-Mariem) were characterized by the highest aril yield (62.74%), while the lowest yield (45.02%) was recorded in pomegranates from Southern Tunisia. The pomegranates characterized by the heaviest and thickest skin had the lowest aril yield.
Regarding the juice yield (JY), values exceeded 70 mL/100 g of arils in different regions (). These values were higher than those reported in previous studies (ranging from 27 to 67 mL/100 g of arils) (Al-Said et al., Citation2009; Labbé et al., Citation2016; Tehranifar et al., Citation2010). The growing location significantly affected the juice yield. The fruits grown in Chott-Mariem presented the juiciest arils (79.73 mL/100 g of arils), while the lowest juice yield was observed in Testour and Zerkine (74.27 and 74.05 mL/100 g of arils, respectively).
The fruit color is a good quality attribute. The geographical origin had a significant effect on the fruit color (). It changed from light pink reddish to red color. The fruits from Southern Tunisia were the most colored while Chott-Mariem (center of Tunisia) produced the less colored fruits. Also, the color coordinates determined by CIE L*a*b* system showed a significant variation among regions. For the red coordinate (a*), the fruits produced in Zerkine and Testour presented the highest values and were more saturated in red color. However, the fruits from Chott-Mariem had the highest yellow coordinate value (b*). The fruit lightness (L*) also varied significantly between regions; the fruits grown in Northern Tunisia (Testour) have a higher light color (54.88), while the less light fruits were found in Zerkine and Chott-Mariem (46.23 and 47.18, respectively). Regarding the hue angle (Hº), fruits from center of Tunisia (Chott-Mariem) presented the highest value (48.52º). The fruit chroma (C) did not show a significant difference among different studied regions. The intense red coloration of pomegranates from southern Tunisia was probably due to higher temperature, particularly during the months of September and October (31°C and 28°C, respectively) as compared to those from other regions. These conditions proved more favorable to the development of the fruit red color. Manera et al. (Citation2012) revealed that the pomegranate skin color turned from green to red between the first and second week of September with mean temperature records of 27°C.
Table 2. Changes in visual and CIE L*a*b* color coordinates of pomegranate fruits according to region
Total Phenolic Content
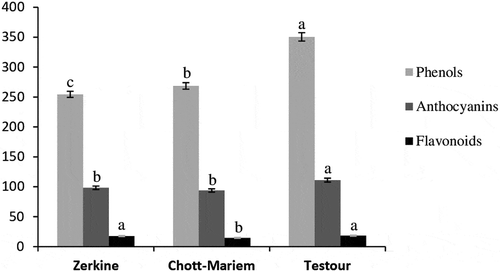
The total phenolic content (TPC) ranged from 254.51 mg GAE 100 mL–1 to 350.52 mg GAE 100 mL–1. These results are in agreement with contents recorded in previous studies for ‘Gabsi’ pomegranate juice (Boussaa et al., Citation2019). However, the total phenolic content in the present study was higher than those reported by Zaouay et al. (Citation2012) for ‘Gabsi’ variety. Significant differences were recorded in total phenolic content among different growing locations (). The highest TPC content was observed in pomegranates grown in Northern Tunisia (Testour), while the fruits from Zerkine (Southern Tunisia) showed the lowest content. Polyphenolic profile is greatly dependent on environmental conditions. Light, radiation, temperature as well as water and nutritional status are the most influential factors of which for phenolic synthesis (Orduña, Citation2010). Our results revealed that lower temperature especially in September and October (28°C and 23°C, respectively) and higher precipitations in August, September, October (41 mm, 57 mm, 61 mm, respectively) resulted in higher phenol content in pomegranates from Northern Tunisia. That supports the findings of Mditshwa et al. (Citation2013) who found that cool climate induce higher total phenolic content in pomegranate juice. However, a recent study revealed that TPC content in pomegranate arils was significantly reduced in cool and wet regions of Sri Lanka (Attanayake et al., Citation2018).
