?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The phenolic compounds and antioxidant activities of natural plant extract have the ability to maintained human health and reduced the risk of disease. The present study investigates the feasibility of the ultra-sonic assisted extraction method and most effective solvents for the extraction of phenolic, flavonoid, and antioxidant activities of freeze-dried pomegranate seed powder. The study also involved investigations of physicochemical characteristics such as edible & waste index, acidity, TSS, moisture, and pH of pomegranate fruits, seed, and dried powder. The pomegranate seed powder was subjected to extraction using five different polar and nonpolar solvents viz., methanol, ethanol, water, acetone, and hexane. In this study, the methanol solvents exhibit the highest value of phenolic contents (18.25 mg/g), flavonoid contents (9.13 mg/g), and antioxidant activity (89.39%). The antioxidant (DPPH), phenolic and flavonoid content activity were analyzed by using standard methods. The physicochemical properties depend on the types of solvents that extract compounds according to polarity and nonpolarity. The obtained results were indicated that the methanol solvent is significantly effective (P < .05) for quality extraction of phenolic and flavonoid contents with high antioxidant activity of pomegranate seed powder.
Introduction
India is one of the largest producers of pomegranate (Punica granatum L.) followed by Iran (Ercisli et al., Citation2011). It has excellent physicochemical and organoleptic properties (Kumar et al., Citation2018). The pomegranate fruits are widely used in the pharmaceutical and food processing industries (Russo et al., Citation2020). Seeds of pomegranate fruits have been used traditionally as a folk medicine due to the presence of phenolics, and antioxidants showing medicinal properties (Fahad et al., Citation2017). The human consumption of pomegranate since ancient times characterized it as a super fruit due to the presence of various beneficial bioactive compounds (Keziban and Alpaslan, Citation2016). shows the chemical structure of major bioactive compounds of the pomegranate seed. Besides these seeds being the good source of powerful health-promoting oil exhibited good physicochemical properties such as carbohydrates, organic acids, vitamins, phytosterols, linolenic acid, sex steroids (Estradiol, Testosterone) antioxidant, antilipidemic, and anticarcinogenic activities with good oxidative stability (Illana et al., Citation2016; Mohammad et al., Citation2016; Nejib et al., Citation2011; Puneeth and Chandra, Citation2020; Walid et al., Citation2011). The pomegranate seed extract is valued for its ability for prevention and management of oxidative stress-induced diseases due to the presence of high amount of phenolic contents (Abiola et al., Citation2018). The several studies reported evidence of anti-cancerogenic effects in prostate cancer pomegranate (Christian et al., Citation2015).
Betwixt the drying methods, the freeze-drying method is the most effective and suitable method to retentions of nutritional, biological compounds as well as organoleptic properties of food materials (Sehrawat et al., Citation2018). It has the ability to drying the plant materials without affecting the chemical properties, color and flavor agents of the pomegranate peel powder (Kennas et al., Citation2020). Al-Sanabani et al. (Citation2016) reported the freeze-drying method has minimum destruction of phenolic, flavonoid, and antioxidant activity of the pomegranate seed.
Extractions are the primary procedure for the extraction of biological and organic compounds from plant extract in the preparations of food ingredient and nutraceutical products. The extraction process directly influences the physicochemical properties of the plant extract (Xia et al., Citation2006). Ultrasonic-assisted extraction is green, inexpensive, eco-friendly, effective, and innovative & conventional technology for the extraction of biological activity (Huang et al., Citation2009). It allowed high extraction yield, efficiency, short extraction time, reduce solvents consumptions and prevent thermal degradation. It provides shear force to facilitate to dispute the cell wall. This technique also helps to increase antioxidant activity and prevent biological compounds of plant extract (Buddin et al., Citation2018).
Among the solvents, the methanol is considered as the most effective solvents to recover higher amounts of the phenolic, flavonoid, tannins, and antioxidant activity. Various studies have investigated the role of solvents in the extractions of phenolic and antioxidant compounds. Methanol solvents are the best for the extracting of phenolic compounds to access the antioxidant activity by using different methods such as 2,2-diphenyl-1-picrylhydrazyl (DPPH), beta carotene bleaching, Ferric reducing antioxidant power (FRRP), and Ferric thiocyanate (FTC) as compared to other polar and nonpolar solvents (Massaud et al., Citation2017; Padmaja and Prasad, Citation2011; Wang et al., Citation2011).
Resources and Methods
Pomegranate fruits of variety Bhagwa were procured from the nearest renowned Azadpur Fruits Mandi, Delhi. All chemicals, solvents (HPLC grade) methanol, ethanol, water, acetone, and hexane and standards reagent were procured from Sigma Aldrich and M/s Hi-tech. The samples subjected to antioxidative analysis were subjected to the analysis in triplicates.
Extraction of Pomegranate Seed `
Pomegranate (Punica granatum L.) fruit seeds were lyophilized overnight and dried in a freeze dryer for 120 h at –45ºC and ground well. The extract of pomegranate seed powder was prepared according to (Kumar and Neeraj, Citation2018). Approximately, 0.2 g powder of pomegranate seed was taken and dissolved in 10 ml different solvents (methanol, ethanol, water, acetone, and hexane) in an ultrasonic bath (CUB-5, Citizen, 40 kHz, 220–240 V) for 30 min at 45ºC (Kumar and Neeraj, Citation2020). The dissolved extracts were centrifuged to obtained clear extract at 8654 rpm at 5ºC for 10 min The extracts were filtered and stored for further use at −20ºC.
Physical and Morphological Characteristics
The physical characteristics and morphological features of pomegranate fruits were determined in the following ways, in terms of the color of fruit & seed, an average of fruit weight, edible & waste index, moisture, acidity, pH, and TSS.
Moisture Content
The moisture content of pomegranate seed was determined by using the AOAC method (AOAC, Citation1995). About 10 g samples were oven-dried at 105 ± 2°C for 16 h in uncovered pre-weighed Petri dish. After the drying process, the Petri dish was covered with a lid and cooled in desiccators containing silica gel for 1 h before weighing. The moisture content of the whole sample was calculated by:
where
W1 = Weight of empty Petri dish,
W2 = Weight of Petri dish + sample before drying
W3 = Weight of Petri dish + sample after drying
TSS (Total Soluble Solids)
TSS of pomegranate seed was determined in terms of °Brix by using Hand Refractometer (Atago 3810-Japan) by using AOAC, method (AOAC, Citation2000a). The measurement of TSS was determined at 25 ± 2°C.
pH
The pH of pomegranate seed was measured with the help of pH meter. The instruments calibrated with standard buffer solutions, i.e., pH 7 and pH 4. Sample’s reading was recorded in three replicates.
Titrable Acidity
Titrable acidity of pomegranate seed was determined by using the NaOH titration method of AOAC (AOAC, Citation2000b). The pomegranate seed was crushed manually to extract juice. About 25 ml juice extract was titrated with 0.01 N NaOH solution. Phenolphthalein indicator was used to mark the endpoint of the titration.
Physicochemical Properties of Freeze-dried Pomegranate Seed
Total Phenolic Content
The phenolic activity of pomegranate freeze-dried seed powder extract was determined by using the Folin-Ciocalteu colorimetric standard method (AOAC, Citation2016a; Singleton and Joseph, Citation1965) with some modifications. About 1 ml of the extract was dissolved with 70ml distilled water and kept for 2 min followed by the addition of 5 ml FC reagents (10-time fold). Sodium carbonate solution (7.5 ml of 20%) was added followed by final volume makeup to 100 ml using distilled water. The gallic acid standard was used for the computed phenolic content of the pomegranate seed powder extract (mg GAE/g) on dry basis. The absorbance was recorded at 765 nm wavelength.
Total Flavonoid Content
Flavonoid content of freeze-dried seed powder extract was determined by using flavonoid–aluminum spectrophotometric method followed by (Kumar and Neeraj, Citation2020). Quercetin was used as a standard to compute the value of flavonoids. In this case extract (1 ml) was added with methanol (1.5 ml), methanolic solution (0.1 ml of 10% AlCl3) and potassium acetate (0.1 ml, 1 M) followed by proper mixing. After that sample tubes were covered with aluminum foil and incubated at 25 ± 2ºC for 40 min. The absorbance was measured at 430 nm wavelength and results were expressed as mg Quercetin equivalents/g on dry basis.
Antioxidant Activity
The antioxidant activity of freeze-dried seed powder extract of pomegranate was determined by using the standard DPPH assay method given by Brand-Williams et al. (Citation1995) with some modifications (AOAC, Citation2016b; Brand-Williams et al., Citation1995; Sehrawat and Nema, Citation2018). Take of freeze-dried seed powder extract of pomegranate (0.1 ml) and added Methanol (0.49 ml) and DPPH Methanolic solution (0.39 ml; 3.94 mg/100 ml). Then, the mixtures were vortexed and allowed to keep in the dark place for 60 min the absorbance was measured at 517 nm wavelength by using UV-spectrophotometer (Sican, 2301, Incarp).