Total Flavonoid Content
Flavonoids are secondary metabolites synthesized by plants with various biological activities (Mierziak et al., Citation2014). The TFC content changed significantly among regions (). The total flavonoid contents were higher in pomegranates from Zerkine and Testour (17.6 mg 100 mL−1 and 18.23 mg 100 mL−1, respectively) than that (14.51 mg 100 mL−1) recorded in pomegranates grown in Chott-Mariem (Center of Tunisia). The TFC contents in pomegranates from Northern and Southern regions were proved statistically similar despite the extreme difference of average rainfall, temperature, and relative humidity during fruit maturation. It is not clear what could have been a major contributor to this behavior. In fact, the TFC variation could be due to light intensity, altitude, or soil type (Downey et al., Citation2006).
Total Anthocyanin Content
Anthocyanins are water-soluble pigments responsible for blue, purple, and red colors of many fruits (Goulas et al., Citation2012). Growing location had a significant effect on total anthocyanin content (). The total anthocyanin content ranged between 93.85 mg L−1 and 111.13 mg L−1, being samples from Testour region which had the highest content. The low temperature and high rainfall average could be responsible for the increase of total anthocyanin content in pomegranates from Northern Tunisia. Wang et al. (Citation2016) revealed that the expression of the anthocyanin biosynthetic genes decreased as temperature increased, thus, reducing the anthocyanin concentration in red fleshed apple. In strawberries, anthocyanin synthesis genes (FaDFR, FaANS, FaUFGT, and FaMYB10) showed a significant decrease under a high air temperature regime (30/15°C, day/night) compared to a control (20/15°C, day/night) (Matsushita et al., Citation2016). However, a considerable up-regulation of ANS, DFR, and F3 H genes in pomegranate arils were observed in the drier and warmer locations of Sri Lanka (Attanayake et al., Citation2018).
Antioxidant Activity
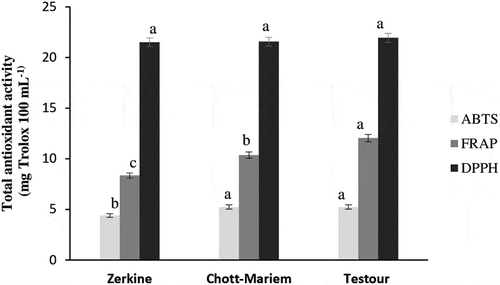
Antioxidants are considered as important bioactive compounds on account of many health benefits along with their pivotal role in delaying oxidative rancidity of numerous foods (Moharram and Youssef, Citation2014). The antioxidant activity evaluated by ABTS and FRAP assays was found significantly different among regions. However, there was no difference for the DPPH activity from region to another (). The ABTS scavenging activity was higher in pomegranate juices from Chott-Mariem and Testour (5.24 mg Trolox 100 mL−1), while the fruits grown in Southern Tunisia presented the lowest activity (4.40 mg Trolox 100 mL−1). Regarding FRAP assay, the pomegranates from Testour, Northern Tunisia, exhibited the highest activity (12.04 mg Trolox 100 mL−1). On the other hand, the lowest ferric reducing antioxidant power (8.35 mg Trolox 100 mL−1) was observed in fruits from Southern Tunisia (Zerkine). The total phenolic and anthocyanin contents could be contributed to the high antioxidant activity of pomegranate juices from Northern Tunisia. These results were in accordance with those reported by Schwartz et al. (Citation2009). A temperature inferior to 30°C during fruit maturation in Northern Tunisia could be the most appropriate conditions to obtain higher fruit antioxidant activity. Similarly, Wang (Citation2006) found that a temperature growing conditions (25/30°C) significantly enhanced antioxidant activity, as well as anthocyanin and total phenolic content.