The antioxidant activity was computed and expressed as the inhibition percentage by using the following formula:
Data Analysis
The recorded data were analyzed by using IBM SPSS Version 24. One-way ANOVA and Duncan’s test to determine the mean values for the different treatments within an experiment. The graphical representation of the data was made by using Origin 2019b. The results were expressed as mean ± SD. All tests were performed in replicates of three.
Results and Observations
Physical and Morphological Characteristics
The morphological and physical characteristics of pomegranate fruit, seed, and dried powder were recorded and results have shown in . The color of fruit and arils was observed red and glossy red, respectively. The edible and waste index of pomegranate fruits was found 52.16% and 47.84% respectively. The moisture of the pomegranate seed and freeze-dried powder was recorded 80.10% and 8.72%, respectively. The acidity, pH, and TSS were recorded of pomegranate seed 0.59%, 4.51, and 13.76 ºBrix, respectively. Results of this study are in line with previous results reported by Wetzstein et al. (Citation2011). He reported the 345 g of an average weight of wonderful pomegranate fruit with 49.57% of edible and 50.43% of the waste index (Wetzstein et al., Citation2011). The edible and waste index of Ganesh cultivars pomegranate fruits were recorded 52 g and 48 g, respectively. The results showed that the Ganesh cultivars have high average fruit weight with the high waste index of pomegranate as compared to Bhagwa cultivars. Similar kind feature results of pomegranate fruit were reported by Hota and Dahiya (Citation2017). He reported the average weight of the pomegranate fruit Bhagwa (193.5 g) and Ganesh (291.5 g) with 40.63% and 32.96% of the rind (Hota and Dahiya, Citation2017). Radunic et al. (Citation2015) investigated the physical and morphological characteristics of 8 different cultivars of pomegranate peel. He reported the fruit weight range between 189.4 and 593.9 g (Radunic et al., Citation2015). Al-Maiman and Ahmed (Citation2002) reported the average weight of Tafil cultivar (216.50 g) of the full-ripened pomegranate fruit. Prasad et al. (Citation2012) reported the average weight of Bhagwa pomegranate fruit 320 g along with 36.80% of waste index (peel). The average weight of Ganesh pomegranate was determined 297 g with 37.54% of waste index. The difference between the waste indexes of these varieties of his study showed a similar range. The Ganesh variety of pomegranate fruit has shown a higher range of waste index (Prasad et al., Citation2012). The results are in line with those previous study (Gadze et al., Citation2012; Mphahlele et al., Citation2016; Kumar and Neeraj, Citation2020) reported similar kind results of morphological features and physical characteristics, i.e., fruit weight, edible & waste index of pomegranate fruit.)
Table 1. Physical characteristics of pomegranate fruit, seed, and dried powder
Table 1. Physical characteristics of pomegranate fruit, seed, and dried powder (Insert here)
Physicochemical Analysis
Total Phenolic Content
The values of total phenolic contents of Bhagwa pomegranate seed extracts are shown in . Various research studies have been investigated the effect of solvents in the extraction of phenolic compounds from pomegranate arils and peel extract (Charles et al., Citation2012; Kumar and Neeraj, Citation2018, Citation2020). The results of the mean comparison were found that methanolic extraction of freeze-dried pomegranate seed powder was significantly higher than other solvents. Methanol has a good ability to extract to both polar and nonpolar phenolic compounds and exhibited the highest phenolic content activity, i.e., 18.25 mg/g followed by ethanolic and aqueous extraction 16.84 and 10.19 mg/g, respectively. The hexane extraction rate was found lowest phenolic content activity 1.39 followed by acetone 2.34 mg/g. The results of this study indicated the nonpolar solvent (hexane) and a polar solvent (acetone) has less potential to extract phenolic compounds from pomegranate seed powder extract. The results are agreed with the previous study done by Singh et al. (Citation2002) and Razali et al. (Citation2012). These studies identified the methanolic fractions of the pomegranate seed were extracted higher amounts of the phenolic and antioxidative compounds as compared to other solvents such as water, ethyl acetate, and hexane. The hexane solvents have lower efficiency to extract phenolic compounds from pomegranate seed (Razali et al., Citation2012; Singh et al., Citation2002). Basiri (Citation2013) investigated the effects of different solvents on the phenolic activity of pomegranate seed. He reported the methanolic extraction (27.93 mg/l) of pomegranate seed showed higher phenolic activity followed by aqueous extraction (22.61 mg/l) due to the higher efficiency and polar nature. The acetone, butanol, ethyl-acetate, and hexane fraction of pomegranate seed showed the least activity of phenolic content due to lower efficiency of extract plant secondary compounds. The hexane fraction of the pomegranate seed showed lower efficiency to extract phenolic compounds (0.29 mg/l) due to nonpolar nature (Basiri, Citation2013). The extraction methods of the plant material have an impact on the quality and quantity of extracted phenolic groups from plant materials. Mphahlele et al. (Citation2016) reported the phenolic activity (215.21 mg/100 ml) of Wonderful pomegranate variety arils. Elfalleh et al. (Citation2012) investigate the effect of sun-drying and methanol and water solvents on the physiochemical characteristics of pomegranate fruit parts, i.e., seed, peel, leaf, and flower. He reported the methanolic fractions of the pomegranate seed were showed higher phenolic activity (11.84 mg/g) as compared to aqueous extraction (7.94 mg/g) of pomegranate seed (Elfalleh et al., Citation2012).
Figure 2. Effect of solvents on phenolic activity (mg/g dry basis) of freeze-dried pomegranate seed extract
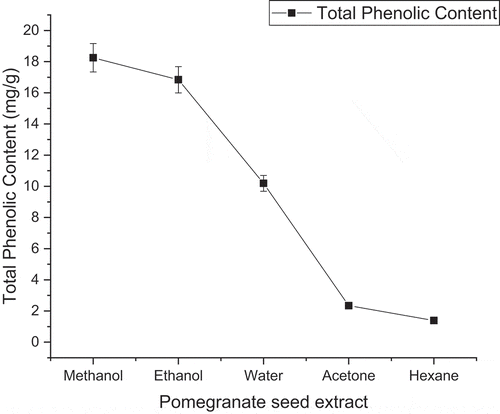
Al-Sanabani et al. (Citation2016) reported the phenolic content activity of the methanolic extraction of freeze-dried pomegranate seed with 491.84 mg 100 g-1 . Kumar and Neeraj (Citation2018) evaluated the effect of drying methods and solvents on the phenolic activity of pomegranate peel. He reported that the freeze-drying method and methanol solvents are the best choice for the extraction of higher amounts of phenolic compounds (Kumar and Neeraj, Citation2018). Chen et al. (Citation2015) reported that the ultrasonic-assisted extraction is a green extraction to extract higher amounts of natural phenolic compounds from plant materials. Deng et al. (Citation2017) investigated the comparison between ultrasonic-assisted and maceration extraction method. His findings concluded that the ultrasonic-assisted extraction method is higher efficient to the extraction of high yield and natural biological activity from fresh olives as compare to maceration extraction methods (Deng et al., Citation2017). The various researchers have been reported, the ultrasonic-assisted extraction method improving the extraction of phenolic compounds from plant extracts (Wen et al., Citation2019). The results of this study are similar to the previous study Kumar and Neeraj (Citation2020) investigated on freeze-dried pomegranate arils. He reported the methanol is a potential solvent to recover phenolic compounds followed by water, ethanol, and acetone (Kumar and Neeraj, Citation2020). The hexane was finding weak solvent to extract phenolic compounds. The results concluded that methanol, ethanol, and water are potential solvents used for the recovery of phenolic compounds (Thouri et al., Citation2017).
Total Flavonoid Content
Total flavonoid contents (TFC) of pomegranate seed powder extract graphical data are depicted in . The result of the mean comparison of this study found that extraction in methanol solvent of pomegranate seed powder extract was significantly higher as compared to other polar and nonpolar solvents. Total flavonoid content was obtained highest 9.13 mg/g in the case of Methanolic extraction whereas ethanolic extraction showed 8.79 mg/g. Hexane extraction showed the lowest total flavonoid content value (0.97 mg/g). Aqueous and acetone extraction showed 5.35 and 1.23 mg/g of total flavonoid content, respectively. The results revealed and indicated that the methanol and ethanol are the best solvents for the extraction of flavonoid contents from pomegranate seed extract. Mphahlele et al. (Citation2016) reported the flavonoid activity (331.92 mg/100 ml) of Wonderful pomegranate variety arils. Elfalleh et al. (Citation2012) reported the methanolic extraction is much potential to extract a higher amount of flavonoid content as compared to aqueous extraction.