Mineral Content
Tunisian pomegranate juice seems to be a good source of minerals. Potassium was the predominant element (2089–2175 mg L−1) in ‘Gabsi’ pomegranates, followed by magnesium (73.32–82.5 mg L−1) and sodium (48.79–65.01 mg L−1). These values were in the range of those obtained in previous studies (El Falleh et al., Citation2011; Gozlekci et al., Citation2011). The mineral contents varied significantly in response to growing location except potassium (). The fruits grown in Chott-Mariem, center of Tunisia, were found the richest in mineral contents and exhibited high levels of magnesium, sodium, zinc, manganese, and copper. Pomegranates from Southern Tunisia presented higher magnesium, sodium, and calcium contents, while those grown in Northern Tunisia showed the highest zinc and copper levels. Nutrients variation in pomegranate juice could originate from agro-climatic conditions (Al-Maiman and Ahmad, Citation2002). High calcium content in pomegranates from Zerkine could be attributed to high temperature, low relative humidity, or low rainfall. Water and minerals transport in plant occur through the transpiration stream as triggered mainly by the environmental conditions. Higher fruit transpiration rates are associated with increased fruit Ca levels (Montanaro et al., Citation2012). Also, Hofman et al. (Citation1997) revealed that increased relative humidity around the fruit can reduce fruit Ca accumulation. However, relative humidity had little effect on accumulation of Mg and K in sweet pepper fruit (Tadesse et al., Citation2001).
Table 3. Variation of mineral content in pomegranate juice var ‘Gabsi’ according to region
Principal Component Analysis
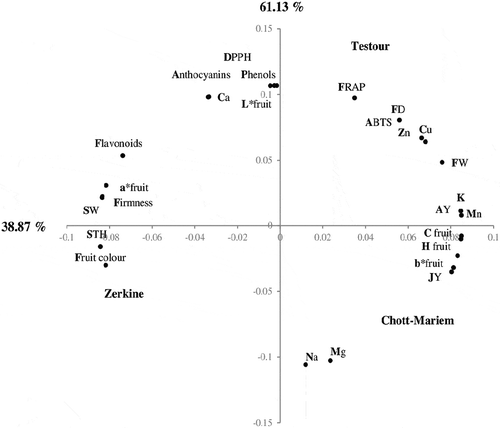
A principal component analysis (PCA) was performed based on physical and nutritional properties of pomegranates from the three regions (). Within the first two principal components (PC), 100% of the total variance can be explained. The first PC, representing 61.13 %, was positively correlated with the fruit weight, aril yield, juice yield, fruit color coordinates (b*, C and Hº), ABTS activity, K, Mg, Na, and Mn contents. These parameters characterized the pomegranates from Chott-Mariem (center of Tunisia). However, Zerkine (Southern Tunisia) depicted negative loadings to PC1 and presented more colorful fruits with heavier and thickest skin as well as juice rich in flavonoids content, calcium, sodium, and magnesium levels. The PC2 axis, representing 38.87% of total variance, was positively related to the region of Testour. This component defined the fruits from Northern Tunisia which are rich in zinc, copper, anthocyanins, phenols and exhibited high antioxidant activity.
Correlation Analysis
To determine the effect of the climatic conditions on studied quality parameters, an annual mean temperature, rainfall, and relative humidity of the selected regions were used for the correlation analysis (). An important correlation was recorded between total phenolic content (TPC) and temperature (r = – 0.995) as well as relative humidity (r = 0.966). A same trend was observed for antioxidant activity determined by FRAP and DPPH that was correlated negatively with temperature and positively with rainfall and relative humidity. Similar results were reported by Mditshwa et al. (Citation2013) and showed that TPC and antioxidant activity were related to rainfall. However, Attanayake et al. (Citation2018) indicated that the total phenolic content was reduced with lower temperature and higher rainfall. Temperature was shown positively affected magnesium and sodium contents, but a negative relationship was noted between these mineral elements and relative humidity. Climate no clearly affected the variation of the other studied quality attributes that could have been more attributed to other factors such as light intensity, agricultural practices, or soil type.
Table 4. Relationship between climatic parameters (temperature, rainfall, relative humidity), physical and nutritional properties
Conclusion
A significant regional effect was observed on the bioactive compounds and antioxidant activity of ‘Gabsi’ pomegranate fruits. Principal component analysis revealed a clear distinction of the different studied regions. Pomegranate fruits grown in northern and center of Tunisia, under relatively lower temperature and higher rainfall and relative humidity, were of higher quality than those produced in semi arid region. These results will provide a better understanding of the effect of environmental conditions in physical properties and synthesis of bioactive compounds in order to obtain pomegranates of good appearance and nutritional quality. Further research is recommended in this area to assess aroma, organic acids, and sugars of pomegranate juice ‘Gabsi’ in the same studied growing locations and determine the link between climate and flavor.