Figure 3. Effect of solvents on flavonoid activity (mg/g dry basis) of freeze-dried pomegranate seed extract
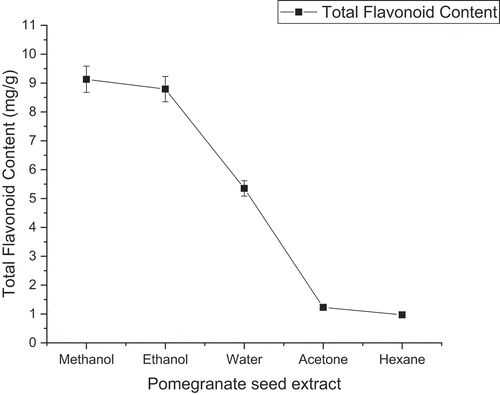
Al-Sanabani et al. (Citation2016) reported the flavonoid content activity of the methanolic extraction of freeze-dried pomegranate seed with 496.04 mg 100 g-1 . Jing et al. (Citation2012) evaluated the flavonoid activity of four different varieties of pomegranate seed extraction. In the 80% methanolic extraction, the flavonoid content range of the pomegranate seed was higher (0.42–0.62 mg/g) as compared to acetone extraction (0.37–0.58 mg/g) (Jing et al., Citation2012). Results are agreed with the previous study Kumar and Neeraj (Citation2020) reported the effect of solvents on flavonoid content activity of freeze-dried pomegranate aril. His study indicated that methanol is a potential solvent to recover flavonoid compounds followed by water, ethanol, and acetone. The hexane was finding weak solvent to extract flavonoid compounds (Kumar and Neeraj, Citation2020).
Antioxidant Activity (DPPH Assay)
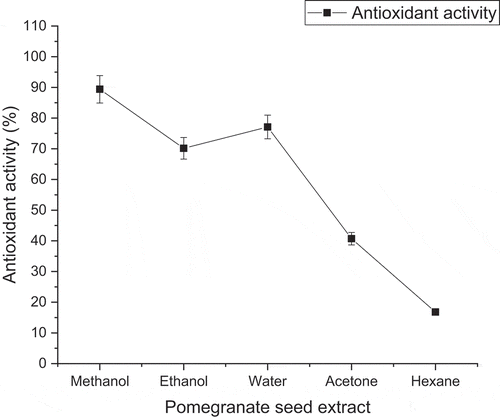
The natural antioxidant of plants has the ability to reduce disease risk due to scavenging activity. The graphical data of antioxidant activity of pomegranate seed powder extract are represented in . Results indicated the highest antioxidant activity value 89.39% as obtained by methanolic extraction followed by the activity value of 77.10% by way of aqueous extraction whereas ethanolic and acetone extracts showed 70.16% and 40.70% free radical scavenging activity. The lowest antioxidant activity 16.80% was shown by seed extracts in the hexane fraction. The results of the study indicated the extraction of antioxidant activity of freeze-dried pomegranate seed powder extract in methanolic and aqueous extraction as the best one.
The mean results of the antioxidant activity of pomegranate seed are agreement with the previous literature and investigations. Sultana et al. (Citation2009) investigated the comparison between absolute and 80% aqueous methanol and ethanol solvents on the antioxidant property of different medicinal plants. He reported the absolute and 80% methanol are the best solvent for the extraction of higher antioxidant activity as compare to absolute and 80% ethanol (Sultana et al., Citation2009). Singh et al. (Citation2002) reported the higher antioxidant activity of pomegranate seed in methanolic extraction as compared to other solvent extraction, i.e., water and ethyl acetate. Elfalleh et al. (Citation2012) reported the methanol solvent is the best choice for the extraction of a higher amount of hydrolyzable tannins from pomegranate flower than water due to higher efficiency and polar nature. Razali et al. (Citation2012) investigated the antioxidant activities of different parts of pomegranate fruits. His results supported our results and showed the higher antioxidant activity of pomegranate seed extract in methanol as compared to other solvents such as water, ethyl acetate, and hexane. The hexane solvent has lower efficiency to extract phenolic compounds and antioxidant capacity of a pomegranate seed (Razali et al., Citation2012). Basiri (Citation2013), evaluated the effect of solvents on the free antioxidant power capacity of pomegranate seed. He reported the higher antioxidant capacity of pomegranate seed with methanol solvent (721.8 μMol Fe2+/l) followed by water (207.6 μMol Fe2+/l), and acetone extraction (155.1 μMol Fe2+/l) respectively. The lowest antioxidant activity was recorded in hexane fraction followed by ethyl acetate and butanol (Basiri, Citation2013). Fernandes et al. (Citation2019) confirmed that the methanol is the best solvent for the extraction of high quality of phenolic fractions and antioxidant activity as compared to other solvents, i.e., water, ethanol, and water: acetone combination.
Conclusion
Ultrasonic-assisted extraction of freeze-dried pomegranate seed powder has good phenolic, flavonoid content with higher antioxidant activity. Methanolic extraction showed the highest values of phenolic, flavonoid content and antioxidant activity followed by ethanol and aqueous extraction of pomegranate seed powder. Hexane solvent showed less extraction potential of phenolic compounds and other compounds with antioxidant activity. The ultrasonic-assisted extraction method (45°C for 30 min) synergized with different solvents such as methanol, ethanol, and water showed effective results related to high content extraction of phenolic, flavonoid compounds and antioxidant activity of freeze-dried pomegranate seed powder.
Acknowledgments
Infrastructural support provided by the National Institute of Food Technology Entrepreneurship and Management (under Ministry of Food Processing Industries), Kundli – 131028 (Sonepat), Haryana, India is also duly acknowledged, Technical and service support from APT Lab, NIFTEM is greatly acknowledged.
Disclosure statement
The authors declare that there is no conflict of interest associated with this article.
References
- Abiola, T., L.K. Falana, and D. Adediji. 2018. Proximate composition, phytochemical analysis and in vivo antioxidant activity of pomegranate seeds (Punica granatum) in female albino mice. Biochem. Pharmacol. 7:250.
- Al-Maiman, A.S., and D. Ahmad. 2002. Changes in physical and chemical properties during pomegranate (Punica granatum L.) fruit maturation. Food Chem. 76:437–441. doi: 10.1016/S0308-8146(01)00301-6.
- Al-Sanabani, A.S., K.M. Youssef, A.A. Shatta, and S.K. El-Samahy. 2016. Impact of freezing and freeze-drying processes on color, phytochemical contents and antioxidant capacity of pomegranate seeds.SCUJFS. Suez Canal Univ. 3(1):27–34. doi: 10.21608/scuj.2016.6659.
- AOAC. 1995. Official methods of analysis, Association of Official Analytical Chemists.
- AOAC. 2000a. Official methods of analysis, Association of Official Analytical Chemists, in Method. 932.12.
- AOAC. 2000b. Official methods of analysis. 15th ed. Association of Official Analytical Chemist, Washington.
- AOAC. 2016a. Official methods of analysis, in method SMPR®. 20th ed. Association of Official Analytical Chemists, 2015.009, Washington.
- AOAC. 2016b. Official methods of analysis, in method SMPR®. 20th ed. Association of Official Analytical Chemists, 2011.011, Washington.
- Basiri, S. 2013. Evaluation of antioxidant and antiradical properties of pomegranate (Punica granatum L.) seed and defatted seed extracts. J. Food Sci. Technol. 52(2):1117–1123. doi: 10.1007/s13197-013-1102-z.
- Brand-Williams, W., M.E. Cuvelier, and C. Berset. 1995. Use of a free radical method to evaluate antioxidant activity. Lebensm.-Wiss. u.-Technol. 28:25–30. doi: 10.1016/S0023-6438(95)80008-5.
- Buddin, S.M.H.M., A.M.Z. Rithuan, A.M.S. Surni, M.N.H. Jamal, and F.M. Faiznur. 2018. Ultrasonic Assisted Extraction (UAE) of moringa oleifera seed oil: kinetic study. ASM Sci. J. 11(3):158–166.
- Charles, H.W.I., P.O.S.V. Manuel, D.M. Gisely, C.T. Solange, and B.G. Ivanise. 2012. Extraction and quantification of phenolic acids and flavonols from Eugenia pyriformis using different solvents. J. Food Sci. Technol. 51(10):2862–2866.
- Chen, M., Y. Zhao, and S. Yu. 2015. Optimisation of ultrasonic-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from sugar beet molasses. Food Chem. 172(1):543–550. doi: 10.1016/j.foodchem.2014.09.110.
- Christian, V., Z.F. Benno, and C.H. Sigrun. 2015. Efficacy and safety of pomegranate medicinal products for cancer. Evidence-Based Complementary Altern. Med. 258598:1–15.
- Deng, J., Z. Xu, C. Xiang, J. Liu, L. Zhou, T. Li, Z. Yang, and C. Ding. 2017. Comparative evaluation of maceration and ultrasonic-assisted extraction of phenolic compounds from fresh olives. Ultrason. Sonochem. 37:328–334. doi: 10.1016/j.ultsonch.2017.01.023.
- Elfalleh, W., H. Hannachi, N. Tlili, Y. Yahia, N. Nasri, and A. Ferchichi. 2012. Total phenolic contents and antioxidant activities of pomegranate peel, seed, leaf and flower. J. Med. Plant Res. 6(xx):4724–4730. doi: 10.5897/JMPR11.995.