Additional information
Funding
Literature cited
- Al-Maiman, S.A., and D. Ahmad. 2002. Changes in physical and chemical properties during pomegranate (Punica granatum L.) fruit maturation. Food Chem. 76:437–441. doi: 10.1016/S0308-8146(01)00301-6.
- Al-Said, F.A., U.L. Opara, and R.A. Al-Yahyai. 2009. Physico-chemical and textural quality attributes of pomegranate cultivars (Punica granatum L.) grown in the Sultanate of Oman. J. Food Eng. 90:129–134. doi: 10.1016/j.jfoodeng.2008.06.012.
- Attanayake, R., R. Eeswaran, R. Rajapaksha, P. Weerakkody, and P.C.G. Bandaranayake. 2018. Biochemical composition and expression analysis of anthocyanin biosynthetic genes of a yellow peeled and pinkish ariled pomegranate (Punica granatum L.) cultivar are differentially regulated in response to agro-climatic conditions. J. Agri. Food Chem. 66(33):8761–8771. doi: 10.1021/acs.jafc.8b02909.
- Benzie, I.F.F., and J.J. Strain. 1996. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 239(1):70–76. doi: 10.1006/abio.1996.0292.
- Boussaaa, F., F. Zaouay, F. Burlo-Carbonell, N. Nuncio-Jáuregui, M. Gmati, B. El Arbie, P. Melgarejo, F. Hernandez, and M. Mars. 2019. Combined effects of cropping system and harvest date determine quality and nutritional value of pomegranate fruits (Punica granatum L. cv. Gabsi). Sci. Hortic. 249:419–431. doi: 10.1016/j.scienta.2019.02.007.
- Brand-Williams, W., M.E. Cuvelier, and C. Berset. 1995. Use of free radical method to evaluate antioxidant activity. Lebensm Wiss. Technol. 28:25–30. doi: 10.1016/S0023-6438(95)80008-5.
- Cheng, G.W., and B.J. Breen. 1991. Activity of phenylalanyl ammonialyase (PAL) and concentrations of anthocyanins and phenolics in developing strawberry fruit. J. Am. Soc. Hortic. Sci. 116:865–868. doi: 10.21273/JASHS.116.5.865.
- Dangcham, S., J. Bowen, I.B. Ferguson, and S. Ketsa. 2008. Effect of temperature and low oxygen on pericarp hardening of mangosteen fruit stored at low temperature. Postharvest Biol. Tec. 50(1):37–44. doi: 10.1016/j.postharvbio.2008.02.005.
- Downey, M.O., N.K. Dokoozlian, and M.P. Krstic. 2006. Cultural practice and environmental impacts on the flavonoid composition of grapes and wine: A review of recent research. Am. J. Enol. Vitic. 57:3.
- El Falleh, W., N. Tlili, M. Ying, H. Sheng-Hua, A. Ferchichi, and N. Nasri. 2011. Organoleptic quality, minerals, proteins and amino acids from two tunisian commercial pomegranate fruits. Int. J. Food Eng. 7 (4):13. Art.12.
- Goulas, V., A.R. Vicente, and G.A. Manganaris. 2012. Structural diversity of anthocyanins in fruits, p. 225–250. In: N. Motohashi (ed.). Anthocyanins: Structure, biosynthesis and health benefits. Nova Science Publishers, New York.
- Gozlekci, S., S. Ercisli, F. Okturen, and S. Sonmez. 2011. Physico- Chemical characteristics at three development stages in pomegranate cv. ‘Hicaznar’. Not. Bot. Hort. Agrobot. Cluj. 39(1):241–245. doi: 10.15835/nbha3918985.
- Gündüz, K., and H. Özbay. 2018. The effects of genotype and altitude of the growing location on physical, chemical, and phytochemical properties of strawberry. Turk. J. Agric. For. 42:145–153. doi: 10.3906/tar-1706-65.