- Ercisli, S., J. Gadze, G. Agar, N. Yildirim, and Y. Hizarci. 2011. Genetic relationships among wild pomegranate (Punica granatum) genotypes from Coruh Valley in Turkey. Genet. Mol. Res. 10(1):459–464. doi: 10.4238/vol10-1gmr1155.
- Fahad, J.A., O.M. Mehmet, and G. Kashif. 2017. Characterization of pomegranate (Punica granatum L.) seed and oils. Eur. J. Lipid Sci. Technol. 119(1700074):1–6.
- Fernandes, L., J.A. Pereira, J. Saraiva, S. Casal, and E. Ramalhosa. 2019. Extraction solvents’ influence on the content of bioactive compounds and antioxidant activity of pansies. Millenium 2(8):89–99.
- Gadze, J., S. Voca, Z. Cmelik, I. Mustac, S. Ercisli, and M. Radunic. 2012. Physico-chemical characteristics of main pomegranate (Punica granatum L.) cultivars grown in Dalmatia region of Croatia. J. Appl. Bot. Food Qal. 85:202–206.
- Hota, M., and D.S. Dahiya. 2017. Physico-chemical properties of some varieties of pomegranate (Punica granatum L.). Ind. J. Pure App. Biosci. 5(5):979–983. doi: 10.18782/2320-7051.2671.
- Huang, W., A. Xue, H. Niu, Z. Jia, and J. Wang. 2009. Optimised ultrasonic-assisted extraction of flavonoids from Folium eucommiae and evaluation of antioxidant activity in multi-test systems in vitro. Food Chem. 114:1147–1154. doi: 10.1016/j.foodchem.2008.10.079.
- Illana, D.M.L.P., D.C.B.T. Eliane, S.M.D. Ana, Y.T. Luciana, S.A.G. José, P.T. Rosângela, and M.F. Jorge. 2016. Characterization of constituents, quality and stability of pomegranate seed oil (Punica granatum L.). Food Sci. Technol. 36(1):132–139. doi: 10.1590/1678-457X.0069.
- Jing, P., T. Ye, H. Shi, Y. Sheng, M. Slavin, B. Gao, L. Liu, and L. Yu. 2012. Antioxidant properties and phytochemical composition of China-grown pomegranate seeds. Food Chem. 132(3):1457–1464. doi: 10.1016/j.foodchem.2011.12.002.
- Kennas, A., H. Amellal-Chibane, F. Kessal, and F. Halladj. 2020. Effect of pomegranate peel and honey fortification on physicochemical, physical, microbiological and antioxidant properties of yoghurt powder. J. Saudi Soc. Agric. Sci. 19(1):99–108.
- Keziban, Y., and S. Alpaslan. 2016. Characterization of pomegranate (Punica granatum L.) hybrids and their potential use in further breeding. Turk. J. Agric. For. 40:813–824. doi: 10.3906/tar-1604-120.
- Kumar, N., K.S. Neeraj, and S. Kumar. 2018. Functional properties of pomegranate (punica granatum l.). pharma. 7(10):71–81.
- Kumar, N., and Neeraj. 2018. Study on physico-chemical and antioxidant properties of pomegranate peel. J. Pharmacogn Phytochem. 7(3):2141–2147.
- Kumar, N., and Neeraj. 2020. Effect of ultrasonic assisted extraction on the properties of freeze-dried pomegranate arils. Curr. Nut. Food Sci. 16:83–89. doi: 10.2174/1573401315666181130100200.
- Massaud, A.I., A.A. Baig, and A.K.M. Rohin. 2017. Exhaustive extraction of compounds from pomegranate peel and flesh using solvents of varying polarity. Asian Biomed. 1(1):10–13.
- Mohammad, B.T., M. Hamid, and A.R. Amir. 2016. Pomegranate seed oil: A comprehensive review on its therapeutic effects. Int. J. Pharm. Sci. Res. 7(2):430–442.
- Mphahlele, R.R., O.A. Fawole, L.M. Mokwena, and U.L. Opara. 2016. Effect of extraction method on chemical, volatile composition and antioxidant properties of pomegranate juice. S Afr. J. Bot. 103:135–144. doi: 10.1016/j.sajb.2015.09.015.
- Nejib, H., M. Messaoud, G. Sana, T. Mokhtar, M. Pablo, and H. Francisca. 2011. Seed and juice characterization of pomegranate fruits grown in Tunisia: Comparison between sour and sweet cultivars revealed interesting properties for prospective industrial applications. Ind. Crops Prod. 33:374–381. doi: 10.1016/j.indcrop.2010.11.006.