- Hofman, P.J., L.G. Smith, D.C. Joyce, G.I. Johnson, and G.F. Meiburg. 1997. Bagging of mango (Mangifera indica cv. ‘Keitt’) fruit influences fruit quality and mineral composition. Postharvest Biol. Tec. 12:83–91. doi: 10.1016/S0925-5214(97)00039-2.
- Khodabakhshian, R., B. Emadi, M. Khojastehpour, and M.R. Golzarian. 2015. Determining quality and maturity of pomegranates using multispectral imaging. J. Saudi Soc. Agri. Sci. 16(4):322–331.
- Labbé, M., P.A. Ulloa, F. López, C. Sáenz, A. Peña, and F.N. Salazar. 2016. Characterization of chemical compositions and bioactive compounds in juices from pomegranates (‘Wonderful’, ‘Chaca’ and ‘Codpa’) at different maturity stages. Chil. J. Agr. Res. 76(4):479–486. doi: 10.4067/S0718-58392016000400012.
- Legua, L., M.A. Forner-Giner, N. Nuncio-Jáuregui, and F. Hernández. 2016. Polyphenolic compounds, anthocyanins and antioxidant activity of nineteen pomegranate fruits: A rich source of bioactive compounds. J. Func. Foods. 23:628–663. doi: 10.1016/j.jff.2016.01.043.
- Liu, Q., L. Luo, and L. Zheng. 2018. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 19:335. doi: 10.3390/ijms19020335.
- Manera, F.J., P. Legua, P. Melgarejo, R. Martínez, J.J. Martínez, and F. Hernández. 2012. Effect of air temperature on rind colour development in pomegranates. Sci. Horti. 134:245–247. doi: 10.1016/j.scienta.2011.11.016.
- Martinez, J.J., P. Melgarejo, R. Legua, R. Martinez, and F. Hernandez. 2012. Diversity of (Punica Granatum L.) germplasm in Spain. II. international symposium on the pomegranate, options Mediterraneennes: Serie A. Seminaires Mediterraneens 103:53–66.
- Matsushita, K., K. Sakayori, and T. Ikeda. 2016. The effect of high air temperature on anthocyanin concentration and the expressions of its biosynthetic genes in strawberry ‘Sachinoka’. Environ. Control Biol. 54(2):101–107. doi: 10.2525/ecb.54.101.
- Mditshwa, A., O.A. Fawole, F. Al-Said, R. Al-Yahyai, and U.L. Opara. 2013. Phytochemical content, antioxidant capacity and physicochemical properties of pomegranate grown in different microclimates in South Africa. S. Afr. J. Plant Soil 30(2):81–90. doi: 10.1080/02571862.2013.802033.
- Mena, P., L. Calani, C. Dall’Asta, G. Galaverna, C. García-Viguera, R. Bruni, A. Crozier, and D. Del Rio. 2012. Rapid and comprehensive evaluation of (poly)phenolic compounds in pomegranate (Punica granatum L.) juice by UHPLC-MSn. Molecules 17(12):14821–14840. doi: 10.3390/molecules171214821.
- Mierziak, J., K. Kostyn, and A. Kulma. 2014. Flavonoids as important molecules of plant interactions with the environment. Molecules. 19:16240–16265. doi: 10.3390/molecules191016240.
- Mirdehgham, S.H., and M. Rahemi. 2007. Seasonal changes of mineral nutrients and phenolics in pomegranate (Punica granatum L.) fruit. Sci. Hortic. 111:120–127. doi: 10.1016/j.scienta.2006.10.001.
- Moharram, H.A., and M.M. Youssef. 2014. Methods for determining the antioxidant activity: A review. Alex. J. Fd. Sci. Technol. 11(1):31–42.
- Montanaro, G., B. Dichio, and C. Xiloyannis. 2012. Fruit transpiration: Mechanisms and significance for fruit nutrition and growth, in advances in selected plant physiology aspects, p. 233–250. ed. by Giuseppe Montanaro, University of Basilicata, Italy.
- Nuncio-Jáuregui, N., S. Munera-Picazo, A. Calın-Sanchez, A. Wojdyło, F. Hernandez, and A.A. Carbonell-Barrachina. 2015. Bioactive compound composition of pomegranate fruits removed during thinning. J. Food Compos. Anal. 37:11–19. doi: 10.1016/j.jfca.2014.06.015.