- Padmaja, A., and B.L.N. Prasad. 2011. Pomegranate (Punica granatum L.) peel extract as a source of natural antioxidant. J. Food Sci. Eng. 1:171–182.
- Prasad, S., G.K. Mukunda, A.B. Mohankumar, and K. Yathiraj. 2012. Comparatives studies of commercially important varieties of pomegranate (Physico-chemical properties). Hind. Agri. Res. Ins. 7(3–4):287–291.
- Puneeth, H.R., and S.S.P. Chandra. 2020. A review on potential therapeutic properties of pomegranate (Punica granatum L.). Plant Sci. 7(1):9–16.
- Radunic, M., M.J. Spika, S.G. Ban, J. Gadze, J.C. Diaz-Perez, and D. MacLean. 2015. Physical and chemical properties of pomegranate fruit accessions from Croatia. Food Chem. 177:53–60. doi: 10.1016/j.foodchem.2014.12.102.
- Razali, N., J.S. Mat, M.A.F. Abdul, S. Subramaniam, and A.A. Abdul. 2012. Effects of various solvents on the extraction of antioxidant phenolics from the leaves, seeds, veins and skins of Tamarindus indica L. Food Chem. 131(2):441–448. doi: 10.1016/j.foodchem.2011.09.001.
- Russo, M., F. Cacciola, K. Arena, D. Mangraviti, L.D. Gara, P. Dugo, and L. Mondello. 2020. Characterization of the polyphenolic fraction of pomegranate samples by comprehensive two-dimensional liquid chromatography coupled to mass spectrometry detection. Nat. Prod. Res. 34(1):39–45. doi: 10.1080/14786419.2018.1561690.
- Sehrawat, R., O.A. Babar, A. Kumar, and P.K. Nema. 2018. Trends in drying of fruits and vegetables, p. 109–132. In: R. Sehrawat, K.A. Khan, M.R. Goyal, and P.K. Paul eds. Technological interventions in the processing of fruits and vegetables. 1st. Apple Academic Press, Waretown.
- Sehrawat, R., and P.K. Nema. 2018. Low pressure superheated steam drying of onion slices: Kinetics and quality comparison with vacuum and hot air drying in an advanced drying unit. J. Food Sci. Technol. 55(10):4311–4320. doi: 10.1007/s13197-018-3379-4.
- Singh, R.P., M.K.N. Chidambara, and G.K. Jayaprakasha. 2002. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J. Agric. Food Chem. 50(1):81–86. doi: 10.1021/jf010865b.
- Singleton, V.L., and J.A.R. Joseph. 1965. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 16:144–158.
- Sultana, B., F. Anwar, and M. Ashraf. 2009. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 14(6):2167–2180. doi: 10.3390/molecules14062167.
- Thouri, A., H. Chahdoura, A.E. Arem, A.O. Hichri, R.B. Hassin, and L. Achour. 2017. Effect of solvents extraction on phytochemical components and biological activities of Tunisian date seeds (var. Korkobbi and Arechti). BMC Complement Altern. Med. 17:248. doi: 10.1186/s12906-017-1751-y.
- Walid, E., N. Maying, S. Nizar, H. He, G. Ferdaus, and F. Ali. 2011. Fatty acids from Tunisian and Chinese pomegranate (Punica granatum L.) seeds. Int. J. Food Sci. Nutr. 62(3):200–206. doi: 10.3109/09637486.2010.526932.
- Wang, Z., Z. Pan, H. Ma, and G.G. Atungulu. 2011. Extract of phenolics from pomegranate peels. Open Food Sci. J. 5:17–25. doi: 10.2174/1874256401105010017.
- Wen, L., Z. Zhang, D. Rai, D.W. Sun, and B.K. Tiwari. 2019. Ultrasound‐assisted extraction (UAE) of bioactive compounds from coffee silverskin: Impact on phenolic content, antioxidant activity, and morphological characteristics. J. Food Process. Eng. 42(6):1–11. doi: 10.1111/jfpe.13191.
- Wetzstein, H.Y., Z. Zhang, N. Ravid, and M.E. Wetzstein. 2011. Characterization of attributes related to fruit size in pomegranate. Hort. Sci. 46(6):908–912. doi: 10.21273/HORTSCI.46.6.908.
- Xia, T., S. Shi, and X. Wan. 2006. Impact of ultrasonic-assisted extraction on the chemical and sensory quality of tea infusion. J. Food Eng. 74:557–560. doi: 10.1016/j.jfoodeng.2005.03.043.