- OECD. 2014. International Standards for Fruit and Vegetables: Pomegranate.
- Orduña, R. 2010. Climate change associated effects on grape and wine quality and production. Food Res. Int. 43:1844–1855. doi: 10.1016/j.foodres.2010.05.001.
- Re, R., N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, and C. Rice-Evans. 1999. Antioxidant capacity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3.
- Schwartz, E., R. Tzulker, I. Glazer, I. Bar-yaakov, Z. Wiesman, E. Tripler, I. Bar-ilan, H. Fromm, H. Borochov-Neori, D. Holland, et al. 2009. Environmental conditions affect the color, taste, and antioxidant capacity of 11 pomegranate accessions’ fruits. J. Agric. Food Chem. 57:9197–9209. doi: 10.1021/jf901466c.
- Simón-Grao, S., V. Gimeno, I. Simón, V. Lidón, M. Nieves, R.M. Balal, A.A. Carbonell-Barrachina, F.J. Manera, F. Hernández, and F. García-Sánchez. 2014. Fruit quality characterization of eleven commercial mandarin cultivars in Spain. Sci. Horti. 165:274–280. doi: 10.1016/j.scienta.2013.11.022.
- Singleton, V.L., R. Orthofer, and R.M. Lamuela-Raventos. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzymol. 299:152–178.
- Tadesse, T., M.A. Nichols, E.W. Hewett, and K.J. Fisher. 2001. Relative humidity around the fruit influences the mineral composition and incidence of blossom-end rot in weet pepper fruit. J. Hortic. Sci. Biotech. 76(1):9–16. doi: 10.1080/14620316.2001.11511319.
- Tehranifar, A., M. Zarei, B. Esfandiyari, and Z. Nemati. 2010. Physicochemical properties and antioxidant activities of pomegranate fruit (Punica granatum) of different cultivars grown in Iran. Hort. Environ. Biotechnol. 51(6):573–579.
- Teixeira da Silva, J.A., T.S. Rana, D. Narzary, N. Verma, D.T. Meshram, and S.A. Ranade. 2013. Pomegranate biology and biotechnology: A review. Sci. Hortic. 160:85–513. doi: 10.1016/j.scienta.2013.05.017.
- Viyar, A.H., R. Qadri, A. Iqbal, N. Nisar, I. Khan, M. Bashir, and F. Shah. 2017. Evaluation of unexplored pomegranate cultivars for physicochemical characteristics and antioxidant activity. J. Food Sci. Technol. 54(9):2973–2979. doi: 10.1007/s13197-017-2736-z.
- Wang, N., Z. Zhang, S. Jiang, H. Xu, Y. Wang, S. Feng, and X. Chen. 2016. Synergistic effects of light and temperature on anthocyanin biosynthesis in callus cultures of red-fleshed apple (Malus sieversii f. niedzwetzkyana). Plant Cell Tiss. Organ Cult. 127:217–227. doi: 10.1007/s11240-016-1044-z.
- Wang, S.Y. 2006. Effect of pre-harvest conditions on antioxidant capacity in fruits. Acta. Hort. 712:299–305. doi: 10.17660/ActaHortic.2006.712.33.
- Wei, H., A.L. Dhanaraj, R. Arora, L.J. Rowland, Y. Fu, and L. Sun. 2006. Identification of cold acclimation-responsive Rhododendron genes for lipid metabolism, membrane transport and lignin biosynthesis: Importance of moderately abundant ESTs in genomic studies. Plant Cell Environ. 29:558–570. doi: 10.1111/j.1365-3040.2005.01432.x.
- Yang, J., T.E. Martinson, and R.H. Liu. 2009. Phytochemical profiles and antioxidant activities of wine grapes. Food Chem. 116:332–339. doi: 10.1016/j.foodchem.2009.02.021.
- Zaouay, F., P. Mena, C. Garcia-Viguera, and M. Mars. 2012. Antioxidant activity and physico-chemical properties of Tunisian grown pomegranate (Punica granatum L.) cultivars. Ind Crops Prod. 40:81–89. doi: 10.1016/j.indcrop.2012.02.045.